Research Article Open Access
Cerebral Oxygenation Using Near-infrared Spectroscopy (NIRS) before, during and after Therapeutic Hypothermia: A Comparison of Cerebral Saturations between those Infants on Sedatives and Anti-Epileptics and those who are not, all of whom are Undergoing Cooling
Dixon CM and Rais-Bahrami K*
Children′s National Medical Center and The George Washington University School of Medicine, Washington, DC, USA
- *Corresponding Author:
- Rais-Bahrami K
Children′s National Medical Center and The George Washington University School of Medicine
Washington, DC, USA
Tel: 202-476-4683
E-mail: kraisbah@childrensnational.org
Received date: March 09, 2016, Accepted date: April 04, 2016, Published date: April 09, 2016
Citation: Dixon CM, Rais-Bahrami K (2016) Cerebral Oxygenation Using Near-infrared Spectroscopy (NIRS) before, during and after Therapeutic Hypothermia: A Comparison of Cerebral Saturations between those Infants on Sedatives and Anti-Epileptics and those who are not, all of whom are Undergoing Cooling. Neonat Pediatr Med 2: 107. doi: 10.4172/2572-4983.1000107
Copyright: © 2016 Rais-Bahrami K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Neonatal and Pediatric Medicine
Abstract
Objectives: The aims of this study include the following: to determine the effect of therapeutic hypothermia (Cooling) on cerebral saturations using Near Infrared Spectroscopy (NIRS) before, during and after therapeutic hypothermia; to compare these values between infants receiving sedative and anti-epileptic medications and those who do not.
Methods: This study is a retrospective chart review of patients from Children’s National Medical Center (CNMC) Neonatal Intensive Care Unit (NICU) who underwent therapeutic hypothermia with NIRS monitoring from July 2009- December 2014. Cerebral tissue saturations (StO2) using NIRS tissue oximeter (FORE-SIGHT, CAS Medical Systems, Branford, CT, USA) were assessed during the cooling period. StO2 were periodically recorded during 3 phases: before cooling was started, during cooling, and after cooling (30 minutes of rewarming) and averaged to a composite value for each event. Data was then compared based on whether the patient received sedatives and/or anti-epileptic medications.
Results: Complete data sets were obtained for 57 subjects, weighting 1.8 kg-4.9 kg, 1-7 days old and gestational age 35.6-42.0 weeks. Cerebral tissue saturations were significantly higher during cooling (paired t-test). Those on Phenobarbital and/or Versed had significantly higher saturations compared to those on no medications. Those on only Phenobarbital also had significantly higher saturations, but to a lesser degree. Subjects where StO2 failed to rise during cooling had a higher chance of dying, perhaps due to critical brain tissue damage from lack of oxygen during birth asphyxia, or failure to recover thereafter.
Conclusions: Data from this study suggests that cerebral tissue saturations increase during therapeutic hypothermia, likely due to suppressed cerebral metabolism. Those on anti-seizures medications
Keywords
Near-infrared spectroscopy; Cerebral oxygenation
Introduction
Therapeutic hypothermia has been used as a neuroprotective therapy in the management of neonates with hypoxic ischemic encephalopathy (HIE). There is established evidence from major multi-center studies that cooling decreases brain energy use, thereby reducing neuronal cell loss and preserving brain structures [1]. These studies have shown reduced death and developmental disabilities in babies who underwent therapeutic hypothermia.
Neonatal encephalopathy occurs in 1.5/1000 live births [2]. Neonates with mild encephalopathy usually do not have an increased risk for neurodevelopmental delays; however, those with moderate to severe encephalopathy have a high risk of death, CP, and mental retardation. The mechanism behind brain injury involves reductions in cerebral blood flow and a buildup of oxygen compounds that leads to disruption in mitochondrial function and an excessive stimulation of neurotransmitter cascades. The release of free-radicals from this cascade eventually causes cell death. Resolution of the hypoxicischemic event within a certain time frame can reverse the release of toxic compounds and neurotransmitter excitation. However, if injury is severe, a second energy failure can occur in which mitochondrial function is again disrupted [3].
This interval between first and second energy failure represents a window for therapeutic intervention. Research has shown that cooling before 6 hours of life can act as a protective mechanism at cellular and vascular sites of injury. Hypothermia halts the neurotransmitter cascade and inhibits accumulation of free-radicals and other toxic metabolites. This process inhibits mitochondrial failure and cerebral inflammation. Overall, therapeutic hypothermia reduces neuronal cell loses and preserves brain structures.
NIRS is a technology that allows non-invasive continuous real-time measurement of cerebral saturations of infants on cooling protocols [3]. This technology allows for non-invasive continuous measurement of regional tissue oxygen saturations of distant organs. The neonatal brain is readily accessible by NIRS and may provide insight into a correlation between inadequate tissue oxygenation and poor clinical outcomes [4].
Therapeutic hypothermia, as described, reduces the metabolic demands of the brain. Cerebral saturations from NIRS devices are of particular interest before, during and after therapeutic hypothermia as they reflect blood flow to the brain during a time when the metabolic state of the brain is changing. This is of particular clinical interest because we expect cerebral blood flow prior to cooling to be decreased and to increase during the re-warming period. Also, infants on cooling protocols often have disorganized brain activity that is clinically represented by seizures. To our knowledge, a study has yet to assess how cerebral blood flow differs between infants on neurologic medications - such as anti-epileptics and sedatives - and those not on these types of medications during cooling.
Methods
The design of this study was to conduct a retrospective chart review of patients in the Cerner database in the NICU at CNMC from July 2009-December 2014 who underwent cooling with cerebral saturation StO2 measurements using a NIRS monitor (FORE-SIGHT, CAS Medical Systems, Branford, CT, USA) using small size sensors for neonates. Cerebral StO2 were recorded before cooling (Normothermia), during cooling (hypothermia), and after cooling (Rewarming 30 minutes) and averaged to a composite value for each event. Data was also recorded on whether the patient received antiepileptics Phenobarbital and/or Versed. Patients′ demographic data collection also included gender, gestational age, birth weight, Apgars, and survival/neurologic outcome.
The cerebral StO2 data collected from Normothermia, Cooling, and Rewarming events were analyzed to determine any changes due to the effects of hypothermia, as well as the influence of anti-epileptic drugs given before NIRS StO2 measurements using repeated measures ANOVA, paired T-Tests, and independent T-tests as appropriate. Since some subjects did not receive anti-epileptic drugs, this also allowed insight on how induced hypothermia alone affects cerebral StO2. Data was also separated to two groups, survivors and subjects who died in case of any confounding effects and further analyzed. Subjects with base deficit values recorded was also compared to cerebral StO2 using regression techniques.
Results
Data was collected for 57 subjects, weighing 1.8 kg-4.9 kg, 1-7 days old, GA of 33.4-43.4 weeks with 30 males and 22 females. 42 subjects survived and 15 died. Normothermia and cooling cerebral StO2 values were recorded for the 57 subjects, but for Rewarming, StO2 was recorded from 45 subjects. 9 of 12 subjects with no recorded Rewarming StO2 values died.
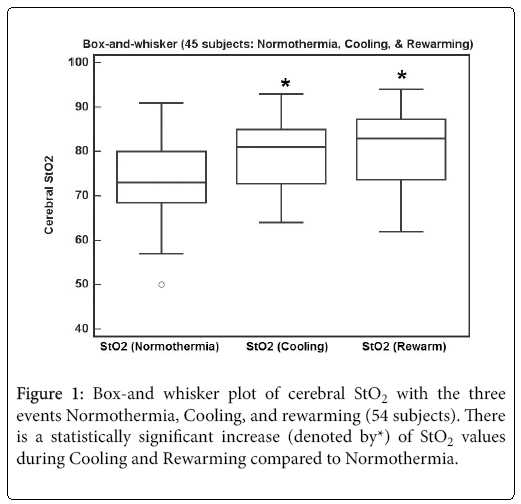
Figure 1 shows a box-and whisker plot of the 45 subjects with the three events Normothermia, Cooling and Rewarming. There is a statistically significant increase of StO2 values during Cooling 79.4 (1.1) and Rewarming 80.6 (1.3), compared to Normothermia 74.0 (1.4) [mean (SE), p<0.001, Repeated measures ANOVA). Since some Rewarming StO2 data was lost, further analysis will be done with Normothermia and Cooling StO2 values only.
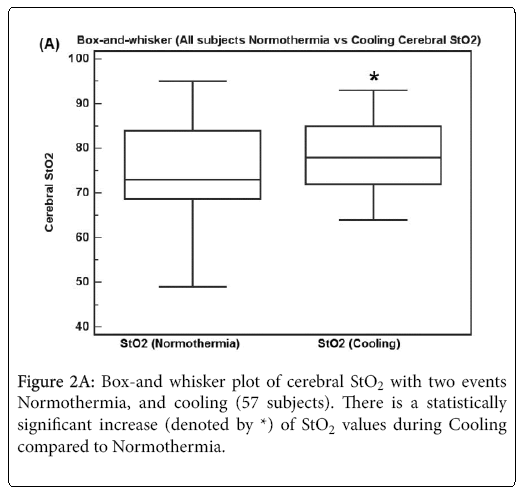
Figure 2A shows a box-and whisker plot of all 57 subjects with two events Normothermia, and Cooling. There is a statistically significant increase of StO2 values by 3 (9.8 sd) during Cooling compared to Normothermia (p = 0.037, Repeated measures T-Test).
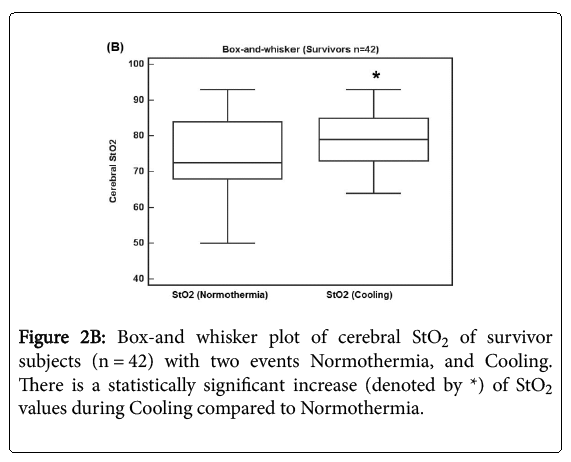
Figure 2B shows a box-and whisker plot of 42 survivor subjects with two events Normothermia, and Cooling. There is a statistically significant increase of StO2 values by 4.6 (9.4 sd) during Cooling compared to Normothermia (p = 0.0029).
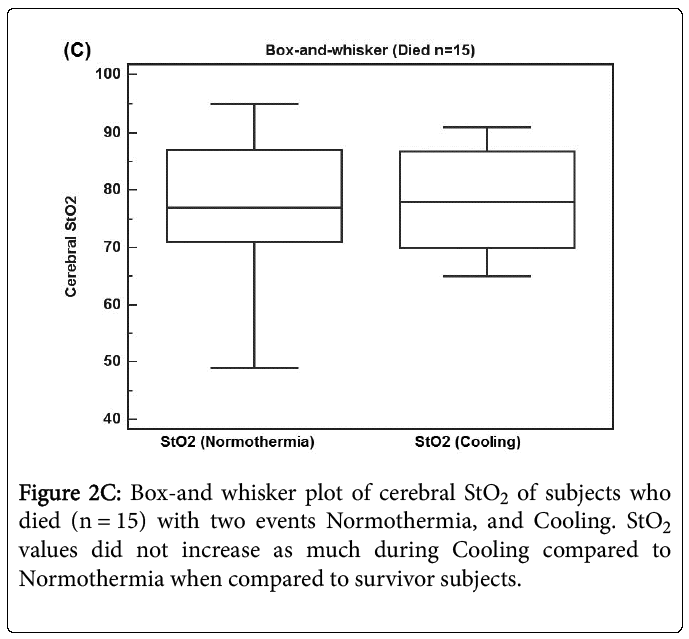
Figure 2C shows a box-and whisker plot of 15 subjects who died with two events Normothermia, and Cooling. StO2 values did not increase as much 2.0 (10.8 sd) during Cooling compared to Normothermia when compared to survivor subjects (p = 0.486). Therefore StO2 data from subjects who died may confound further analysis and so both survivor and deceased subjects data will be reported separately for the next comparisons.
To evaluate hypothermia effects alone and checking for any confounding effects for mortality, subjects who were not given antiepileptic drugs were analyzed.
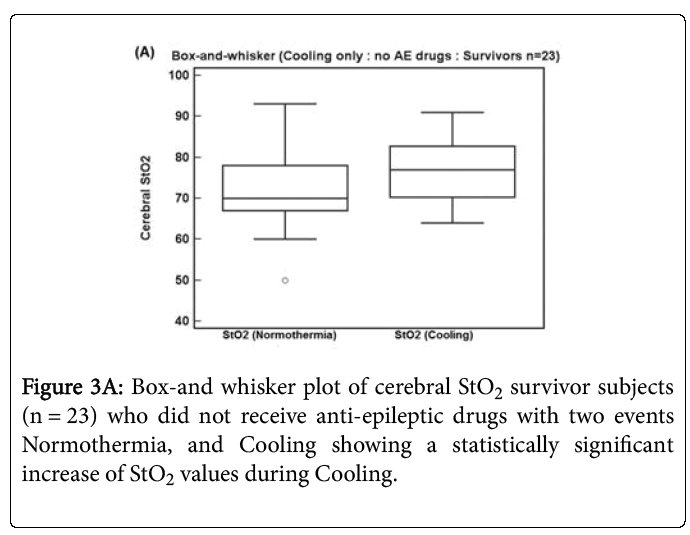
Figure 3A shows a box-and whisker plot of 23 survivor subjects who did not receive anti-epileptic drugs with two events Normothermia, and Cooling. There is now a statistically significant increase of StO2 values by 4.3 (9.0 sd) during Cooling compared to Normothermia (p = 0.034, Repeated measures T-Test).
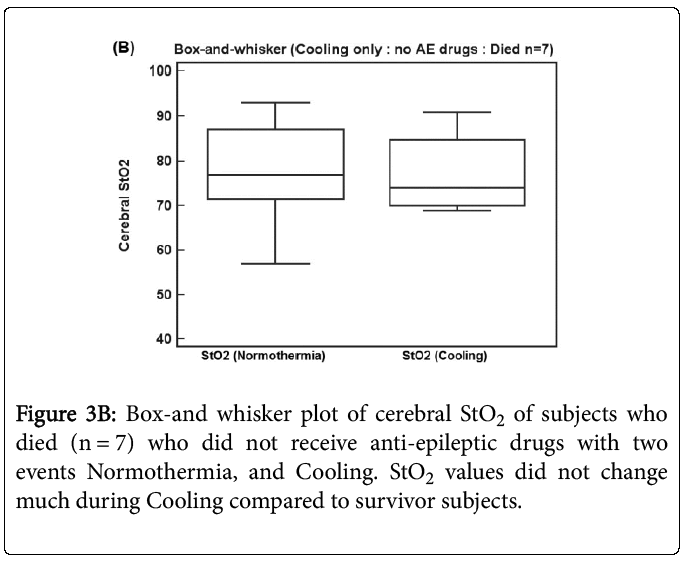
Figure 3B shows a box-and whisker plot of 7 subjects who died and did not receive anti-epileptic drugs with two events Normothermia, and Cooling. StO2 values did not change much -0.3 (9.8 sd) during Cooling compared to Normothermia when compared to survivor subjects (p = 0.94). Therefore there is evidence that hypothermia alone without anti-epileptic drugs increases StO2 during Cooling, which seems to be muted for subjects who died.
To evaluate for anti-epileptic drug effects and any confounding effects for mortality, Normothermia and Cooling StO2 values were compared for subjects receiving anti-epileptic drugs vs. subjects not receiving drugs for the next analysis using Independent T-Test with Equal Variances. Table 1 shows the results, along with Figures 4-6.
| Cerebral StO2 Mean (SE) difference | Event | ALL Subjects (n=57) | Survivors (n=42) | Died (n=15) |
|---|---|---|---|---|
| Anyanti-epilepticdrug | Normothermia | 3.5(2.8) n=27 | 4.9(3.0) n=19 | 0.9(6.9) n=8 |
| Cooling | 5.1(1.9) n=27 * | 5.7(2.1) n=19 * | 3.4(4.7) n=8 | |
| Phenobarbital | Normothermia | 1.9(2.9) n=20 | 0.4(3.3) n=13 | 6.5(6.6) n=7 |
| Cooling | 4.5(2.1) n=20 * | 5.0(2.3) n=13 * | 3.9(4.7) n=7 | |
| Versed | Normothermia | 5.3(3.1) n=15 | 6.4(3.2) n=12 | 2.3(8.6) n=3 |
| Cooling | 2.8(2.3) n=15 | 1.7(2.5) n=12 | 6.5(4.6) n=3 |
Table 1: Increase of cerebral StO2 for Normothermia and Cooling for subjects given anti-epileptic drugs, with statistical significance denoted by*. Data also shown in Figures 4-6.
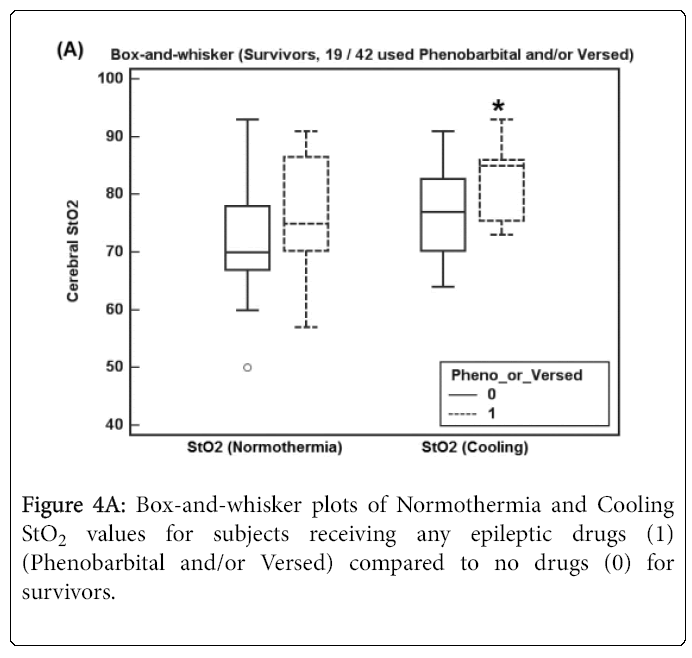
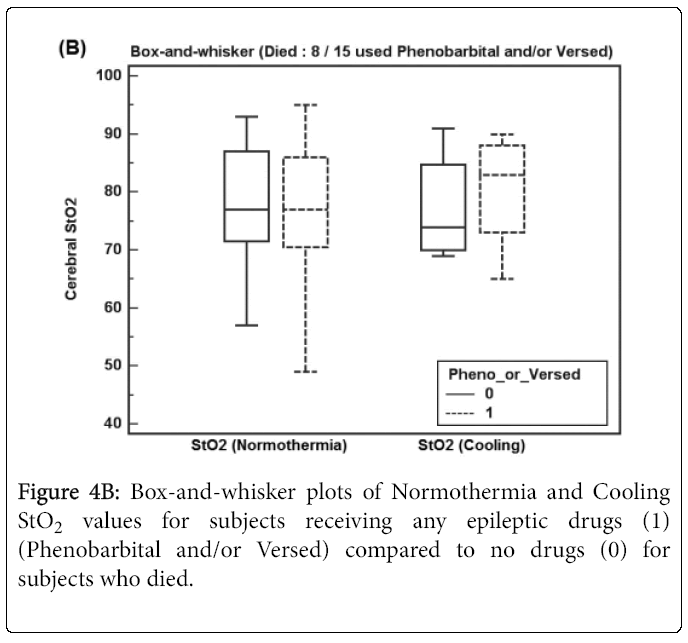
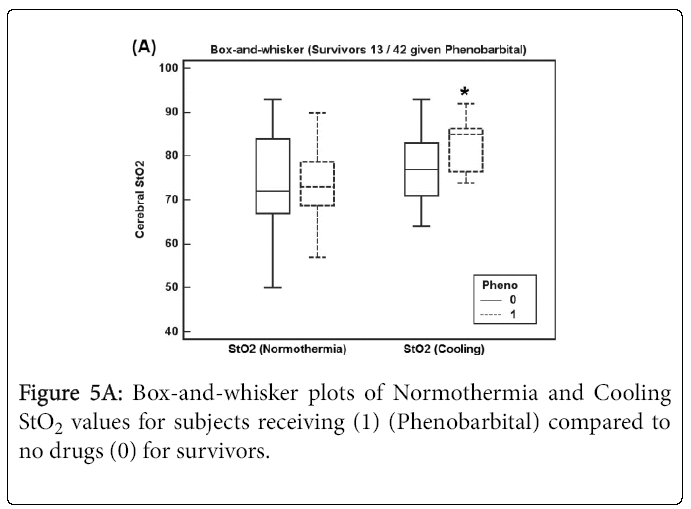
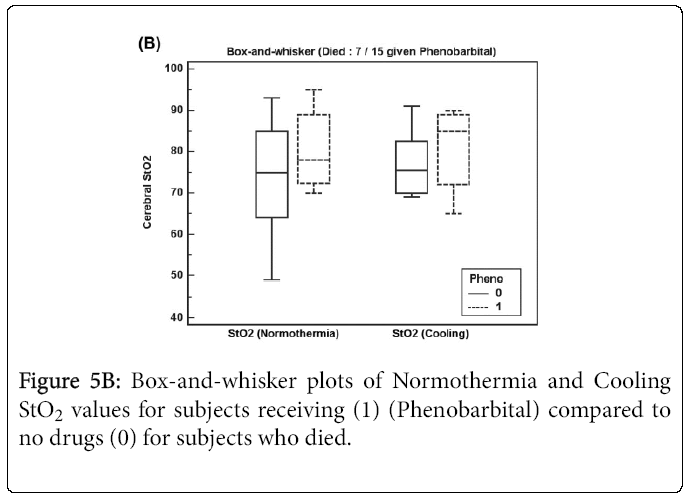
Figures 4A and 4B show box-and-whisker plots of Normothermia and Cooling StO2 values for subjects receiving any epileptic drug before StO2 recordings (Phenobarbital and/or Versed) for (A) survivors and (B) subjects who died.
Figure 4A shows Normothermia and Cooling StO2 values for survivor subjects receiving any anti-epileptic drugs (19 of 42 subjects) where Cooling StO2 increased significantly (p = 0.009).
Figure 4B shows Normothermia and Cooling StO2 values for subjects who died receiving any anti-epileptic drugs (8 of 15 subjects) where Cooling StO2 did not increase significantly.
Therefore there is evidence that anti-epileptic drugs increases StO2 during Normothermia and especially Cooling, as an additive effect over hypothermia subjects alone without drugs. The effect of antiepileptic drugs on StO2 appears muted for subjects who died.
Figure 5A shows Normothermia and Cooling StO2 values for survivor subjects receiving Phenobarbital (13 of 42 subjects) where Cooling StO2 increased significantly (p = 0.039). For subjects who died shown in Figure 5B, Normothermia and Cooling StO2 values increased but not significantly for subjects receiving Phenobarbital (7 of 15 subjects) perhaps due to low sample size.
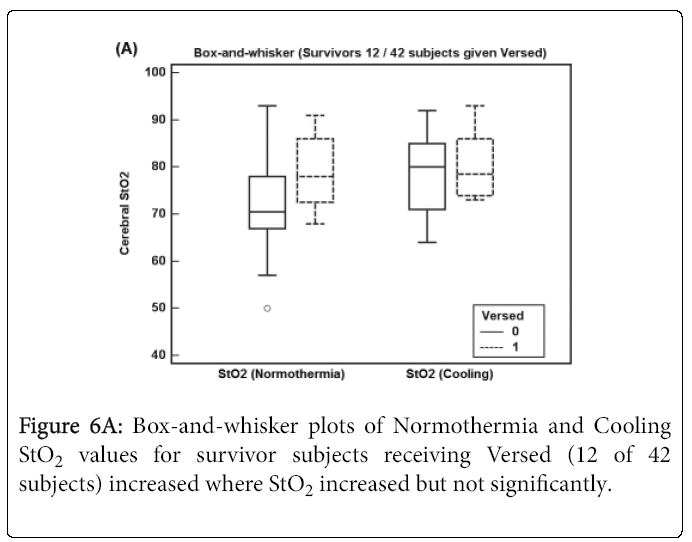
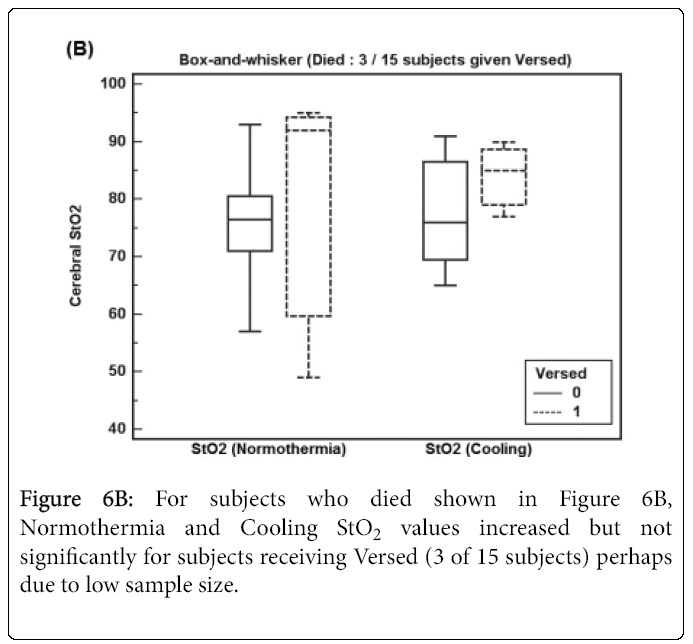
Figure 6A shows Normothermia and Cooling StO2 values for survivor subjects receiving Versed (12 of 42 subjects) where StO2 increased but not significantly. For subjects who died shown in Figure 6B, Normothermia and Cooling StO2 values increased but not significantly for subjects receiving Versed (3 of 15 subjects) perhaps due to low sample size.
The results suggest that for subjects who died, hypothermia and anti-epileptic drugs did not increase StO2 as much as survivor subjects. Another view is to determine if StO2 increased during Cooling compared to Normothermia for survivors and deceased subjects, where the change of StO2 from Normothermia to Cooling is categorized as >0 (increases) to < (no increase). A Chi-Square test shows a statistically significant difference (p = 0.022) as shown in Table 2. This suggests that if StO2 does not increase during hypothermia, the subject has a higher chance of death.
| Outcome | |||
|---|---|---|---|
| Change in StO2 during Cooling | Survivor | Died | |
| DStO2>0 | 30 | 5 | 35 (61.4%) |
| DStO2≤0 | 12 | 10 | 22 (38.6%) |
| 42(73.7%) | 15(26.3%) | 57 | |
| Chi-square | 5.256 | ||
| DF | 1 | ||
| Significance level | P=0.0219 | ||
| Contingency coefficient | 0.291 | ||
Table 2: Chi-Square Results comparing mortality outcomes vs. whether cerebral StO2 increased during Cooling. The statistically significant results suggest that if StO2 does not increase, there is a higher chance of subject death.
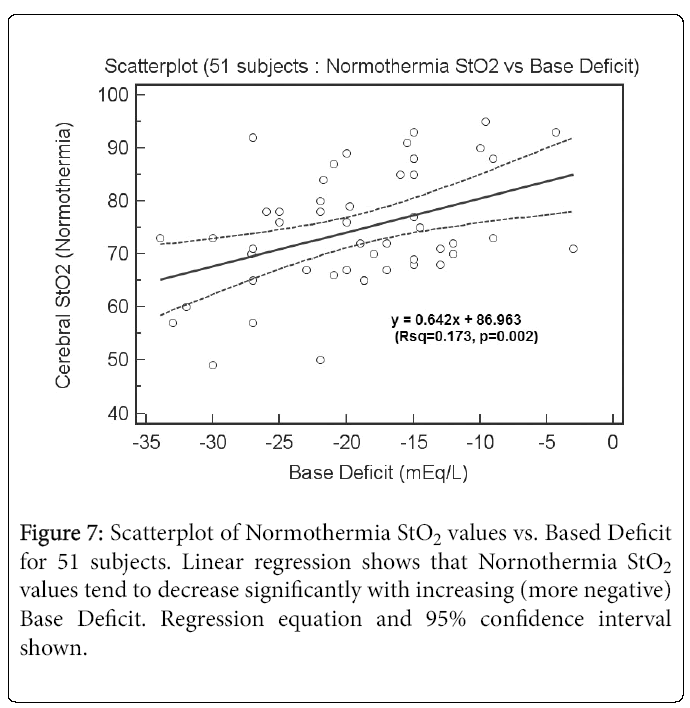
Figure 7 shows a scatterplot of Normothermia StO2 values vs. Base Deficit for 51 subjects. Linear regression shows that Nornothermia StO2 values tend to decrease significantly with increasing (more negative) Base Deficit. The regression equation was y = 0.642x+86.963 (R2 = 0.173, p = 0.002). For Cooling StO2, there is no correlation (data not presented) perhaps because the Base Deficit issue has been corrected clinically.
Discussion
The results show that cerebral tissue saturation StO2 increase during therapeutic hypothermia, and even more so for patients on Phenobarbital and Versed perhaps as an additive effect. This is likely due to suppressed cerebral metabolism during cooling, which there is increased cerebral oxygenation and decreased oxygen utilization. The results suggest that if cerebral StO2 fails to increase during therapeutic hypothermia, these subjects have a higher chance of death, perhaps due to critical brain tissue damage from lack of oxygen during birth asphyxia, or failure to recover thereafter.
The onset of the secondary energy failure - which cooling is meant to halt - has been characterized by cerebral vasodilation inducing delayed hyperemia and higher cerebral oxygenation, which is consistent with our results [3].
Others have postulated that our finding of increased saturations during cooling is due to the secondary energy failure and is particularly worse in infants with adverse outcomes. Toet et al. [5] evaluated infants with NIRS after birth asphyxia - who were not part of a cooling protocol - and measured oxygen saturation and oxygen extraction in the first days of life. Similar to our study, they also showed an increase in oxygen saturation beyond 24 hours of life and a decrease of oxygen extraction. This suggests that the secondary energy failure described above is associated with neuronal cell death - as evidenced by reduced oxygen extraction - and further points to the importance of therapeutic hypothermia to halt this process.
A study by Meek et al. evaluated 27 infants with perinatal asphyxia using NIRS devices and saw an increase in blood flow afterwards and their results interpreted this as a poor prognostic indicator. Our findings are consistent with this study. The Meek study also reports that infants with the most severe adverse outcomes had higher cerebral blood flow during the first 24 hours; our study similarly shows that infants on anti-seizure medications - which suggest greater severity of acute encephalopathic signs - had higher cerebral tissue saturations [1]. Higher blood flow could be secondary to an initial hyperperfusion of the brain after injury and recovery of oxidative metabolism. This also suggests the importance of therapeutic hypothermia to halt further metabolic cascades that could be damaging to neuronal cells.
Increased neuronal activity - as represented by seizures - is a particularly interesting cerebral hemodynamic change to study with NIRS monitoring. This is the first study to document comparisons between infants on anti-epileptic medications and those who are not. Our study confirms that seizures are consistent with fluctuations in cerebral perfusion. This further confirms that seizures are associated with changes in oxygen uptake by increased neuronal activity and also points to the need to monitor these asphyxiated infants on EEG or aEEG.
Limitations of this study include the fact that it was a retrospective chart review and during cooling period the cerebral saturation is an average of many data points recorded in the Cerner database. This means that not every infant had NIRS measurements every hour; there it is also the possibility of reader error during data recording as there were up to 20 + data points for each 24 hour period. However, the averages for the 57 infants before, during, and after cooling were consistent and did reach statistical significance.
The future direction for this project would ideally include a correlation of cerebral saturations with outcome. Nearly all infants in this data collection have an MRI that could offer interpretation as to possible severity of injury and outcome. An interesting future direction would be to correlate NIRS saturations with neurodevelopmental outcomes throughout toddlerhood.
Conclusion
Our study supports the use of NIRS in the routine management of sick preterm and term infants for monitoring of cerebral hemodynamics. NIRS has the potential to provide us with important clinical information in a variety of settings - in the delivery at resuscitation, during cooling, and during decompensation of ill infants. It allows one to measure trends in cerebral tissue oxygenation together with pulse oximetry. Since newborns are particularly susceptible to suffer from a range of cardiopulmonary issues that can affect their oxygenation, this seems like an ideal population for routine use of NIRS monitoring.
Acknowledgments
We gratefully acknowledge CAS Medical System for supporting this research and Paul Benni, PhD. whom contributed enormously to the success of this study.
Disclosure Statement
The authors declare there is no competing interest. Authors have no financial interest in the product discussed in this manuscript to disclose.
References
- Meeka JH, Elwellb CE, McCormickc DC, Edwardsd AD, Townsenda JP, et al. (1999) Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal 81: F110-F115.
- Rais-Bahrami K, Rivera O, Short BL (2006) Validation of a noninvasive neonatal optical cerebral oximeter in veno-venous ECMO patients with a cephalad catheter. Journal of perinatology 26: 628-635.
- Ancora G, Maranella E, Grandi S, Sbravati F, Coccolini E, et al. (2013) Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain and Development 35: 26-31.
- Fuchs H, Lindner W, Buschko A, Almazam M, Hummler H (2012) Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. Journal of Perinatology 32: 356-362.
- Toet MC, Lemmers PM, van Schelven LJ, van Bel F (2006) Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics 117: 333-339.
Relevant Topics
- About the Journal
- Birth Complications
- Breastfeeding
- Bronchopulmonary Dysplasia
- Feeding Disorders
- Gestational diabetes
- Neonatal Anemia
- Neonatal Breastfeeding
- Neonatal Care
- Neonatal Disease
- Neonatal Drugs
- Neonatal Health
- Neonatal Infections
- Neonatal Intensive Care
- Neonatal Seizure
- Neonatal Sepsis
- Neonatal Stroke
- Newborn Jaundice
- Newborns Screening
- Premature Infants
- Sepsis in Neonatal
- Vaccines and Immunity for Newborns
Recommended Journals
Article Tools
Article Usage
- Total views: 12435
- [From(publication date):
June-2016 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 11593
- PDF downloads : 842