Research Article Open Access
Centrifugal Microfluidic Integrated Optical System for Sensing Pesticide Residues
Jingjing Liu1*, Guoqing song1,2, Tengfei Wang1, Chunfei Hu1, Hongmei Chen1, and Fuqiang Nie1*1Division of Nano Bionic Research, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, P. R. China
2Department of Chemical Engineering, Northeast Dianli University, Jilin, P.R. China
- *Corresponding Author:
- Jingjing Liu and Fuqiang Nie
Division of Nanobionic Research, Suzhou Institute of Nano-Tech and Nano-Bionics
Chinese Academy of Sciences, Suzhou, 215123
P. R. China
Tel: 86-512-62872583
E-mail: jjliu2015@sinano.ac.cn, fqnie2012@sinano.ac.cn
Received date: March 31, 2017; Accepted date: April 17, 2017; Published date: April 21, 2017
Citation: Liu J, Song G, Wang T, Hu C, Chen H, et al. (2017) Centrifugal Microfluidic Integrated Optical System for Sensing Pesticide Residues. Biosens J 6: 144. doi:10.4172/2090-4967.1000144
Copyright: © 2017 Liu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
We proposed and demonstrated an effective and non-destructive centrifugal microfluidic sensor (CMS) for analysis of organophosphorus pesticides (OPPs). The CMS was designed to have a sequential sample injection with two capillary valves. The actuation mechanism of CMS was controlled by centrifugal force, i.e. relative centrifugal force (×g). The OPPs analyte in CMS could be successfully derived into a mixer at 34 g and then spined into a detection reservoir at 537 g. The experiment results indicated that limit of detection for OPPs using CMS was 0.05 ppm, a linear relationship between OPPs logarithmic concentration (ppm) and inhibition rate (%) was 0.9215. Furthermore, series fresh vegetables were extracted and assayed for OPPs residue detection by using CMS. It was effective for OPPs detection. A non-destructive, rapid, reliable, and sensitive approach to OPPs analysis was demonstrated. This in turn opens up opportunities for healthcare applications, notably environmental monitoring, foods safety and other fields.
Keywords
Centrifugal microfluidic chip; Organophosphorus pesticide detection; Capillary valve
Introduction
Organophosphorus pesticides (OPPs) are very toxic which can be released into the environment through agriculture work. The conventional methods involving high-performance liquid chromatography (HPLC) and gas chromatography (GC) were usually performed to detect the micro-concentrations of OPPs and complex matrices [1]. However, these analytic methods require large instruments, processing time and consumption of solvents. Therefore, the development of low cost, point-of-need sensors become more eager for improving exposure assessment [2]. The microfluidic analysis platform could integrate multiple analysis procedures into a unit operation of microfluidic such as liquid transport, metering, mixing and valving [3]. This concept of micro total analysis (μTAS) has been proposed for several decades [4]. Towards the development of the microfluidic technology, scientists consider it has the potential to become a standard tool in the application of biological assays [5], metallic risk analysis [6], cells handling and analysis [7], etc. Among of chip-based microfluidics [8], the centrifugal microfluidic chip could offer many merits, such as it does not require large high-voltage power supplies to create the forces rather than external syringe pumps [9-11]. Centrifugal pumping also has the advantages with the electro-kinetic methods. Due to the fluid in centrifugal microfluidic chip is insensitive to physicochemical properties such as pH or ionic strength [12], centrifugal microfluidic chip has been successfully applied in rapid (PCR) amplification [13], immunoassay in the whole blood [14].
With the capability of efficiently transforming chemical or biological reaction into centrifugal microfluidic chip, optical detection methods are widely used in chemical industry [15], biological process [16], medical diagnostics [17], healthcare [18], environmental monitoring [19], military defense [20], and scientific research. Optical analysis possesses higher sensitivity and immunity to electromagnetic interference compared with other analytical methods. This is considered as the most valuable in real-time in-situ applications due to their advantages of miniaturization and integration.
In this article, we proposed and demonstrated a non-destructive, rapid method for analysis of OPPs detection by using centrifugal microfluidic sensor (CMS) via optical analysis. The analyte in CMS was controlled by centrifugal force, i.e. relative centrifugal force (×g) when it reached to burst frequency that fluid will be spined out. The analyte will be collected in detection reservoir and analyzed by using visible spectrometer. The experiment results indicated the use of CMS was a high detection sensitivity and low limit of detection (LD). Eventually, CMS was performed the detection of fresh vegetables in real applications.
Experimental Section
Reagents
All the reagents were prepared in distilled deionized water (DDW, 18 MΩ, Merck Millipore, Darmstadt, Germany) and pH 7.71 phosphate buffer solution (PBS). PBS was prepared from 0.07 M Na2HPO4 in 100 ml and KH2PO4 in 100 ml with volume ratio of 9:1. Omethoate and phorate with a stock concentration of 100 μg/ml (ppm) was an analytical standard. All chemicals were purchased from Sinopharm chemical reagent company, China. Enzyme acetylcholineesterase (AchE) was stored at -20°C, 500 units/mg protein was extracted from electric eel (sigma-aldrich, CA), the stock solution of enzyme was prepared in DDW with the concentration of 70 U/ml. Substrate (S-Butyrylthiocholine iodide, ATCI) and chromatic reagent (DTNB, 5,5'-Dithio bis-(2-nitrobenzoic acid)) were prepared in PBS with stock concentration of 0.2 g/ml. Real sample vegetables were purchased from local market and stored at 4â??. Extraction process was performed by using PBS and Triton-X100 with diluted 5% solution.
Centrifugal microfluidic sensor (CMS) design
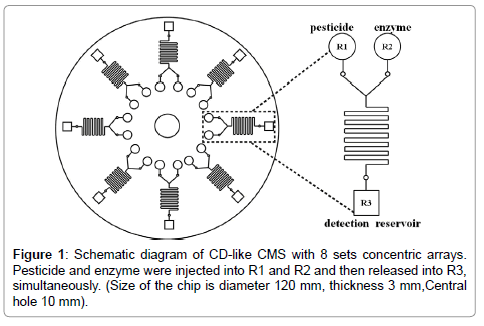
CMS was designed as compact disk (CD)-like by commercial AutoCAD software as shown in Figure 1. Eight identical microfluidic structures were arrayed around a circular plate with a 3 mm in thickness and 120 mm in diameter, respectively, where the diameter of center hole was 10 mm. Channels and reservoirs were engraved on poly-methylmetharylate (PMMA) substrate by a computer numerically controlled (CNC) machine. All solutions were injected into reservoirs via pipetting, i.e. OPPs and enzyme were injected into reservoir_1 (R1) and reservoir_2 (R2), respectively. In this CMS design, the feature sizes of first capillary valve were 730 μm in width and 360 μm in depth. They were equidistant from the center of CMS. The fluid in microchannel was expected to be laminar, where R2 and reservoir_3 (R3) were released simultaneously, and then mixed through the serpentine structure under certain frequencies of the centrifugal force. The width and depth of serpentine structure were designed as 200 μm. This type of structure can induce chaotic mixing due to the serpentine sharp with 90° turns and then resulted in fluids disturbing. The second capillary valve was designed as 400 μm in width and 200 μm in depth. The chromatic reaction was occurred in R3 embedded substrate beforehand. The chromatic reagent was the final destination of the fluid in CMS for OPPs detection.
Optical analysis
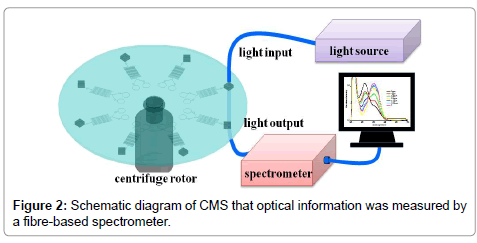
The CD-like CMS was placed on the centrifugal rotor actuated by a controller (0-2147×g). The whole optical testing setup was shown in Figure 2. The controller was adjusted for changing angular frequencies of CD-like CMS and then the fluids could be released and collected in R3 areas of CMS. Finally, the CD-like CMS was decelerated and then performed the optical analysis.
The enzyme inhibition-based determination of pesticide residues was a multistep process requiring a precise control of timing and liquid movement. This precise sequential interaction required irreversible interaction of the enzyme with the pesticide before the substrate and chromatic reagent adding. The operation procedures were briefly introduced into four steps. Firstly, enzyme and pesticide were injected into R1 and R2, respectively, and then mixed in the serpentine structure for 15 mins controlled by angular frequency of controller. Secondly, the inhibition interaction occurred in the mixer. Meanwhile, capillary valve was used to control the releasing volume of mixing fluids. The fluids could not flow into R3 due to the capillary valve derived by angular frequency. Thirdly, the chromatic reaction occurred by increasing the angular frequency of CMS to release the fluids into R3. Finally, the pesticide concentrations on 8 sets concentric arrays were measured on R3 areas of CMS by using a broadband spectrometer in the wavelength range from 200 nm to 1000 nm. Lens fibers in 230 μm diameter with SMA connector were mounted on a holder and placed close to edge of CMS to collect the spectral responses.
Results and Discussion
Capillary valve
The capillary forces are utilized to control the flow sequence of different reaction [21,22]. The pumping pressure of fluid generated by centrifugal force is expressed as Eq. (1) [12,13].
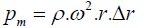 (1)
(1)
Where ρ is liquid density, ω is angular velocity, and r is radius of CMS. The capillary force is given by Equation (2).
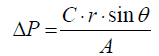 (2)
(2)
Where r is surface tension of fluid, θ is contact angle, A is the crosssectional area of the microchannel, and C is the associated contact line length. When ΔP is larger than Pm, the solution in microchannel can be released at certain rotational speed (×g). This frequency is denoted as burst frequency which is expressed as Equation. (3).
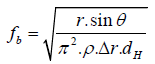 (3)
(3)
Where dH is equal to 4A/C. Some researches using the principle of the capillary forces to achieve the sequential injection, especially for the multi-steps of enzyme reaction of ELISA (Enzyme-Linked Immunosorbent Assays) [22].
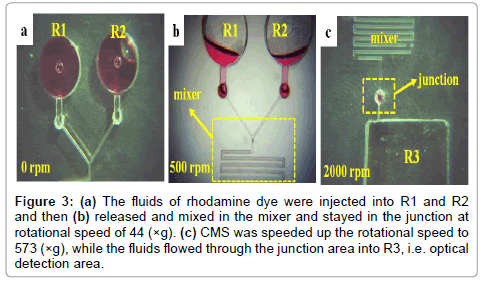
The predicted angular frequency or rotational speed in our design was calculated as 44 (×g) and 573 (×g) for first and second burst frequency, respectively. R1 and R2 were the same design corresponding to burst frequency and equidistant from the center of CD-like CMS. In order to test the theory of valve function by capillary force, solutions of rhodamine dye were used to visually investigate the releasing characteristics of fluids. The capillary valves were performed according to the theory and experiments. In Figure 3(a), the fluids of rhodamine dye were injected into R1 and R2 without starting the CMS rotation. The setup of rotational speed for first burst frequency was 34 (×g) with a ramp of 0.2 (×g)/s. The fluids were held at the end of R1 and R2 channels by the capillary force as shown in Figure 3(b). The fluids flowed along the serpentine structure and released and mixed in the mixer and stayed in the junction. The first burst frequency was lower than the theoretical value (44 ×g). This difference was attributed to the deviation of CMS manufacturing [23,24]. As increasing the rotational speed to 573(×g) to reach the second burst frequency, the fluids were released into R3 and harvested for optical analysis as shown in Figure 3(c).
Figure 3: (a) The fluids of rhodamine dye were injected into R1 and R2 and then (b) released and mixed in the mixer and stayed in the junction at rotational speed of 44 (×g). (c) CMS was speeded up the rotational speed to 573 (×g), while the fluids flowed through the junction area into R3, i.e. optical detection area.
Enzyme inhibition-based determination
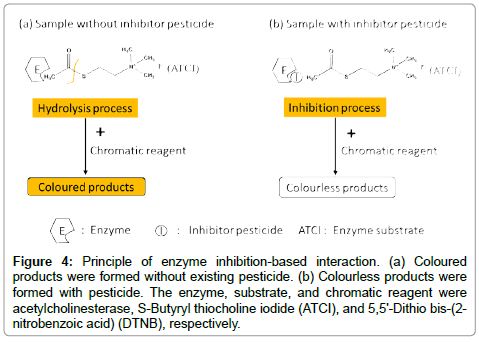
The schematic principle of the enzyme inhibition-based interaction [25] was shown in Figure 4. The hydrolysis process of enzyme substrate, i.e. S-Butyryl thiocholine iodide (ATCI) was reacted with Enzyme (acetylcholinesterase, AchE) and then produced S-Acetylthiocholine iodide. The product will be chelated with certain chromatic reagent (DTNB, 5,5'-Dithio bis-(2-nitrobenzoic acid)) and then generated a colored product (azoic compound) as shown in Figure 4(a). This colored compound can be detected by optical absorbance at 410 nm wavelength. However, the existing pesticide can prohibit the hydrolysis process. That will result in the absence of the colored compound as shown in Figure 4(b). Therefore, it can reflect the relative pesticide concentration directly by detecting the absorbance intensity of colored compound. The multiple analyses included enzyme inhibition-based assay for pesticide detection could be performed by using CMS. The stability of the colored compound was an important role in sensitivity by using this method. To further examine it, the central spectrum of the colored compound was kept at 410 nm wavelength for 10 mins and then did six tests spanned one hour to obtain the Time-Absorbance curve. The inhibition time was 15 mins to keep the linear relationship of logarithmically concentration (lgC) and spectral absorbance, which will be discussed in next session [26,27].
Figure 4: Principle of enzyme inhibition-based interaction. (a) Coloured products were formed without existing pesticide. (b) Colourless products were formed with pesticide. The enzyme, substrate, and chromatic reagent were acetylcholinesterase, S-Butyryl thiocholine iodide (ATCI), and 5,5'-Dithio bis-(2- nitrobenzoic acid) (DTNB), respectively.
Analyte measurements
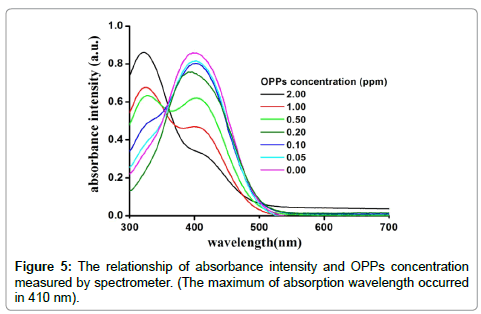
The pesticide could prohibit the activities of the enzyme molecule. Consequently, that can decay the interaction between enzyme and substrate to vanish the colored azoic compound in R3 of CMS. The stable colored azoic compound can be detected by spectrometer. The relationship of absorbance intensity and OPPs concentration measured by spectrometer was shown in Figure 5. The absorbance intensity of colored compound was decreased by increasing the OPPs concentration. The inhibition rate (In%) can be calculated from a comparison of absorbance intensity without (A0) and with (Ai) pesticide as expressed as Equation (4).
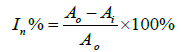 (4)
(4)
The inhibition rate is logarithmically proportional to the pesticide concentration, which can be converted into Equation (5) given as follows.
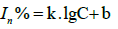 (5)
(5)
Where k and b are slope and intercept, respectively.
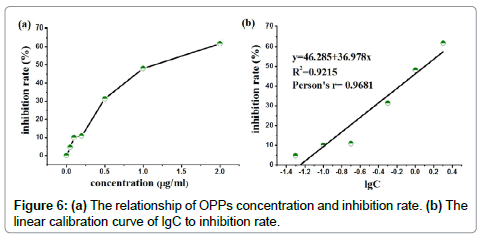
Therefore, the pesticide concentration can be calculated by measurement of absorbance intensity according to Equations (4 and 5). The relationship of OPPs concentration and inhibition rate was shown in Figure 6(a). The correlation coefficient (R-Square) was 0.9215 and Pearson correlation coefficient (Pearson’s r) was 0.9681. The slope of lgC and inhibition rate was 36.978 and an intercept was 46.285 (lgC=0), respectively, as shown in Figure 6(b). The sensitivity of CMS was higher than that reported in literatures as summarized in Table 1. Among of these approaches, paper-based devices were relied on the intensity or brightness on colored paper. The experimental results will be distortion and uncertainty. For PMMA centrifugal microchip, whose liner range was wider than that of other approaches, but LD was quite higher. Herein, the LD for this enzyme-based method using CMS was 0.05 ppm, i.e. 0.05 microgram per 1 gram of OPPs concentration can be detected from the absorbance intensity in Figure 5, which was satisfied with the safety limits in China (0.05 ppm) for OPPs in vegetables (GB 2763-2014). In this approach, the LD was calculated as 0.05 ppm according to the conventional three standard deviations of the blank sample (n≥7).
| Microfluidic biosensor | Detection theory | Pesticide kinds | Detection limit (LD) (ppm) | Liner range (ppm) | References |
|---|---|---|---|---|---|
| Paper-based devices | Enzyme based | Methyl-paraoxon | 0.05 | 0-0.9 | [26] |
| PDMS microchip | Electrochemical | Doazinon | 0.1 | 0.1-0.9 | [27] |
| PMMA centrifugal microchip | Enzyme based | Carbofuran | 0.1 | 0.1-5 | [25] |
| PMMA centrifugal microchip | Enzyme based | Phorate | 0.05 | 0.05-2 | This work |
Table 1: Comparison of approaches for analysis of pesticide residues.
In order to realize the application of this study, series fresh vegetables were bought from market and extracted and assayed for pesticide residue by using CMS. Several vegetables spiked with pesticide standard were tested and listed in Table 2. Two types of OPPs (phorate, omethoate) were evaluated the reliability by using CMS. The vegetable samples spiked with OPPs standards of phorate and omethoate as NO.1 to NO.4 were tested, respectively. Each of them was in triplicate. The recovery percentage of No. 2 for omethoate was higher than 100% as same as other samples due to the OPPs residue of original vegetables. For No 4 sample, there was no OPPs spiked in and the OPPs residue was detected as 0.13 ± 0.09. This value was higher than that of maximum residue limits (0.05 ppm) for pesticides in food (National foods safety standard No. GB 2763-2014). Although the recovery percentage of spiked samples for phorate is was in a reasonable range, CMS turns out to be a reliability and sensitivity for agriculture detection.
| Sample No. | Spiked level (ppm) | Measured level (ppm) | Recovery percentage (%) | |||
|---|---|---|---|---|---|---|
| Phorate | Omethoate | Phorate | Omethoate | Phorate | Omethoate | |
| 1 | 0.1 | 0.05 | 0.08±0.015 | 0.06±0.013 | 80±15 | 120±26 |
| 2 | 1 | 5 | 0.92± 0.15 | 5.16±0.16 | 92±15 | 103±3.4 |
| 3 | 2 | 1 | 1.86±0.05 | 1.76±0.15 | 93±2.5 | 176±15 |
| 4 | 0 | 0 | 0.13±0.09 | - | - | |
Table 2: Two kinds of OPPs residue detection from spiked vegetable samples.
Conclusion
In conclusion, we demonstrated the CMS for analysis of OPPs detection. The OPPs analyte can be controlled to stop or flow forward by changing the rotational speed, such as fluid was collected in two capillary valves at 44 (×g) and released into detection reservoir at 573 (×g), respectively. The LD of OPPs was 0.05 ppm and the relationship of lgC (ppm) and inhibition rate (%) was linear (R2=0.9215). In real vegetables analysis, the OPPs (phorate, omethoate) can be successively detected that recovery percentage of omethoate was larger than 100%. The OPPs detection by using CMS is a non-destructive, rapid, reliable, sensitive approach. The potential feasibility is the integration of CMS into a CD by using optical signal to assay and record all information of each detection reservoir. Therefore, the use of CMS can achieve the multi-functions of detection. The future work will exploit the minimized CMS with multi-functions for highly efficient environmental sensors and for new optofluidic sensor concepts.
Acknowledgement
This research work was supported by National Natural Science Foundation of China (No. 21271182), Major National Science Research Program (973 Program, No. 2013CB933000), Natural Science Foundation of Jiangsu Province (No. BK20160398) and Suzhou Science and Technology Project (No. SYG201633).
References
- Shamsipur M,Yazdanfar N, Ghambarian N(2016) Combination of solid-phase extraction with dispersive liquid-liquid microextraction followed by GC-MS for determination of pesticide residues from water, milk, honey and fruit juice. JFood chemistry 204:289-297.
- CateMD, Noblitt SD, Volckens J, Henry SC(2015) Multiplexed paper analytical device for quantification of metals using distance-based detection. C S Lab on a chip 15:2808-2818
- Strohmeier, Keller O, Schwemmer M,Zehnle F, Mark S, et al.(2015) Centrifugal microfluidic platforms: advanced unit operations and applications. Chem Soc Rev44: 6187-2220159.
- Manz A, Burggraf N, Effenhauser CS, Harrison DJ, Seiler K, etal. (1994) Electroosmotic pumping and electrophoretic separations for miniaturized chemical analysis systems. J MicromechMicroeng 4:257-265.
- Kim TH, Abi-Samra K, Sunkara V, Park DK, Amasia M, et al. (2013) Flow-enhanced electrochemical immunosensors on centrifugal microfluidic platforms. Lab on a chip 13:3747-3754.
- Chang HC, ChenYH, Lo AT, Hung SS, Lin SL, et al.(2014)Modified nanoporous membranes on centrifugal microfluidic platforms for detecting heavy metal ions. Mater Res Innov 18: 685-690.
- Burger R, Ducrée J (2012) Handling and analysis of cells and bioparticles on centrifugal microfluidic platforms. J Expert review of molecular diagnostics 12: 407-421.
- Sun Y, Zhang Y, Liu J, Nie F(2016) Integrated microfluidic device for the spherical hydrogel pH sensor fabrication RSC Adv 6: 11204-11210.
- Cai ZL, Xiang JW, Chen HL, Wang W(2016)Membrane-based valves and inward-pumping system for centrifugalmicrofluidic platforms. J Sens Actuator B-Chem 228: 251-258.
- Gilmore J, Islam M, DuarteMR (2016)Challenges in the use of compact disc-based centrifugal microfluidics for healthcare diagnostics at the extreme point of care. MicromachinesBasel 7: 52.
- Xi Y, Templeton EJ, Salin ED(2010) Rapid simultaneous determination of nitrate and nitrite on a centrifugal microfluidic device.Talanta 82: 1612-1615.
- Gorkin R, Park J, Siegrist J, Amasia M, Lee BS, et al. (2010) Centrifugal microfluidics for biomedical applications Lab on a chip 10: 1758-1773.
- Amasia M, Cozzens M, Madou M(2012) Centrifugal microfluidic platform for rapid PCRamplification using integrated thermoelectric heating and ice-valving.J Sens Actuator B-Chem 161: 1191-1197.
- Lee BS, Lee YU, Kim HS, Kim TH, Park J, et al.(2011) Fully integrated lab-on-a-disc for simultaneous analysis of biochemistry and immunoassay from whole blood. Lab on a chip 11: 70-78.
- Hirankittiwong P, Chattham N, Limtrakul J, Haba O, Yonetake K, et al. (2014)Optical manipulation of the nematic director field around microspheres covered with an azo-dendrimer monolayer. Opt express 22:20087-20093.
- Koh CY, Schaff UY, Piccini ME, Stanker LH, Cheng LW, et al. (2015) Centrifugal microfluidic platform for ultrasensitive detection of Botulinum toxin analytical chemistry 87: 922-928.
- Bison G, Wynands R, Weis A (2003) Dynamical mapping of the human cardiomagnetic field with a room-temperature, laser-optical sensor. Opt express 11: 904-909.
- Kim TH, Park J, Kim CJ, Cho YK (2014) Fully integrated lab-on-a-disc for nucleic acid analysis of food-borne pathogens. Anal chem86: 3841-3848
- Lafleur JP, Salin ED(2009) Pre-concentration of trace metals on centrifugal microfluidic discs with direct determination by laser ablation inductively coupled plasma mass spectrometry.J Anal At Spectrom 24: 1511-1516.
- Joo HW, Moon HJ, Choi Y, Kang HK (2015)Optical and optomechanical design of multiscale gigapixel camera system. Opt Soc Am 26P_118.
- Moorthy J, Mensing GA, Kim D, Mohanty S, Eddington DT, et al. (2004) Microfluidic tectonics platform: A colorimetric, disposable botulinum toxin enzyme-linked immunosorbent assay system. J Electrophoresis 25: 1705-1713.
- Lai S, Wang S, Luo J, Lee LJ, Yang TS, et al. (2004) Design of a compact disk-like microfluidic platform for enzyme-linked immunosorbent assay. Anal chem 76: 1832-1837.
- Chen JM, Huang PC, Lin MG (2007) Analysis and experiment of capillary valves for microfluidics on a rotating disk. Microfluid Nanofluidics4: 427-437.
- Ducrée J, Haeberle S, Lutz S, Pausch S,Stetten FV, et al. (2007) The centrifugal microfluidic Bio-Disk Platform.JMicromechMicroeng17:S103-S115.
- Duford DA, Xi Y, Salin ED(2013) Enzyme inhibition-based determination of pesticide residues in vegetable and soil in centrifugal microfluidic devices. Anal chem 85: 7834-7841.
- Nouanthavong S, Nacapricha D, Henry CS, Sameenoi Y (2016)Pesticide analysis using nanoceria-coated paper-based devices as a detection platform. Analyst141: 1837-1846.
- Han YD, Jeong CY, Lee JH, Lee DS, Yoon HC(2012) Microchip-based organophosphorus detection using bienzymebioelectrocatalysis. Jpn J Appl Phys 51: 06FK01.
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 4085
- [From(publication date):
June-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 3119
- PDF downloads : 966