Research Article Open Access
Ceramide and Sphingosine-1-Phosphate/Sphingosine act as Photodynamic Therapy-Elicited Damage-Associated Molecular Patterns: Release from Cells and Impact on Tumor-Associated Macrophages
Mladen Korbelik1*, Judit Banáth1, Wei Zhang1, Fred Wong1, Jacek Bielawski2 and Duska Separovic31British Columbia Cancer Agency, Vancouver BC, Canada
2Department of Biochemistry and Molecular Biology, Medical University of South Carolina, Charleston, Carolina, USA
3Department of Pharmaceutical Sciences, Eugene Applebaum College of Pharmacy and Health Sciences, Wayne State University, Detroit, USA
- *Corresponding Author:
- Dr. Mladen Korbelik
B.C. Cancer Research Centre, 675 West 10th Avenue
Vancouver BC, V5Z 1L3 Canada
Tel: 604-675-8084
Fax: 604-675-8099
E-mail: mkorbelik@bccrc.ca
Received date: June 18, 2014; Accepted date: July 29, 2014; Published date: July 31, 2014
Citation: Korbelik M, Banáth J, Zhang W, Wong F, Bielawski J, et al. (2014) Ceramide and Sphingosine-1-Phosphate/Sphingosine act as Photodynamic Therapy-Elicited Damage-Associated Molecular Patterns: Release from Cells and Impact on Tumor-Associated Macrophages. J Anal Bioanal Tech S1:009. doi: 10.4172/2155-9872.S1-009
Copyright: © 2014 Korbelik M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A recent finding showed that ceramide and sphingosine-1-phosphate (S1P) become exposed on the surface of cells treated by photodynamic therapy (PDT) and acquire the capacity to act as danger-associated molecular patterns (DAMPs). To explore this further, the present study examined whether ceramide and S1P can be released from PDTtreated cells and investigated changes in the levels of these sphingolipids in tumor-associated macrophages (TAMs) left in contact with PDT-treated tumor cells. Massspectroscopy-based analysis detected increased levels of C16- ceramide and dihydroC16-ceramide in media supernatants from SCCVII cells collected three hours after they were treated by PDT, compared to untreated cell supernatants. While no release of S1P was detected, elevated levels of its precursor sphingosine were found in the supernatants of PDT-treated cells. The co-incubation of TAMs-containing primary cultures derived from mouse SCCVII tumors with PDT-treated SCCVII cells was followed by ceramide and S1P analysis in these cells based on staining with specific antibodies and flow cytometry. Levels of both ceramide and S1P as well as inflammasome protein NLRP3 were found to rise in TAMs when they were co-cultured with PDT-treated SCCVII cells, while no significant change was seen with cancer cells. Such changes were induced also in TAMs incubated with supernatants from PDT-treated cells. The findings of the present study affirm the potential of sphingolipids including ceramide, S1P, and sphingosine to act, either exposed on cell surface or released in the microenvironment, as DAMPs in the response of tumors to PDT.
Keywords
Photodynamic therapy; Ceramide; Sphingosine-1- phosphate; Sphingosine; Damage-associated molecular patterns (DAMPs); Sphingolipid metabolism-modulating drugs (SMMDs)
Introduction
Sphingolipids (SLs) comprise one of the principal groups of bioactive lipids that besides their basic role in constituting cellular membranes mediate many key processes in cell physiology, including cell proliferation/death and immune responses [1-3]. Their key members, ceramide, sphingosine, and sphingosine-1-phosphate (S1P), are critical mediators of cellular stress response [4]. Various types of stress were found to induce regulated changes in the SL metabolism altering the levels of ceramide and other key SLs that control specific pathways of cellular survival/growth or programmed death [5]. This particularly pertains to cancer cells sustaining stress from anti-tumor modalities such as chemotherapy, ionizing radiation, hyperthermia, cytokine treatment or photodynamic therapy (PDT) [6,7].
The eradication of malignant tumors by PDT is achieved by the induction of oxidative stress due to the localized activation of photoreactive drugs with light that generates reactive oxygen species (mainly singlet oxygen) in targeted lesions [8]. The resulting phototoxic lesions elicit a complex response that produces cancer cell death, damage to tumor vasculature and other stromal elements, as well as inflammatory/immune responses [9-11]. Recent findings reveal that SLs belong to biomolecules with a potential to strongly influence PDT response [7,12,13]. It was documented that PDT has a distinct signature effect on the SL profile in treated cells and tumor tissue, which is dominated by the induction of de novo ceramide synthesis [13,14]. This de novo ceramide was shown to be involved in the initiation of tumor cell apoptosis after PDT.
The levels of SLs in cells can be altered by various sphingolipid metabolism-modulating drugs (SMMDs) developed as prospective anti-cancer agents or for other therapeutic interventions [15,16]. We have identified several such agents as effective for use in combination with PDT for improving the cure-rates of treated tumors [13,17].
Our studies aimed at monitoring ceramide and S1P levels in major cellular populations of PDT-treated tumors have revealed that, compared to cancer cells considerably higher levels of these SLs can be found in tumor-associated macrophages (TAMs) both before and after PDT [18]. Interestingly, treatment of SCCVII tumor-bearing mice with LCL29 (C6-ceramide analogue and established SMMD [19]) that increased PDT-mediated cure rates resulted also in the rise in ceramide levels in TAMs but not in cancer cell population of these tumors [18]. This raises the possibility that there is an interaction between ceramide activity in cancer cells and TAMs, bestowing ceramide and perhaps other SLs the function of intercellular signals. Indeed, S1P was suggested to represent one of the “find-me” signals released from apoptotic cells to mobilize immune effector cells with S1P receptors [20]. Several types of cells were also reported to recognize ceramide as one of the agonists of their Toll-like receptor-4 (TLR4) [21]. Recently, we have demonstrated that ceramide and S1P become expressed on the surface of PDT-treated tumor cells acquiring the capacity of acting as damage-associated molecular patterns (DAMPs) [22]. As such they can serve as alarm signals that alert immune cells to the presence of endogenous damage and prompt the activation of inflammatory/ immune responses [23].
The aim of the present study was to establish whether ceramide and S1P can become not only surface-expressed but also released from PDT-treated tumor cells, and how could PDT affect their levels in neighboring TAMs.
Materials and Methods
SCCVII tumor and cell culture
Murine squamous cell carcinoma SCCVII is an accepted model of head and neck cancer of spontaneous origin with established record of the absence of strong immunogenicity [24]. Subcutaneous SCCVII tumors were grown in syngeneic C3H/HeN mice. For experiments, the tumors were excised from sacrificed mice when they reached around 10 mm in largest diameter and immediately subjected to enzymatic digestion for obtaining single cell suspensions following a routine standard protocol [25]. Primary cultures formed from these tumor cell suspensions as well as long-term SCCVII cell line were maintained in vitro using alpha minimal essential medium (Life Technologies, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (FBS) obtained from Life Technologies. The mouse procedures were in compliance with the protocol approved by the Animal Care Committee of the University of British Columbia.
Photodynamic treatment
Cultured SCCVII cells, growing either in 35 mm diameter Petri dishes or in 30 mm diameter Millicell cell culture inserts with 0.4 μm pore polycarbonate membrane (Millipore Ltd., Carrigtwohill, County Cork, Ireland) were first exposed to Photofrin (Axcan Pharma, Mont- Saint-Hilaire QC, Canada) at 20 μg/ml concentration for 18 hours. The cells were then washed in PBS and kept in that buffer while irradiated with 1 J/cm2 of 630 ± 10 nm light (15 mW/cm2). The light was produced by a FB-QTH high throughput illuminator (Sciencetech, London ON, Canada) based on a 150 W QTH lamp and equipped with integrated ellipsoidal reflector. It was delivered through an 8 mm core diameter liquid light guide (Oriel Instruments, Stratford CT, USA).
Electrospray ionization/double MS
Release of key sphingolipids from PDT-treated SCCVII cells was monitored by analysis of their supernatants after they were kept for various post-treatment time intervals in culture conditions at 37°C. In initial experiments, PDT-treated cells were maintained in their growth medium that is supplemented with 10% FBS. Since the levels of some SLs, particularly S1P, were relatively high in this medium, in additional experiments SCCVII cells were maintained for two days in serum-free medium Ex-cell NS0 (Sigma 14650C) and then kept in this medium for generating supernatants. In order to assess possible autocrine/paracrine re-internalization of S1P by binding to S1P receptors on SCCVII cells, anti-S1PR1 or anti-S1PR2 (both rabbit polyclonal antibodies) purchased from Biorbyt Limited (Cambridge, UK) were added to the cell medium in some samples at 20 μg/ml during the post-PDT incubation. Some supernatants were after collection further processed for exosome isolation using P100 PureExo® Exosome Isolation kit (101Bio, Palo Alto, CA) following manufacturer instructions. The exosomes appear as a separate fluffy layer after vortexing of cell medium sample with solutions provided by the kit and microcentrifugation. This kit isolates >95% pure exosomes with the yield of up to 100 μg exosomal material per ml of medium. After extraction, SLs from cell culture supernatants were introduced to electrospray ionization source and analyzed by double mass spectrometry using an ACCELA-TSQ Quantum Access LC-MS-MS system (Thermo-Fisher Scientific, Waltham MA, USA) as described earlier [12]. This allowed simultaneous measurement of various ceramides, dihydroceramides and sphingoid bases employing internal standards and using comparison with the calibration curves based on a linear regression model [26].
Flow cytometry-based determination of cellular ceramide, S1P and NLRP3 levels
Cellular levels of ceramide and S1P were determined by intracellular staining with specific antibodies followed by flow cytometry as described in detail in an earlier report [18]. Briefly, cells detached using a cell scraper were first exposed to surface staining with either FITC-conjugated anti-mouse F4/80 or FITC anti-Ly-6G known as GR1 (eBioscience Inc., San Diego CA, USA). As repeatedly proven in our previous studies [7,18] staining intensity of SCCVII tumor cell suspensions for myeloid marker GR1 allows gating them into two major populations, TAMs (GR1+) and cancer cells (GR1-). In addition to these two populations, SCCVII tumors usually contain less than 1% of other cell types; among them neutrophils and myeloidderived suppressor cells can be gated out as GR1++. Hence, TAMs were routinely identified as GR1+ and F4/80+ cells. Surface staining was followed by intracellular staining of fixed and permeabilized cells with mouse anti-ceramide monoclonal antibody 15B4 (Enzo Life Sciences, Plymouth Meeting PA, USA) or mouse anti-S1P monoclonal antibody (clone NHS1P) (Cosmo Bio USA, Carlsbad CA, USA) used as primary antibodies. Goat anti-mouse IgM antibody conjugated with phycoerythrin (Santa Cruz Biotechnology Inc., Santa Cruz Ca, USA) served as the secondary antibody. Staining with normal mouse IgM (Santa Cruz) was employed as the isotype control. Under physiological conditions the used anti-ceramide antibody is highly specific for C16- and C24-ceramide and does not cross-react with sphingomyelin, cholesterol or other phospholipids [27], while anti-S1P antibody shows no-cross-reactivity with ceramide, sphingosine, sphingomyelin or other phospholipids [28]. The inflammasome activity in TAMs was assessed by monitoring levels of the prominent protein of this complex, NLRP3, based on intracellular staining with rabbit anti-NLRP3 (Boster Biological Technology, Pleasanton, CA) followed by PE-conjugated chicken anti-rabbit IgG (Santa Cruz) with non-specific rabbit IgG as isotype control. Flow cytometry was performed by a Coulter Epics Elite ESP (Coulter Electronics, Hialeah FL, USA) with at least 2×104 cells included for each test.
Statistical analysis
Experimental groups contained quadruplicate samples (N=4). The results were evaluated based on Mann-Whitney test and the significance level threshold of 5% (two-tailed test) was set for determining whether the groups were statistically different.
Results
Levels of SLs in cell supernatants
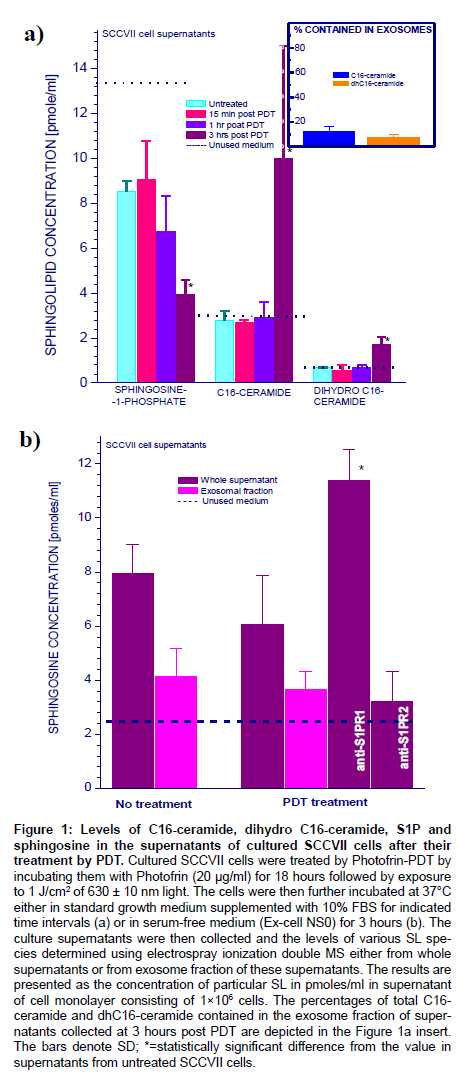
Cultures of SCCVII cells (2×106 per sample) were exposed to the standard dose of Photofrin-PDT that resulted in 80-90% cell kill [22]. The cells were then left in the 37°C incubator with fresh regular growth medium (containing 10% FBS) for different time intervals before the supernatants were collected for the analysis of SL contents by electrospray ionization/double MS. No significant changes were found in the levels of C14-, C18-, C18:1-, C20-, C20:1-, C22-, C22:1-, C24-, C24:1-, C26-, and C26:1-ceramide, as well as in dihydrosphingosine, dihydrosphingosine-1-phosphate, and sphingosine (not shown). Conversely, significant changes were found with C16-ceramide, C16- dihydroceramide and S1P (Figure 1a). The levels of C16-ceramide and C16-dihydroceramide in the unused cell medium were approximately 3 and 0.6 pmoles/ml, respectively, and they remained unchanged in the supernatants of untreated cells and PDT-treated cells during the first hour after treatment. However, a marked rise in both C16-ceramide and C16-dihydroceramide content was found at three hours after PDT. Ceramide levels reached 10 pmoles/ml implying that at least 5 pmoles were released from one million cells. The analysis of exosomal fractions revealed that only around 12% of C16-ceramide and 7% C16- dihydroceramide were contained in exosomes. Quite different changes were seen with S1P. Its content, which in the unused medium was over 13 pmoles/ml, lowered to around 8 pmoles/ml during the first hour of incubation with PDT-treated cells or upon incubation with untreated cells. An additional significant decrease in S1P levels to less than 4 pmoles/ml was detected in supernatants of PDT-treated cells kept in culture for 3 hours.
Figure 1: Levels of C16-ceramide, dihydro C16-ceramide, S1P and sphingosine in the supernatants of cultured SCCVII cells after their treatment by PDT. Cultured SCCVII cells were treated by Photofrin-PDT by incubating them with Photofrin (20 μg/ml) for 18 hours followed by exposure to 1 J/cm2 of 630 ± 10 nm light. The cells were then further incubated at 37°C either in standard growth medium supplemented with 10% FBS for indicated time intervals (a) or in serum-free medium (Ex-cell NS0) for 3 hours (b). The culture supernatants were then collected and the levels of various SL species determined using electrospray ionization double MS either from whole supernatants or from exosome fraction of these supernatants. The results are presented as the concentration of particular SL in pmoles/ml in supernatant of cell monolayer consisting of 1×106 cells. The percentages of total C16- ceramide and dhC16-ceramide contained in the exosome fraction of supernatants collected at 3 hours post PDT are depicted in the Figure 1a insert. The bars denote SD; *=statistically significant difference from the value in supernatants from untreated SCCVII cells.
Relatively high levels of S1P in 10% FBS-containing cell growth medium may mask the above analysis. Hence, additional testing was done with cells cultured in serum-free medium Ex-cell NS0. Except for a slower growth-rate, SCCVII cells tolerated this medium well. The unused medium contained no detectable S1P and in all cell supernatants its amounts were very low (never exceeding 0.2 pmoles/ ml). In contrast, significant levels of sphingosine were detectable in these supernatants, while its levels in the unused medium were about 2.5 pmoles/ml (Figure 1b). Close to 8 pmoles/ml of sphingosine was found in the medium maintaining one million of untreated SCCVII cells for 3 hours, and this level has not significantly changed in the supernatants of PDT-treated cells. Over 50% of sphingosine in the supernatants of untreated and PDT-treated cells was found contained in exosomes (Figure 1b), and thus even a higher percentage might have been in the material released from cells given the presence of sphingosine in unused medium. Notably, the supernatant sphingosine levels rose significantly when antibodies blocking S1PR1 (but not S1PR2) were present during the post-PDT incubation confirming the well-known dynamic equilibrium between S1P and sphingosine [29]. Since both anti-S1PR1 and anti-S1PR2 are rabbit antibodies, the latter can serve as isotype control confirming the specificity of action of anti-S1PR1. This finding is consistent with a PDT-induced S1P translocation out of cells involving autocrine/paracrine release followed by uptake mediated by this receptor.
Inflammasome activity in TAMs co-incubated with PDTtreated SCCVII cells
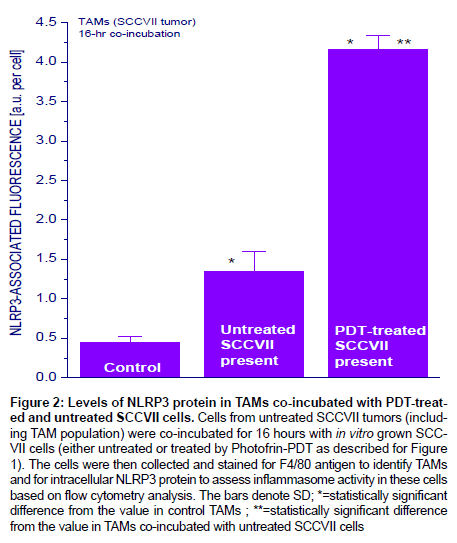
Since sphingosine was reported to activate inflammasome in macrophages [30], it was next examined whether such event can be detected in macrophages co-incubated with PDT-treated cells. This was done using primary cultures with cells disaggregated from untreated mouse SCCVII tumors that have sizable TAMs content (20-30% [31]). Indeed, the levels of key inflammasome protein NLRP3 increased markedly in SCCVII tumor-derived TAMs after PDT-treated SCCVII cells were added to their culture, while this increase was much less pronounced upon addition of untreated SCCVII cells (Figure 2).
Figure 2: Levels of NLRP3 protein in TAMs co-incubated with PDT-treated and untreated SCCVII cells. Cells from untreated SCCVII tumors (including TAM population) were co-incubated for 16 hours with in vitro grown SCCVII cells (either untreated or treated by Photofrin-PDT as described for Figure 1). The cells were then collected and stained for F4/80 antigen to identify TAMs and for intracellular NLRP3 protein to assess inflammasome activity in these cells based on flow cytometry analysis. The bars denote SD; *=statistically significant difference from the value in control TAMs ; **=statistically significant difference
from the value in TAMs co-incubated with untreated SCCVII cells
Increased ceramide and S1P levels in TAMs co-incubated with PDT-treated SCCVII cells
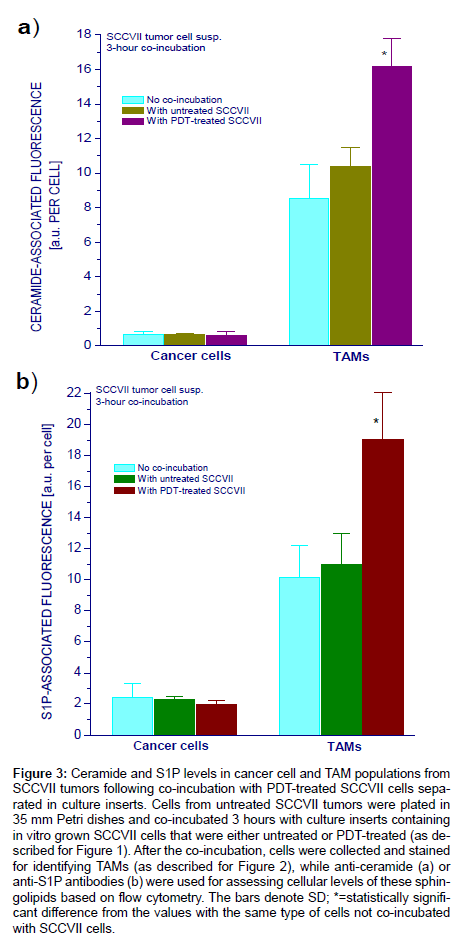
The cultures of cells from SCCVII tumors containing TAMs were further used in the remainder of the study to assess ceramide and S1P levels in macrophages encountering PDT-treated tumor cells. In the first set-up, Petri dishes with TAMs-containing primary cultures were co-incubated 3 hours in presence of either untreated or PDTtreated SCCVII cells held in separate tissue culture inserts with porous membrane base. The inserts were then discarded and primary culture cells collected for surface antibody staining with anti-F4/80 or anti- GR1 followed by intracellular staining with antibodies raised against ceramide or S1P. Analysis of changes in ceramide and S1P levels in cancer cells and TAMs after co-incubation with untreated or PDTtreated SCCVII cells is shown in Figure 3. As reported earlier, cancer cells contained less ceramide and S1P than TAMs [18]. With regards to ceramide, there were no significant changes in cancer cells induced by co-incubation with either untreated or PDT-treated SCCVII cells (Figure 3a). In contrast, ceramide levels in TAMs increased significantly after the co-incubation with PDT-treated SCCVII cells whilst no significant change was found for co-incubation with untreated SCCVII cells. Similar changes were found with cellular levels of S1P (Figure 3b). While again no obvious difference was detected in cancer cells, there was a significant increase in S1P levels in TAMs co-incubated with PDT-treated but not with PDT-untreated SCCVII cells.
Figure 3: Ceramide and S1P levels in cancer cell and TAM populations from SCCVII tumors following co-incubation with PDT-treated SCCVII cells separated in culture inserts. Cells from untreated SCCVII tumors were plated in 35 mm Petri dishes and co-incubated 3 hours with culture inserts containing in vitro grown SCCVII cells that were either untreated or PDT-treated (as described for Figure 1). After the co-incubation, cells were collected and stained for identifying TAMs (as described for Figure 2), while anti-ceramide (a) or anti-S1P antibodies (b) were used for assessing cellular levels of these sphingolipids based on flow cytometry. The bars denote SD; *=statistically significant difference from the values with the same type of cells not co-incubated with SCCVII cells.
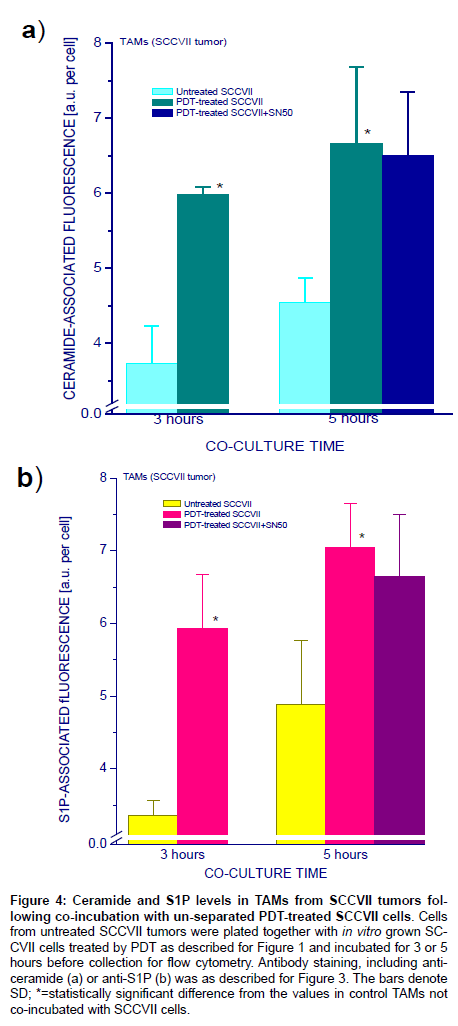
In next experiments, untreated or PDT-treated SCCVII cells were directly added to Petri dishes with TAMs-containing SCCVII tumorderived culture for 3- or 5-hour co-incubation. Flow cytometry-based analysis in cells identified as TAMs again showed elevated ceramide levels when these cells were co-incubated with PDT-treated, compared to untreated, SCCVII cells (Figure 4a) and very similar changes were seen with S1P (Figure 4b). Presence of NFκB inhibitor SN50 in the co-incubation medium had no significant impact on the induced rise in ceramide or S1P levels in TAMs. Both ceramide and S1P levels were after 5 hours not significantly higher than after 3 hours of coincubation, although average values were higher for the 5 hour timepoint.
Figure 4: Ceramide and S1P levels in TAMs from SCCVII tumors following co-incubation with un-separated PDT-treated SCCVII cells. Cells from untreated SCCVII tumors were plated together with in vitro grown SCCVII cells treated by PDT as described for Figure 1 and incubated for 3 or 5 hours before collection for flow cytometry. Antibody staining, including anticeramide (a) or anti-S1P (b) was as described for Figure 3. The bars denote SD; *=statistically significant difference from the values in control TAMs not co-incubated with SCCVII cells.
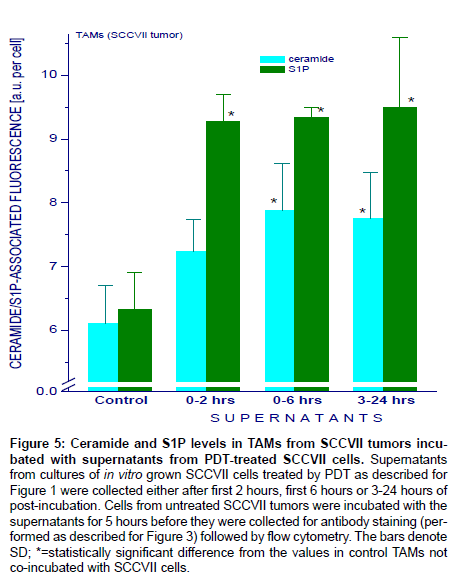
Further insights were gained from the study of media supernatants collected from PDT-treated SCCVII cells. The supernatants were added to TAMs-containing SCCVII tumor-derived cultures and left for 5 hours before sample collection for flow cytometry analysis. Similarly as with the direct contact with PDT-treated cells or by way of signals passing through the porous membrane of culture inserts, the supernatants triggered a rise in both ceramide and S1P levels in TAMs (Figure 5). For ceramide, this rise was induced by supernatants collected either after the first 6 hours or from 3 to 24 hours post PDT treatment but was not significant with supernatants collected after the first 2 hours post PDT treatment. For S1P, however, this rise in TAMs was induced by all types of supernatants tested.
Figure 5: Ceramide and S1P levels in TAMs from SCCVII tumors incubated with supernatants from PDT-treated SCCVII cells. Supernatants from cultures of in vitro grown SCCVII cells treated by PDT as described for Figure 1 were collected either after first 2 hours, first 6 hours or 3-24 hours of post-incubation. Cells from untreated SCCVII tumors were incubated with the supernatants for 5 hours before they were collected for antibody staining (performed as described for Figure 3) followed by flow cytometry. The bars denote SD; *=statistically significant difference from the values in control TAMs not co-incubated with SCCVII cells.
Discussion
The findings of this study support the assumption that ceramide and S1P, the two key members of SL family, are involved in the reaction of TAMs to the appearance of PDT-induced damage in cancer cells. This is consistent with the property of ceramide and S1P to function as DAMPs or alarmins, normally hidden molecules becoming either exposed on and/or /released from damaged cells, or actively elaborated by immune cells [22,32]. They function as signals recruiting and stimulating cells of innate immune system to instigate inflammatory/ immune responses. Among cancer therapies PDT is highly prominent in its effectiveness in engaging various established DAMPs, including heat shock proteins, calreticulin, high mobility group box-1, and ATP [8,33-35].
The choice of TAMs to represent macrophages in this study was governed by their direct origin in tumor microenvironment, which makes them authentic participants of the situation in vivo in close proximity to PDT-treated cancer cells. The presence of other tumorderived cellular populations in the used primary cultures of TAMs added to the credibility of mimicking elements/influences occurring in vivo.
This study shows that, compared to their control range, the levels of both ceramide and S1P increase in TAMs in the presence of cancer cells that have sustained PDT-mediated injury, while no such change is induced in the presence of untreated cancer cells (Figure 3). This could reflect the escalated SLs biosynthesis related to the activation of TAMs, but also the uptake of ceramide and S1P released from PDT-treated SCCVII cells. Indeed, our data demonstrate that C16-ceramide and its de novo synthesis precursor C16-dihydroceramide were released from PDT-treated SCCVII cells within three hours after PDT although this did not happen during the first hour after treatment (Figure 1). As a lipophilic molecule, ceramide is unlikely to have been released as a free entity [36] but only around 10% was found secreted in exosomes (Figure 1). This leaves the possibility of its presence in membrane fragments shed from PDT-damaged cells.
With regards to S1P, its secretion from cells is a well-established phenomenon [37]. Moreover, the released S1P can be taken up by cells in an autocrine manner, which is a well-known occurrence termed “inside-out” signaling [38,39]. Five known dedicated receptors for S1P (S1PRs) ubiquitously expressed on various cells including macrophages are well characterized [40]. Nonetheless, it proved more difficult to corroborate the release of S1P from PDT-treated cells. To begin with, its levels are relatively high in unused cell growth medium supplemented with 10% FBS and this masked S1P release/uptake by cells. However, the analysis of supernatants with serum-free medium uncovered no significant levels of S1P in samples from either untreated or PDT-treated cells but revealed a substantial presence of its precursor sphingosine. Given the fact that S1P and sphingosine are present in a dynamic equilibrium controlled by the activities of sphingosine kinase and S1P phosphatase [29], these changes in sphingosine levels may closely reflect events with S1P including its decrease with time after PDT depicted in Figure 1a. This is supported by the fact that blocking the uptake of S1P by antibodies raised against its receptor S1PR1 results in the increase in the sphingosine levels in supernatants of PDT-treated cells (Figure 1b). This finding is consistent with S1P release from PDT-treated cells followed by its prompt autocrine re-internalization through the engagement of S1PR1 (which is indicated as the dominant S1P receptor involved). Activation of membranous S1P phosphatases induced by PDT treatment would then result in converting this S1P into sphingosine that is discharged from cells, largely inside exosomes. A support for such a conception is also the finding that the activation of NFκB in TAMs co-incubated with PDT-treated SCCVII cells can be abrogated by the presence of S1P-neutralizing antibodies [22].
One of pattern recognition receptors dedicated for recognizing DAMPs is the NOD-like receptor pyrin domain-containing 3 (NLRP3), which is expressed predominantly in TAMs and other leukocytes [41]. Sphingosine is prominent among ligands of NLRP3 and was because of this property proposed to belong among DAMPs [30]. Activated NLRP3 assembles and oligomerizes into multimolecular complex named inflammasome that activates caspase-1 cascade leading to the production of pro-inflammatory cytokines [42]. Thus sphingosine released from PDT-treated SCCVII cells can be assumed to play a major role in NLRP3 inflammasome activation observed in TAMs co-incubated with PDT-treated SCCVII cells (Figure 2). This inflammasome activity in TAMs is probably involved in the upregulated production of ceramide and S1P found in these cells. However, our data reveal that one of prominent events following inflammasome activation, the mobilization of nuclear transcription factor NFκB, is not responsible for the elevation of ceramide and S1P levels in these TAMs.
As shown in our earlier studies [33,43-46], PDT-treated cancer cells emit signals into their environment that can be recognized by TAMs (and other cells). Among responses we detected in TAMs or other macrophages co-incubated with PDT-treated cancer cells are the upregulation of various receptors including TLR2, TLR4, C3aR [43,44] and in the present work NLRP3, activation of TIRAP-mediated signaling leading to NFκB activation with consequent induction of the production of inflammatory cytokines like TNFα [33], and the upregulation of various genes including those encoding for various complement and pentraxin proteins [45,46]. The present work adds the enhancement of ceramide and S1P production in these TAMs. Although presented first in TAMs left in the direct contact with PDT-treated cancer cells (Figures 2 and 4), such changes can also be induced with supernatants from PDT-treated cells (Figure 5) revealing the recognition of soluble signals. Our testing with the supernatants revealed important differences with regards to the upregulation in ceramide and S1P production in these TAMs. For triggering S1P production in TAMs, sufficient signals appear to be emitted into the supernatants of PDT-treated cells during the first two hours after PDT although they continue to be released at later time intervals during the first day post treatment. The latter holds also true for ceramide upregulation, but signals emitted during the first 2 hours were not sufficient to trigger a significant rise in the production of this SL in TAMs. It is obvious that further work needs to be done to elucidate the nature of these soluble signals, which form their impact appear compatible with DAMPs.
In conclusion, our recent finding that ceramide and S1P become DAMPs after PDT treatment is re-affirmed in the present study revealing the capacity of ceramide and S1P and/or its precursor sphingosine to function as soluble signals released from PDT-treated cells. Although S1P is known to be released from cells, PDT treatment may render cells more active in its re-internalization. Moreover, this seems to be associated with a rise in the conversion of S1P into sphingosine (presumably reflecting membranous S1P phosphatase activation). Sphingosine, subsequently found largely in discharged exosomes, can be also included among PDT-induced DAMPs. Rising levels of ceramide and S1P in TAMs co-incubated with PDT-treated SCCVII cells or their supernatants are probably associated with inflammasome activation in these TAMs.
Acknowledgement
This work was supported by US Public Health Service Grant R01 CA77475 from the National Cancer Institute (NCI), National Institutes of Health (NIH) and NCI Grant IPO1CA097132.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139-150.
- Morad SA, Cabot MC (2013) Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13: 51-65.
- Cinque B, Di Marzio L, Centi C, Di Rocco C, Riccardi C, et al. (2003) Sphingolipids and the immune system. Pharmacol Res 47: 421-437.
- Hannun YA, Luberto C (2000) Ceramide in the eukaryotic stress response. Trends Cell Biol 10: 73-80.
- Young MM, Kester M, Wang HG (2013) Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res 54: 5-19.
- Senchenkov A, Litvak DA, Cabot MC (2001) Targeting ceramide metabolism--a strategy for overcoming drug resistance. J Natl Cancer Inst 93: 347-357.
- Korbelik M, Zhang W, Separovic D (2011) Amplification of cancer cell apoptosis in photodynamic therapy-treated tumors by adjuvant ceramide analog LCL29. Lasers Surg Med 43: 614-620.
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, et al. (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61: 250-281.
- Allison RR, Moghissi K (2013) Photodynamic Therapy (PDT): PDT Mechanisms. Clin Endosc 46: 24-29.
- Mroz P, Yaroslavsky A, Kharkwal GB, Hamblin MR (2011) Cell death pathways in photodynamic therapy of cancer. Cancers (Basel) 3: 2516-2539.
- Korbelik M (2013) Tumor-localized insult delivered by photodynamic therapy and the breakdown of tumor immunotolerance. In: Keisari Y Tumor Ablation - The Tumor Microenvironment volume 5, Springer, Dordrecht 121-132.
- Separovic D, Bielawski J, Pierce JS, Merchant S, Tarca AL, et al. (2009) Increased tumour dihydroceramide production after Photofrin-PDT alone and improved tumour response after the combination with the ceramide analogue LCL29. Evidence from mouse squamous cell carcinomas. Br J Cancer 100: 626-632.
- Separovic D, Bielawski J, Pierce JS, Merchant S, Tarca AL, et al. (2011) Enhanced tumor cures after Foscan photodynamic therapy combined with the ceramide analog LCL29. Evidence from mouse squamous cell carcinomas for sphingolipids as biomarkers of treatment response. Int J Oncol 38: 521-527.
- Wispriyono B, Schmelz E, Pelayo H, Hanada K, Separovic D (2002) A role for the de novo sphingolipids in apoptosis of photosensitized cells. Exp Cell Res 279: 153-165.
- Kester M, Kolesnick R (2003) Sphingolipids as therapeutics. Pharmacol Res 47: 365-371.
- Adan-Gokbulut A, Kartal-Yandim M, Iskender G, Baran Y (2013) Novel agents targeting bioactive sphingolipids for the treatment of cancer. Curr Med Chem 20: 108-122.
- Korbelik M, Zhang W, Saw KM, Szulc ZM, Bielawska A, et al. (2013) Cationic ceramides and analogues, LCL30 and LCL85, as adjuvants to photodynamic therapy of tumors. J Photochem Photobiol B 126: 72-77.
- Korbelik M, Zhang W, Separovic D (2012) Monitoring ceramide and sphingosine-1-phosphate levels in cancer cells and macrophages from tumours treated by photodynamic therapy. Photochem Photobiol Sci .
- Senkal CE, Ponnusamy S, Rossi MJ, Sundararaj K, Szulc Z, et al. (2006) Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther 317: 1188-1199.
- Peter C, Wesselborg S, Herrmann M, Lauber K (2010) Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis 15: 1007-1028.
- Fischer H, Ellström P, Ekström K, Gustafsson L, Gustafsson M, et al. (2007) Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell Microbiol 9: 1239-1251.
- Korbelik M, Banáth J, Sun J, Canals D, Hannun YA, et al. (2014) Ceramide and sphingosine-1-phosphate act as photodynamic therapy-elicited damage-associated molecular patterns: cell surface exposure. Int Immunopharmacol 20: 359-365.
- Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, et al. (2010) Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 1805: 53-71.
- Khurana D, Martin EA, Kasperbauer JL, O'Malley BW Jr, Salomao DR, et al. (2001) Characterization of a spontaneously arising murine squamous cell carcinoma (SCC VII) as a prerequisite for head and neck cancer immunotherapy. Head Neck 23: 899-906.
- Korbelik M (1993) Distribution of disulfonated and tetrasulfonated aluminum phthalocyanine between malignant and host cell populations of a murine fibrosarcoma. J Photochem Photobiol B 20: 173-181.
- Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, et al. (2009) Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol 579: 443-467.
- Cowart LA, Szulc Z, Bielawska A, Hannun YA (2002) Structural determinants of sphingolipid recognition by commercially available anti-ceramide antibodies. J Lipid Res 43: 2042-2048.
- Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, et al. (2008) Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke 39: 3411-3417.
- Mandala SM (2001) Sphingosine-1-phosphate phosphatases. Prostaglandins Other Lipid Mediat 64: 143-156.
- Luheshi NM, Giles JA, Lopez-Castejon G, Brough D (2012) Sphingosine regulates the NLRP3-inflammasome and IL-1β release from macrophages. Eur J Immunol 42: 716-725.
- Korbelik M, Krosl G (1996) Photofrin accumulation in malignant and host cell populations of various tumours. Br J Cancer 73: 506-513.
- Srikrishna G, Freeze HH (2009) Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 11: 615-628.
- Korbelik M, Sun J, Cecic I (2005) Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res 65: 1018-1026.
- Korbelik M, Zhang W, Merchant S (2011) Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: surface expression of calreticulin and high-mobility group box-1 release. Cancer Immunol Immunother 60: 1431-1437.
- Garg AD, Krysko DV, Vandenabeele P, Agostinis P (2011) DAMPs and PDT-mediated photo-oxidative stress: exploring the unknown. Photochem Photobiol Sci 10: 670-680.
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, et al. (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244-1247.
- Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S (2010) Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol 688: 141-155.
- Takabe K, Paugh SW, Milstien S, Spiegel S (2008) "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60: 181-195.
- Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22: 50-60.
- Rivera J, Proia RL, Olivera A (2008) The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 8: 753-763.
- Cassel SL, Sutterwala FS (2010) Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol 40: 607-611.
- Brough D, Rothwell NJ (2007) Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci 120: 772-781.
- Korbelik M (2009) Complement upregulation in photodynamic therapy-treated tumors: Role of Toll-like receptor pathway and NFkB. Cancer Lett 281: 232-238.
- Cecic I, Sun J, Korbelik M (2006) Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of host neutrophils and other immune cells. Photochem Photobiol 82: 558-562.
- Stott B, Korbelik M (2007) Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol Immunother 56: 649-658.
- Merchant S, Sun J, Korbelik M (2007) Dying cells program their expedient disposal: serum amyloid P component upregulation in vivo and in vitro induced by photodynamic therapy of cancer. Photochem Photobiol Sci 6: 1284-1289.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14801
- [From(publication date):
November-2014 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 10138
- PDF downloads : 4663