Cerebral Amyloid Angiopathy or Frontotemporal Dementia? A Case Study
Received: 31-Aug-2017 / Accepted Date: 04-Sep-2017 / Published Date: 11-Sep-2017 DOI: 10.4172/2161-0460.1000373
Abstract
A 67 year old patient developed progressive behavioral abnormalities and executive dysfunction corresponding to the diagnosis of behavioral variant of frontotemporal dementia (bvFTD). Unexpectedly, MRI showed multiple vascular ischemic and hemorrhagic lesions as typically found in sporadic cerebral amyloid angiopathy (SCAA). Based on clinical, laboratory and imaging findings the diagnosis of possible SCAA was made. SCAA may represent another important differential diagnosis among the various underlying disease pathologies of the bvFTD syndrome.
Keywords: Behavioral variant frontotemporal dementia; Cerebral amyloid angiopathy; Behavioral abnormalities; Dysexecutive syndrome
Introduction
The behavioral variant of frontotemporal dementia (bvFTD) is characterized by neuropsychiatric abnormalities (disinhibition, apathy, loss of empathy, stereotyped behavior, hyperorality) and neuropsychological deficits (predominantly dysexecutive impairments), combined with frontal and/or anterior temporal atrophy [1]. Several conditions may mimick bvFTD due to their overlapping clinical presentation, such as, e.g. Alzheimer’s disease (AD) [2], vascular cognitive impairment [3], particularly in patients with subcortical vascular lesions [4,5], mixed dementia [6] and also psychiatric conditions [7,8]. Sporadic cerebral amyloid angiopathy (SCAA) is an important clinical entity which is present in up to 40% of elderly brains and 80% or more in patients with concomitant Alzheimer’s disease [9,10]. SCAA is associated with meningeal or parynchymal amyloid-ß deposition within cerebral blood vessels causing non-traumatic lobar hemorrhages, microbleeds, focal superficial or sulcal siderosis and diffuse white matter lesions. Spontaneous microbleeds are found in neocortical, posterior regions, and later progress to other cortical areas [9,11]. Due to variable involvement of neural structures, SCAA presents heterogeneously. Clinical correlates of vascular dysfunction in SCAA include recurrent focal neurological symptoms and signs, and progressive cognitive and functional decline [12-14]. The cognitive impairment of SCAA through hemorrhagic and ischemic pathomechanisms comprises mainly memory, executive functions and perceptual speed [15]; it may progress to MCI [16] and eventually to dementia [10]. Prominent psychopathology may also be present in SCAA [17,18]. SCAA appears as a distinct cerebrovascular condition, or comorbid with AD [19,20], Here we report a case of SCAA whose striking behavioral abnormalities and neuropsychological test results closely resembled bvFTD.
Case Report and Methods
The patient is a 67 year old right handed male (11y formal education), founder and manager of a large business, with a 2 year history of progressive behavioral abnormalities and cognitive dysfunction. He has no family history of dementia or strokes and reported no vascular risk factors such as hypertension, cardiac, metabolic or vessel disease. He never complained of headache. Over the past 24 months he had two short episodes of a sudden, slight left sided hemiparesis with full recovery within hours which were interpreted as TIAs. Neuropsychiatric abnormalities comprised apathy and social withdrawal, loss of spontaneous ideas, motivation and long-standing interests, excessive periods of daytime sleep and neglect of hygiene and clothing. He developed a new preference for sweets, alcohol and sexual encounter, a peculiar attachment to schedules and stereotypies like, e.g. starting his car repeatedly over the day to prevent fading of the battery. These traits were in strong contrast to his premorbid personality which was described as active, interested, highly motivated, conscientious, flexible, well organized and behaviorally controlled. Topographical disorientation and problems with more complex, e.g. financial or household tasks were noted as cognitive impairments. Progressive memory decline was reported over the past 12 months, but there was no history of amnesic episodes before that time. The patient remained completely anosognosic despite the progression of impairments and his functional decline. There was no gait disorder, bladder dysfunction or history of seizures. Neurological examination was normal and the Hachinski score was 2/10. Because of the striking neuropsychiatric and cognitive abnormalities, the patient was diagnosed with possible bvFTD and underwent a detailed assessment of psychopathology, neuropsychology, as well as MRI, FDG-PET, EEG and laboratory investigation.
Results
Assessment of behavior and cognition (Tables 1 and 2). Behavior was rated on standard scales by a knowledgeable family member and partly also by self-rating. In sum, the patient was characterized as apathetic, poorly motivated, hyperoral, with problems to plan, selforganize, execute and monitor more complex routines and to handle personal care. Also notified were problems to make proper decisions or to interpret the behavior of other people correctly. Self and proxy ratings differed considerably in the judgement of executive abilities, behavioral control and apathy. Neuropsychological testing revealed severe impairments of global cognition (MMSE), verbal and figural memory (CERAD [21]) and executive functions (FAB [22]) verbal and figural fluency, trail making tests, clock drawing, Stroop color-word task [23]). On a gambling task (Game of Dice Task [24]) the patient made many risky decisions. Recognition of facial emotions [25] and more complex mental arithmetics [26] were also impaired. Object naming was defective and comprehension (Token test) was mildly impaired, whereas other standard language functions were normal. Dementia severity (CDR [27]) was estimated as mild.
| Hospital Anxiety and Depression Scale [48] | Anxiety | 4 | Cut-off: 11 |
| Depression | 0 | Cut-off: 11 | |
| Apathy Evaluation Scale [49] | Patient | 35 | Possible |
| Caregiver | 49 | Present | |
| The Dysexecutive Questionnaire [50] | Self-rating | 13 | PR 18 |
| Caregiver’s rating | 41 | PR 69 | |
| Frontotemporal Dementia Scale [51] | Behaviour | 1 | |
| Outing and shopping | 2 | ||
| Household chores and phone | 0 | ||
| Finances | 1 | ||
| Medication | 1 | ||
| Meal preparation and eating | 3 | ||
| Self-care and mobility | 3 | ||
| Total score/28 | 11 | Moderate | |
| Frontotemporal Behavioral Scale [52] | Self-monitoring dyscontrol | 1 | |
| Self-neglect | 1 | ||
| Self-centered behaviour | 1 | ||
| Affective disorders | 1 | ||
| Total score/4 | 4 | FTD present | |
| Clinical Dementia Rating [27] | Memory | 1 | |
| Orientation | 1 | ||
| Judgement and problem solving | 1 | ||
| Community affairs | 0,5 | ||
| Home and hobbies | 1 | ||
| Personal care | 1 | ||
| Composite score | 1 | Mild dementia | |
| FTDL-modified CDR [53] | Language | 0,5 | |
| Bahviour and personality | 1 |
Table 1: Assessment of behavior.
| Raw score | Percentiles/cut-off | ||
|---|---|---|---|
| Global cognition | MMSE | 16 | PR 0 |
| Verbal memory, sum of learning | 4 | PR 0 | |
| trials 1-3 | |||
| Verbal free recall | 2 | PR 1,4 | |
| Recognition correct minus false | 6 | PR 1 | |
| positives | |||
| Intrusions | 0 | PR 79 | |
| Dementia screening | |||
| Constructional praxis | 10 | PR 21 | |
| (CERAD [21]) | |||
| Constructional praxis recall | 4 | PR 0 | |
| Animals/min | 5 | PR 0 | |
| S - words/min | 2 | PR 0 | |
| Trail A | 99 s | PR 0 | |
| Trail B | Prematurely | ||
| terminated | |||
| Token - Test errors | 15 | PR 74 | |
| Language | |||
| Repetition (trial 2-5) | 119 | PR 99 | |
| (Aachener Aphasie Test [54]) | |||
| Comprehension Total Score | 85 | PR 59 | |
| Short-term Storage | Digits forward/backward | 6/3 | PR 28/5 |
| Conceptualization | 1 | ||
| Lexical Fluency | 0 | ||
| Frontal Assessment Battery | Motor programming | 0 | |
| [22] | |||
| Sensitivity to interference | 1 | ||
| Inhibitory Control | 1 | ||
| Environmental autonomy | 3 | ||
| Total Score/18 | 6 | Cut-off: 13 | |
| Total number of designs | 20 | PR 18 | |
| Figural Fluency [55] | |||
| Total number of unique designs | 10 | PR 3 | |
| Figural conceptualization | Clock Drawing Task | 8 | Cut-off: 11 |
| Inhibition of Interference [23] | Stroop Colour Word Test | 72 sec | PR<10 |
| Risky Decision | 9 | ||
| Game of Dice Task [24] | Unrisky decisions | 9 | |
| Net Score | 0 | PR 14 | |
| Addition | 4 | ||
| Number Processing and | |||
| Subtraction | 3 | ||
| Calculation Battery, Short | |||
| Version [26] | Multiplication | 3 | |
| Division | 1 | ||
| Total Score/20 | 11 | Cut-off: 14 | |
| Anger | 7 | Cut-off: 4 | |
| Disgust | 1 | Cut-off: 6 | |
| Facial Expressions of Emotion | Fear | 1 | Cut-off: 3 |
| [25] | |||
| Happiness | 10 | Cut-off: 9 | |
| Sadness | 6 | Cut-off: 5 | |
| Surprise | 4 | Cut-off: 6 | |
| Grand total/66 | 29 | Cut-off: 41 | |
Table 2: Neuropsychology.
Structural MRI
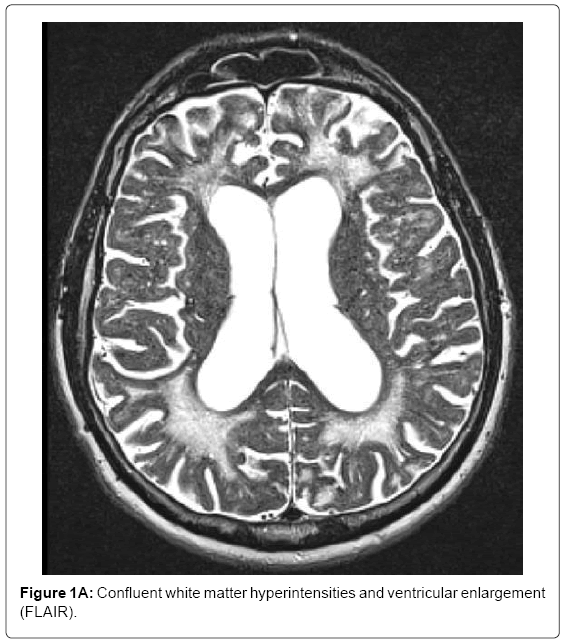
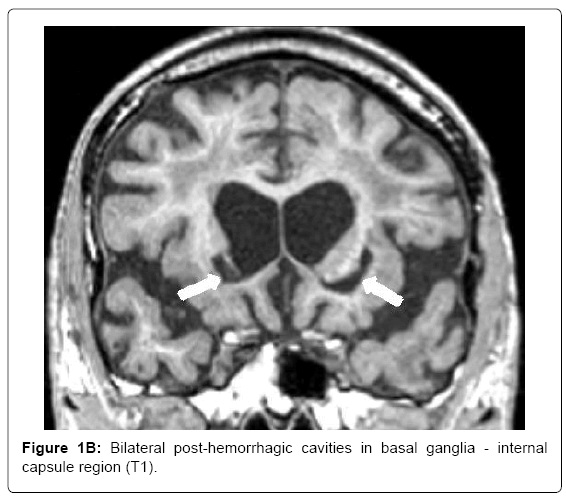
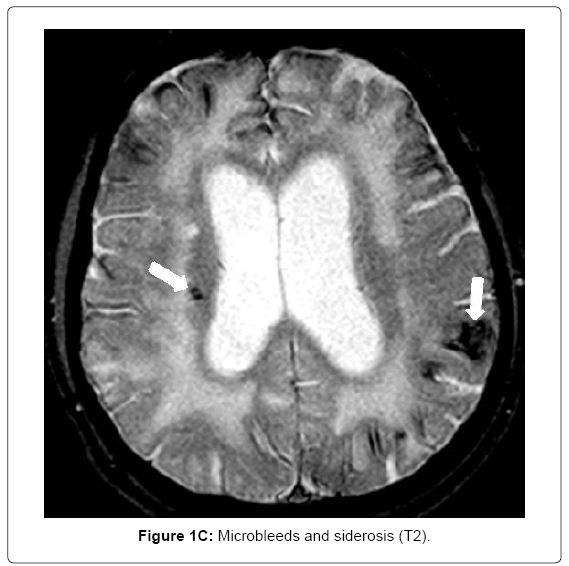
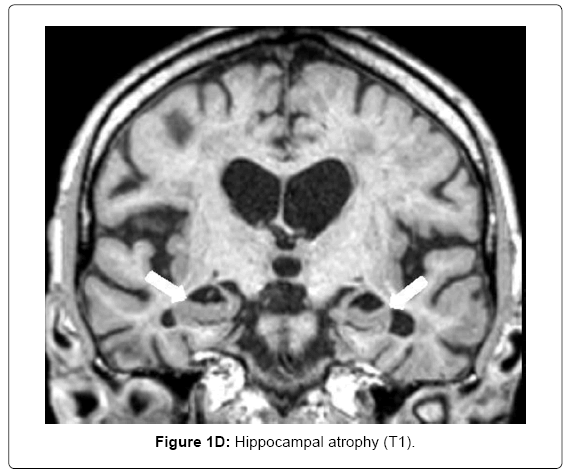
MRI (Siemens Magnetom Symphony TIM 1.5 Tesla Scanner) showed no signs of large vessel pathology (TOF) but markedly confluent white matter hyperintensities, particularly in the frontal, left temporal and right temporo-parietal areas (Figure 1A). Infarctions and posthemorrhagic cavities were found in the frontal area, basal ganglia, left and right temporal regions (Figure 1B) and the PICA territory. There were several microbleeds and areas of superficial siderosis as well as old bifrontal laminar hemorrhages (T2*, Figure 1C). TOF-MRA showed no evidence for sclerosis in the circle of Willis. Hippocampal atrophy was rated as 3 on the Scheltens score, Figure 1D).
Volumetric analysis
High-resolution structural scans were obtained with a T1-weighted magnetization-prepared rapid gradient echo sequence and were segmented into 184 subcortical and cortical as well as 70 white matter brain regions using FreeSurfer (version 5.3) [28]. Standard deviation of patient’s volumetric measures were calculated by z-transformation using mean centering and unit-variance scaling of age adjusted healthy male subjects (n=59). The analysis revealed a pattern of cortical, subcortical and white matter atrophy. Significant bilateral atrophy (>2 SDs below control mean) was found in the thalamus and amygdala (range -2 to -2.8 SD), in cortical areas (superior and inferior frontal, inferior and middle temporal, orbital, cingulate, precuneus, superior parietal and angular region, hippocampal formation, cerebellum; range -2 to -6.6 SD) and in the white matter (orbitofrontal, fusiform, inferior and superior temporal, parahippocampal, superior frontal; range -2 to -8.25 SD) of both hemispheres; atrophy of grey and white matter was more advanced in the right hemisphere.
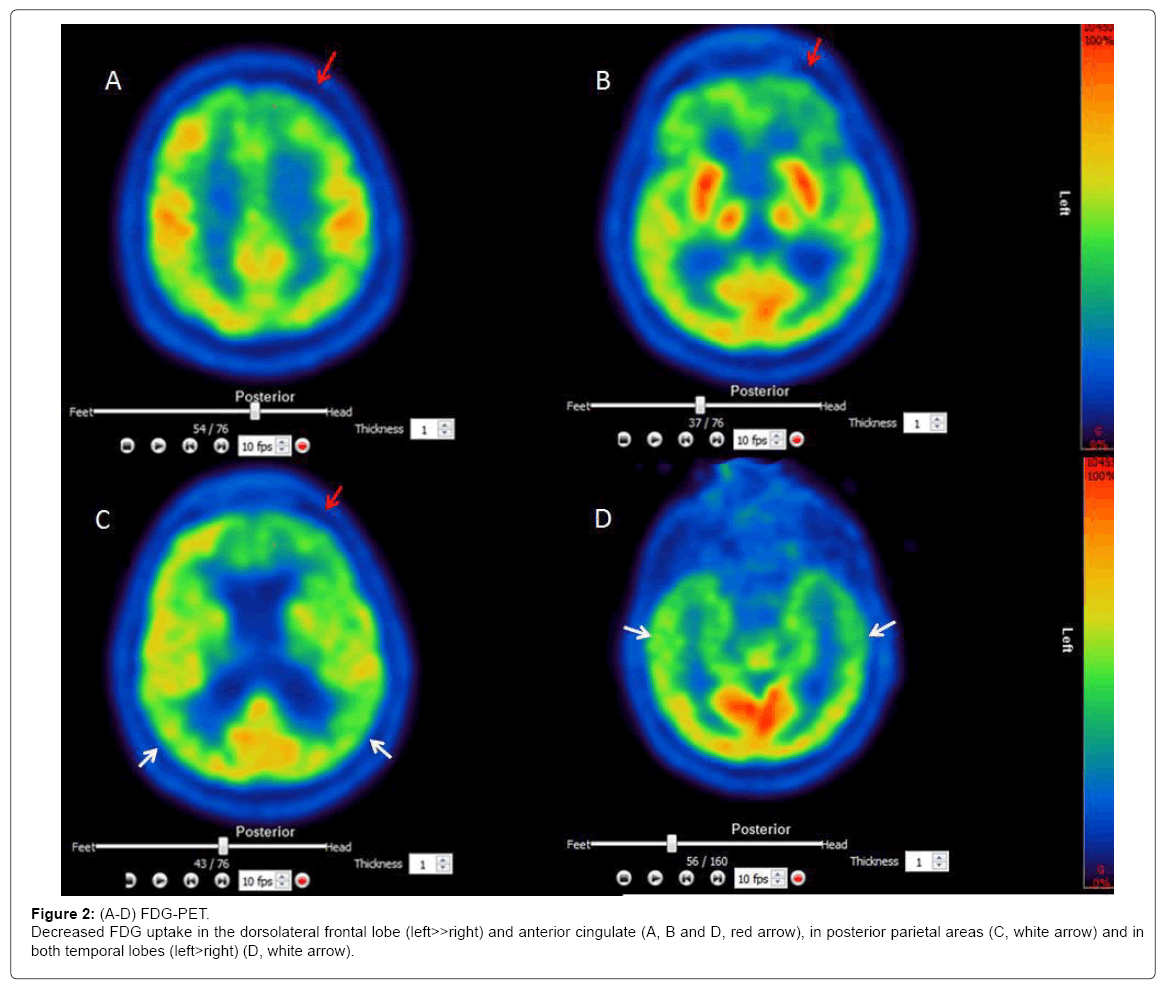
FDG-PET
[18F]-FDG Brain PET images showed markedly decreased FDG uptake in frontal, temporal and parietal brain areas (Figures 2A-2D.)
Laboratory findings
Routine laboratory findings including measures of coagulation were all normal. Analysis of CSF revealed normal total tau (201 pg, n.v. 149+/-149) and phospho-tau (34 pg, n.v. 30+/-5), but low ß-amyloid (246 pg, n.v. 744+/-20). The APOE genotype was ε 2/2. Serum lipids (cholesterol, LDL, HDL and triglycerides) were all in the normal range. EEG was moderately abnormal with intermittent slowing of Alpha (6-8 Hz) and a high content of intermittent irregular diffuse delta-theta waves.
Discussion
Progressive neuropsychiatric and dysexecutive features as core symptoms in a relatively young patient strongly suggest the diagnosis of bvFTD, which is commonly caused by tau, TDP-43 or Pick pathology.
However, other neurological disorders such as atypical AD [29], subcortical ischemic vascular [4] or mixed type dementia [6,30] may have a similar clinical presentation. SCAA is frequently found in elderly subjects; it often appears together with other neuropathologies, mostly AD. It is a known risk factor for cognitive decline [13,31]. Although definite SCAA can only be diagnosed by post-mortem examination of the brain, a probable clinical diagnosis can be made with imaging support [32,33]. In our case, the diagnosis of SCAA was based on clinical (reversible ‘amyloid spells’), behavioral, cognitive and imaging abnormalities. Behavioral abnormalities were apathy, altered food preferences, disinhibition and stereotyped behaviors. Dysexecutive impairment was pronounced and mainly comprised flexibility, inhibitory control, risk estimation, effortful self-initiation and psychomotor speed. Moreover, episodic memory and emotion recognition were also defective. MRI revealed major leukoaraiosis, microbleeds and siderosis. A volumetric analysis showed combined atrophy in subcortical structures (basal ganglia, amygdala), white matter tracts and cortical regions (frontal, temporal, parietal, hippocampal cortex). FDG-PET showed regions of hypoperfusion mainly in the frontal, but also temporal and parietal lobes. This lesion pattern matches the clinical picture due to disrupted communication of brain regions [34], necrosis, vascular dysfunction and SCAA-related cortical thinning [35]. The patient’s APOE genotype was ε2/2; both APOE ε4 and ε2 alleles are associated with more severe SCAA [36]. Decreased CSF levels of ß-amyloid implicate vascular amyloid deposition [37,38]. However, approximately 80% of all subjects with moderate to severe SCAA have a pathologic diagnosis of AD [13,36,39] and the question of SCAA as distinct condition vs. SCAA associated with AD remains unclear without further examination [11].
Although clearly resembling bvFTD, the exact diagnosis, type and number of underlying pathologies remain unclear in the present case without neuropathological confirmation. However, in addition to bvFTD and atypical AD [29,40]. SCAA appears to be an important differential diagnosis in patients with progressive neuropsychiatric symptoms and executive impairment [17,18,41]. The diagnosis of SCAA is crucial and can have essential therapeutic consequences with regard to thrombolytic or anticoagulation therapy [42,43], blood pressure control [44] and prognosis due to their risk of further spontaneous brain hemorrhages and consequent dementia [45]. MRI depicting vascular lesions currently represents the best disease marker of SCAA [46]. Our patient never had symptoms of a documented stroke and the disease course was slowly progressive. This suggests that MRI should also be performed in cases without stroke-like presentation. Details regarding the overlap between SCAA with other degenerative syndromes [47] are presently unclear and need further study.
References
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, et al. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: 2456-2477.
- Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, et al. (2014) New criteria for frontotemporal dementia syndromes: Clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry 85: 865-870.
- O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, et al. (2003) Vascular cognitive impairment. Lancet Neurol 2: 89-98.
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC (2002) Subcortical ischaemic vascular dementia. Lancet Neurol 1: 426-436.
- Jung NY, Kim HJ, Kim YJ, Kim S, Seo SW, et al. (2016) Neuropsychiatric characteristics of PiB-negative subcortical vascular dementia versus behavioral variant frontotemporal dementia. Arch Gerontol Geriatr 67: 86-91.
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, et al. (2006) Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol 60: 677-687.
- Dols A, Krudop W, Moller C, Shulman K, Sajatovic M, et al. (2016) Late life bipolar disorder evolving into frontotemporal dementia mimic. Neuropsychiatr Dis Treat 12: 2207-2212.
- Ducharme S, Price BH, Larvie M, Dougherty DD, Dickerson BC (2015) Clinical approach to the differential diagnosis between behavioral variant frontotemporal dementia and primary psychiatric disorders. Am J Psychiatry 172: 827-837.
- Attems J, Jellinger K, Thal DR, Van Nostrand W (2011) Review: Sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 37: 75-93.
- Keage HA, Carare RO, Friedland RP, Ince PG, Love S, et al. (2009) Population studies of sporadic cerebral amyloid angiopathy and dementia: A systematic review. BMC Neurol 9: 3.
- Brenowitz WD, Nelson PT, Besser LM, Heller KB, Kukull WA (2015) Cerebral amyloid angiopathy and its co-occurrence with Alzheimer's disease and other cerebrovascular neuropathologic changes. Neurobiol Aging 36: 2702-2708.
- Greenberg SM, Gurol ME, Rosand J, Smith EE (2004) Amyloid angiopathy-related vascular cognitive impairment. Stroke 35: 2616-2619.
- Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, et al. (2011) Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol 69: 320-327.
- Xiong L, Davidsdottir S, Reijmer YD, Shoamanesh A, Roongpiboonsopit D, et al. (2016) Cognitive profile and its association with neuroimaging markers of non-demented cerebral amyloid angiopathy patients in a stroke unit. J Alzheimers Dis 52: 171-178.
- Schrag M, Kirshner H (2016) Neuropsychological effects of cerebral amyloid angiopathy. Curr Neurol Neurosci Rep 16: 76.
- Case NF, Charlton A, Zwiers A, Batool S, McCreary CR, et al. (2016) Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke 47: 2010-2016.
- Gleason A, Hayhow B, Emmanuel J, Gaillard F (2014) Cerebral amyloid angiopathy presenting with neuropsychiatric symptoms. Aust N Z J Psychiatry 48: 779-780.
- Coppola C, Saracino D, Califano F, Barbarulo AM, Di FG, et al. (2015) A case of progressive frontal lobe syndrome in a sporadic form of cerebral amyloid angiopathy: A singular overlap with fronto-temporal dementia? J Neurol Sci 359: 247-249.
- Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ (2002) Cerebral amyloid angiopathy and cognitive function: The HAAS autopsy study. Neurology 58: 1629-1634.
- Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, et al. (2015) Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 85: 1930-1936.
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, et al. (1994) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44: 609-614.
- Benke T, Karner E, Delazer M (2013) FAB-D: German version of the frontal assessment battery. J Neurol 260: 2066-2072.
- Oswald WD, Fleischmann UM (1997) Nürnberger-Alters-Inventar (4.unveränderte Auflage). Göttingen: Hogrefe. Preis 28: 296-298.
- Brand M, Kalbe E, Labudda K, Fujiwara E, Kessler J, et al. (2005) Decision-making impairments in patients with pathological gambling. Psychiatry Res 133: 91-99.
- Young A, Perrett D, Calder ASR, Ekman P (2002) Facial expressions of emotion-stimuli and tests (FEEST). Thames Valley Test Company, Bury St. Edmunds, England.
- Delazer M1, Girelli L, Granà A, Domahs F (2003) Number processing and calculation--normative data from healthy adults. Clin Neuropsychol 17: 331-350.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140: 566-572.
- Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97: 11050-11055.
- Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, et al. (2015) The behavioural/dysexecutive variant of Alzheimer's disease: Clinical, neuroimaging and pathological features. Brain 138: 2732-2749.
- Torralva T, Sposato LA, Riccio PM, Gleichgerrcht E, Roca M, et al. (2015) Role of brain infarcts in behavioral variant frontotemporal dementia: Clinicopathological characterization in the National Alzheimer's Coordinating Center database. Neurobiol Aging 36: 2861-2868.
- Attems J, Jellinger KA (2014) The overlap between vascular disease and Alzheimer's disease--lessons from pathology. BMC Med 12: 206.
- Knudsen KA, Rosand J, Karluk D, Greenberg SM (2001) Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 56: 537-539.
- Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, et al. (2010) Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 74: 1346-1350.
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357-381.
- Fotiadis P, van RS, van der Grond J, Schultz A, Martinez-Ramirez S, et al. (2016) Cortical atrophy in patients with cerebral amyloid angiopathy: A case-control study. Lancet Neurol 15: 811-819.
- Yu L, Boyle PA, Nag S, Leurgans S, Buchman AS, et al. (2015) APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol Aging 36: 2946-2953.
- Verbeek MM, Kremer BP, Rikkert MO, Van Domburg PH, Skehan ME, et al. (2009) Cerebrospinal fluid amyloid beta (40) is decreased in cerebral amyloid angiopathy. Ann Neurol 66: 245-249.
- Noguchi-Shinohara M, Komatsu J, Samuraki M, Matsunari I, Ikeda T, et al. (2017) Cerebral amyloid angiopathy-related microbleeds and cerebrospinal fluid biomarkers in alzheimer's disease. J Alzheimers Dis 55: 905-913.
- Viswanathan A, Greenberg SM (2011) Cerebral amyloid angiopathy in the elderly. Ann Neurol 70: 871-880.
- Godefroy O, Bakchine S, Verny M, Delabrousse-Mayoux JP, Roussel M, et al. (2016) Characteristics of Alzheimer's Disease Patients with Severe Executive Disorders. J Alzheimers Dis 51: 815-825.
- Gahr M, Nowak DA, Connemann BJ, Schönfeldt-Lecuona C (2013) Cerebral Amyloidal Angiopathy--a disease with implications for neurology and psychiatry. Brain Res 1519: 19-30.
- Rosand J, Hylek EM, O'Donnell HC, Greenberg SM (2000) Warfarin-associated hemorrhage and cerebral amyloid angiopathy: A genetic and pathologic study. Neurology 55: 947-951.
- Dannenberg S, Scheitz JF, Rozanski M, Erdur H, Brunecker P, et al. (2014) Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke 45: 2900-2905.
- Dufouil C, Chalmers J, Coskun O, Besançon V, Bousser MG, et al. (2005) Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Sub study. Circulation 112: 1644-1650.
- Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, et al. (2016) Dementia risk after spontaneous intracerebral haemorrhage: A prospective cohort study. Lancet Neurol 15: 820-829.
- Greenberg SM, Al-Shahi SR, Biessels GJ, van BM, Cordonnier C, et al. (2014) Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol 13: 419-428.
- Whitwell JL, Jack CR, Duffy JR, Strand EA, Gunter JL, et al. (2014) Microbleeds in the logopenic variant of primary progressive aphasia. Alzheimers Dement 10: 62-66.
- Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361-370.
- Lueken U, Seidl U, Schwarz M, Volker L, Naumann D, et al. (2006) Psychometric properties of a German version of the apathy evaluation scale. Fortschr Neurol Psychiatr 74: 714-722.
- Wilson B, Emslie H, Evans J, Alderman N, Burgess P (1996) Behavioural assessment of the dysexecutive syndrome. Thames Valley Test Company, Bury St. Edmunds, England.
- Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR (2010) Clinical staging and disease progression in frontotemporal dementia. Neurology 74: 1591-1597.
- Lebert F, Pasquier F, Souliez L, Petit H (1998) Frontotemporal behavioral scale. Alzheimer Dis Assoc Disord 12: 335-339.
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, et al. (2008) Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 131: 2957-2968.
- Huber W, Poeck K, Weniger D, Willmes K (1983) Aachener Aphasietest. Hogrefe, Göttingen,
- Haid T, Martl C, Schubert F, Wenzl M, Kofler M, et al. (2002) Der HAMASCH-Fünfpunktettest. Erste Normierungsergebnisse. Z Neuropsychologie 13: 233.
Citation: Benke T, Karner E, Scherfler C, Dazinger F, Donnemiller E (2017) Cerebral Amyloid Angiopathy or Frontotemporal Dementia? A Case Study. J Alzheimers Dis Parkinsonism 7: 373. DOI: 10.4172/2161-0460.1000373
Copyright: © 2017 Benke T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6620
- [From(publication date): 0-2017 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 5715
- PDF downloads: 905