Research Article Open Access
Central Venous Oxygen Saturation above 75% on Day Three of Septic Shock is Associated with Tripled Mortality
Timo Sturm1,2*, Jan Dertinger1,2, Michael Hagmann1,2, Manfred Thiel1,2 and Verena Schneider-Lindner1,2,3
1University Medical Centre Mannheim, Department of Anaesthesiology and Surgical Intensive Care Medicine, Mannheim, Germany
2TRACC-Group (Translational Research in Anaesthesiology and Critical Care), Canada
3Department of Community Health Sciences, University of Manitoba, Winnipeg, Canada
- *Corresponding Author:
- Timo Sturm
University Medical Centre Mannheim
Department of Anaesthesiology and Surgical Intensive Care Medicine
Theodor-Kutzer-Ufer 1-3 68167, Mannheim, Germany
Tel: 0049-6213832415
E-mail: timo.sturm@med.uni-heidelberg.de
Received date: September 26, 2017; Accepted date: October 10, 2017; Published date: October 12, 2017
Citation: Sturm T, Dertinger J, Hagmann M, Thiel M, Schneider-Lindner V (2017) Central Venous Oxygen Saturation above 75% on Day Three of Septic Shock is Associated with Tripled Mortality. J Infect Dis Ther 5:338. doi: 10.4172/2332-0877.1000338
Copyright: © 2017 Sturm T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Background: Central venous oxygen saturation (ScvO2) is commonly used to identify global oxygen uptakedelivery mismatches. A level above 70% was the declared goal in early resuscitation of septic shock for over a decade. Recent evidence leads to doubts and levels higher than 80% may represent harmful conditions. This study ´s aim was to identify favourable ScvO2 levels in treatment of septic shock.
Methods: Electronic data of patients suffering from septic shock who have been admitted to the surgical intensive care unit of a university hospital were analysed surveying a period of six years with focus on the association of ScvO2 levels with in-hospital mortality.
Results: Data from 238 individuals were included. No difference was found comparing initially measured values of ScvO2 of survivors to non-survivors. Patients whose levels of ScvO2 never exceeded 70% (n=28) had a higher mortality rate (73.2% vs. 54.3%, p<0.05). On day three patients with values above 75% (n=32) had higher mortality rates (59.4% vs. 38.5%, p<0.05). A mortality rate of 100% was detected if ScvO2 levels exceeded 84% (n=6).
Conclusions: ScvO2 develops from a therapy guiding parameter to a prognostic marker. Reaching levels of at least 70% within the first 72 h of disease is favourable in regard to prognosis. Exceeding 75% after day two is associated with higher mortality. These findings require further confirmation. At this point it can be assumed that a varying, time-dependent ScvO2 approach might be required for clinical decision-making.
Keywords
Central venous; Saturation; Mortality; Septic shock; Prognosis
Introduction
Despite intensive efforts of improving therapy in treatment of septic shock mortality rates are still reported about 36.5% [1]. A number of 5.3 million deaths annually worldwide is estimated [2]. Treatment recommendations [3,4] focus on infection control and cardiorespiratory resuscitation to restore oxygen supply, optimize tissue perfusion, avoid hypoxia, and at least prevent organ failure [5]. Central venous oxygen saturation (ScvO2) and serum lactate concentration are commonly accepted indicators of oxygen supply and global oxygen balance [3,6,7] and have been suggested as goals of treatment for more than a decade. Serum lactate has traditionally been interpreted as a marker of tissue hypoxia due to inadequate oxygen delivery. Particularly during prolonged episodes of sepsis, however, high levels of serum lactate can also result from impaired hepatic clearance or increased pyruvate production which is irresponsive to an optimized oxygen supply [8]. By now there are doubts concerning treatment strategies which are focused on serum lactate levels [9].
A level of ScvO2 above 70% has been a conventional goal in early septic shock therapy since first early goal directed therapy (EGDT) studies have been released [10,11]. Recent trials failed to demonstrate a survival benefit for these classic treatment goals compared to usual care [12-15]. Particularly in later stages of disease, interpretation of ScvO2 is challenging for clinicians [7]. Reviews attest “no benefit of early resuscitation guided by venous oxygen saturation” [16] or suggest that a subset of patients could benefit [15]. Consequently, adjusting ScvO2 is no longer part of the current surviving sepsis campaign (SSC) guidelines [4]. In contrast, the SSC guidelines state that the use of the previous targets is still safe and may be considered as no harm was associated with the interventional strategies and clinicians still need guidance for approaching this group of patients with significant mortality and morbidity. This raises the question whether ScvO2 should still be measured in septic shock at all.
ScvO2 values are considered to reflect mixed venous oxygen saturation (SvO2) [11]. SvO2 is an indirect index of global oxygen supply-to-demand ratio [11]. In early septic shock with inadequate oxygen delivery, mostly due to anaemia, absolute or relative hypovolemia, or myocardial dysfunction, systemic extraction ratio rises and SvO2 decreases [17]. In prolonged septic shock oxygen utilization is compromised by oedema induced diffusion limitation and by toxic respiratory chain uncoupling [5,18,19]. As a result less oxygen is consumed and returned back to the central circulation as indicated by increasing SvO2 values. Increased risk of multiple organ dysfunction and death, hence, are likely reflected by both low and high ScvO2 values. This suggests there may be ranges or corridors of ScvO2 values, which are associated with favourable patient outcome. Moreover, the significance of specific ScvO2 values may vary in the course of septic shock and thus a single fixed goal or corridor may be inadequate for treatment guidance. Data supporting these hypotheses are scarce, retrospective analyses suggest reduced survival for patients with venous saturations below 70% and above 90% in the first 6 h [19], as well as above 80% up to day three of septic shock therapy [20]. Prognostic studies evaluating ScvO2 with a time-dependent approach, however, are lacking.
The aim of this study therefore was to link the level of ScvO2 with mortality rates and identify ScvO2 levels in defined time corridors potentially beneficial for patient outcome from septic shock.
Materials and Methods
This investigation is single centre retrospective observational, using the patient data management system (PDMS) database (IntelliVue Clinical Information Portfolio, Phillips Healthcare, Netherlands) of a surgical intensive care unit (ICU). The study is designed corresponding to the declaration of Helsinki and ethical approval has been granted by a local committee (Medical Faculty of Mannheim, Germany, chairman Prof. JP Striebel, No. 2014-816R-MA). Design and reporting are according to the CONSORT statement recommendations (www.consort-statement.org).
Cohort inclusion and exclusion criteria
All patients admitted with septic shock between 1st January 2007 and 31st October 2013 were included (Figure 1). Septic shock was operationalized according to the SSC guidelines of 2012 focussing on sepsis-induced hypotension despite appropriate volume resuscitation [3].
Patients admitted to the ICU with an International Classification of Disease 10-code implying sepsis who had received norepinephrine and at least one measurement of ScvO2 within 72 h after ICU admission were identified automatically from the PDMS. Afterwards all matching electronic records as well as additional clinical data were examined by a medical expert. Clinical records of previous ICU stays were also reviewed. Patients who were readmitted due to ongoing septic disease were excluded as well as cases in which septic shock developed after admission to the ICU. Patients treated with norepinephrine for less than 3 h after having received a fluid bolus, whose venous catheter was not centrally located, or who have been receiving extracorporeal gas exchange therapy within their first 72 h were also excluded.
Cohort characteristics and outcome
Of all patients meeting the cohort of septic shock basic demographic data, sequential organ failure score (SOFA) and simplified acute physiology score 2 (SAPS 2) [21,22] were extracted from the PDMS. ScvO2 measurements as well as hemodynamic monitoring parameters and routine laboratory parameters have been determined five times – first 6,7 to 12,13 to 24,25 to 48 and 49 to 72 h after ICU admission. If more than one value was available, the one closer to the upper time interval limit was considered with exception of the first interval in which first measurements were considered. This procedure allowed comparability to previous investigations [19,23-26]. Based on recent literature [10,20] and our receiver operating characteristic (ROC) analysis, ScvO2 levels of each time interval were categorized as below, within or above corridors of 66-75% and 71-80%. Furthermore, the minimum and maximum ScvO2 values during the first 72 h were extracted.
Necessity of organ replacement therapy as well as ICU length of stay were also determined. Follow-up ended at hospital discharge or inhospital death, which were extracted from the local hospital information system (IS-H med, SAP-Industry Solution, Germany).
Note on clinical practice during the investigation
Moderate sedation, lung protective ventilator settings supporting spontaneous breathing, empirical antibiotics, early microbiological and radiological diagnostics as well as interventional and surgical focus control were ensured like recommended by the SSC [3]. For hemodynamic stabilization balanced crystalloid solutions, norepinephrine and dobutamine were preferred choices. A MAP of>65 mmHg, urine output of >0.5 ml/kg/h, ScvO2>70% and serum lactate concentration<2 mmol/l were declared as primary goals. If required, hemodynamic monitoring was augmented by echocardiography or pulse contour cardiac output (therapeutic aim: cardiac index (CI)>2.5 l/min*m2) and to estimate volume status and contractility. ScvO2 measurements were part of routine care, determined by a point-of-care blood gas analyzer (ABL800 Radiometer, Copenhagen, Denmark) and directly transmitted to the local PDMS.
Statistical analyses
Descriptive statistics were used to summarize demographic, clinical, hemodynamic and laboratory parameters of the cohort and also for survivors and non-survivors separately. Characteristics of the latter two groups were compared with two-tailed t-tests and chi-square tests. Survivors and non-survivors of the cohort were classified according to their ScvO2-level at each time interval. For each of the fivetime intervals, odds ratios (OR) with 95% confidence intervals (CI) for ScvO2-dependent mortality risk in those still alive at the beginning of the respective interval were obtained from logistic regression. ROC-curves were used to determine the maximum value for the sum of sensitivity and 1-specificity of potential ScvO2 cut-off values for mortality prediction at the different time intervals. The probability of survival for different ScvO2 levels was depicted in Kaplan-Meier plots. For all analyses a p-value<0.05 (two-tailed) was considered statistically significant. SAS 9.4 (SAS Cooperation, Cary, North Carolina, USA) was used for data analysis.
Results
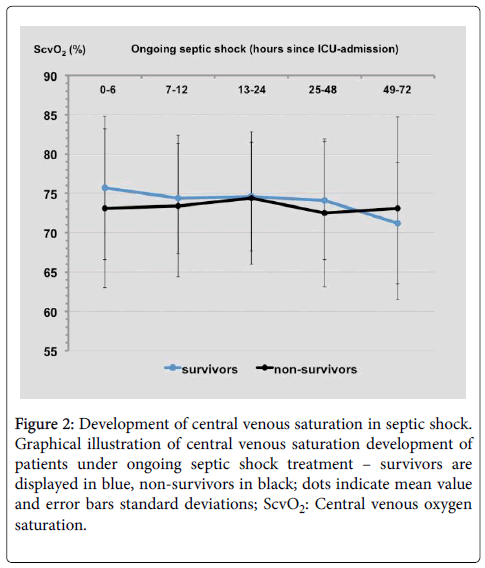
Of 16044 ICU admissions during the study period 466 potential shock patients fulfilled the inclusion criteria. After examination by an expert the final cohort of 238 patients with septic shock on admission was set (Figure 1). Overall hospital mortality of 57.6% and observed disease severity of the first day after ICU admission based on SAPS 2 and SOFA score in these patients are consistent with manifest multiple organ failure (Table 1). Survivors had higher haemoglobin levels at day one (p<0.05) and lower plasma lactate levels in any time interval (p<0.05 for each interval). A nonsignificant trend to higher initial ScvO2 measurement values in survivors compared to non-survivors (mean ± SD: 75.7% ± 9.1% vs. 73.1% ± 10.1%, p=0.13; median: 77% vs. 74%) and lower ones on day three (71.2% ± 7.7% vs. 73.1% ± 11.6%, p=0.37; median: 72% vs. 75%) was detected (Figure 2).
| n | All patients 238 |
Survivors 101 |
Non-survivors 137 |
p-value |
|---|---|---|---|---|
| Gender male (%) | 159 (77) | 67 (76) | 92 (77) | 0.89 |
| Age – years | 64 ± 15 | 59 ± 16 | 67 ± 14 | <0.0001* |
| Medical history: | ||||
| Diabetes (%) | 48 (20) | 19 (19) | 29 (21) | 0.63 |
| Hypertension (%) | 114 (48) | 49 (49) | 65 (47) | 0.87 |
| Impaired immunity (%) | 22 (9) | 10 (10) | 12 (9) | 0.76 |
| Malignancies (%) | 66 (28) | 18 (18) | 48 (35) | <0.01* |
| Focus side: | ||||
| Abdomen (%) | 115 (48) | 45 (45) | 70 (51) | 0.32 |
| Lung (%) | 82 (34) | 37 (37) | 45 (33) | 0.54 |
| Urogenital (%) | 11 (5) | 8 (8) | 3 (2) | <0.05* |
| Soft tissue (%) | 10 (4) | 3 (3) | 7 (5) | 0.42 |
| Others or unknown (%) | 20 (8) | 8 (8) | 12 (9) | 0.82 |
| Clinical data: | ||||
| SAPS 2 | 65.8 ± 14.6 | 61.4 ± 14.2 | 69.7 ± 13.9 | <0.001* |
| SOFA | 13.3 ± 2.5 | 12.5 ± 2.3 | 14.0 ± 2.5 | <0.0001* |
| Temperature (°C) | 36.9 ± 1.2 | 37.0 ± 1.0 | 36.8 ± 1.3 | 0.52 |
| CRP (mg/l) | 205 ± 108 | 196 ± 101 | 214 ± 113 | 0.44 |
| PCT (mg/l) | 22.5 ± 44.5 | 30.8 ± 55.9 | 16.8 ± 34.1 | 0.12 |
| Bilirubin (mg/dl) | 1.2 ± 1.2 | 1.2 ± 1.4 | 1.2 ± 1.0 | 0.85 |
| Creatinine (mg/dl) | 2.2 ± 2.2 | 2.2 ± 2.6 | 2.2 ± 1.8 | 0.91 |
| Haemoglobin (g/dl) | 10.3 ± 1.9 | 10.7 ± 1.9 | 9.9 ± 1.8 | <0.05* |
| MAP (mmHg) | 76 ± 14 | 77 ± 14 | 74 ± 13 | 0.37 |
| Heart rate (/min) | 106 ± 21 | 105 ± 20 | 107 ± 22 | 0.52 |
| Cardiac index (l/min/m2) | 3.7 ± 1.3 | 4.1 ± 1.4 | 3.4 ± 1.2 | 0.22 |
| Fluid balance first day (l) | + 6.8 ± 5.0 | + 6.8 ± 4.9 | + 6.8 ± 5.0 | 0.93 |
| Norepinephrine (mg/kg/min) | 0.37 ± 0.43 | 0.33 ± 0.37 | 0.39 ± 0.48 | 0.41 |
| Lactate (mmol/l) | 3.7 ± 3.5 | 2.8 ± 2.5 | 4.4 ± 0.5 | <0.05* |
| SaO2 (%) | 95.2 ± 4.6 | 95.8 ± 3.4 | 94.9 ± 5.3 | 0.12 |
| ScvO2 (%) | 74.3 ± 9.8 | 75.7 ± 9.1 | 73.1 ± 10.1 | 0.13 |
| Mechanical ventilation (%) | 222 (93.3) | 93 (92.1) | 129 (94.2) | 0.53 |
| Renal replacement (%) | 46 (19.3) | 12 (11.9) | 34 (24.8) | <0.05* |
| ICU days | 14.1 ± 18.0 | 17.6 ± 18.6 | 11.3 ± 17.0 | <0.01* |
| Hospital days | 30.6 ± 45.5 | 49.0 ± 46.3 | 17.0 ± 39.7 | <0.0001* |
Table 1: Study population – basic characteristics. First six hours data are shown except for clinical scores (first day). Data are given as number and percentage or mean ± SD, respectively; SAPS: Simplified acute physiology score; SOFA: Sequential organ failure assessment score; CRP: C-reactive protein; PCT: Procalcitonin; MAP: Mean arterial pressure; SaO2: Arterial blood oxygen saturation; ScvO2: Central venous oxygen saturation; * significant result by two-tailed t-test comparison.
Figure 2: Development of central venous saturation in septic shock. Graphical illustration of central venous saturation development of patients under ongoing septic shock treatment – survivors are displayed in blue, non-survivors in black; dots indicate mean value and error bars standard deviations; ScvO2: Central venous oxygen saturation.
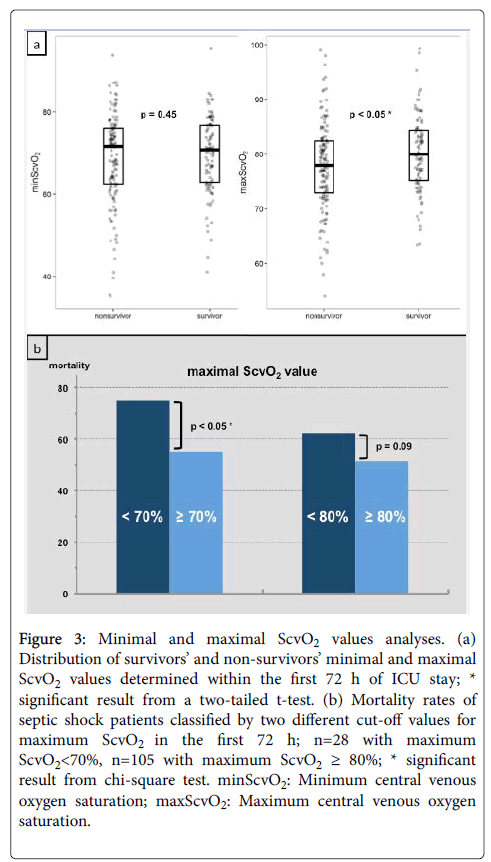
The minimum value of ScvO2 was 69.03% on average in all patients (range 35.2%-95.3%). While ScvO2 minima did not differ in survivors and non-survivors, maximum ScvO2 values, on average, were significantly higher in survivors compared to non-survivors (Figure 3a). Hospital mortality was higher if maximal ScvO2 values recorded at any time within the first three days of therapy were below 70%, whereas no impact on outcome was detected for maximum values ≥ 80% (Figure 3b).
Figure 3: Minimal and maximal ScvO2 values analyses. (a) Distribution of survivors’ and non-survivors’ minimal and maximal ScvO2 values determined within the first 72 h of ICU stay; * significant result from a two-tailed t-test. (b) Mortality rates of septic shock patients classified by two different cut-off values for maximum ScvO2 in the first 72 h; n=28 with maximum ScvO2<70%, n=105 with maximum ScvO2 ≥ 80%; * significant result from chi-square test. minScvO2: Minimum central venous oxygen saturation; maxScvO2: Maximum central venous oxygen saturation.
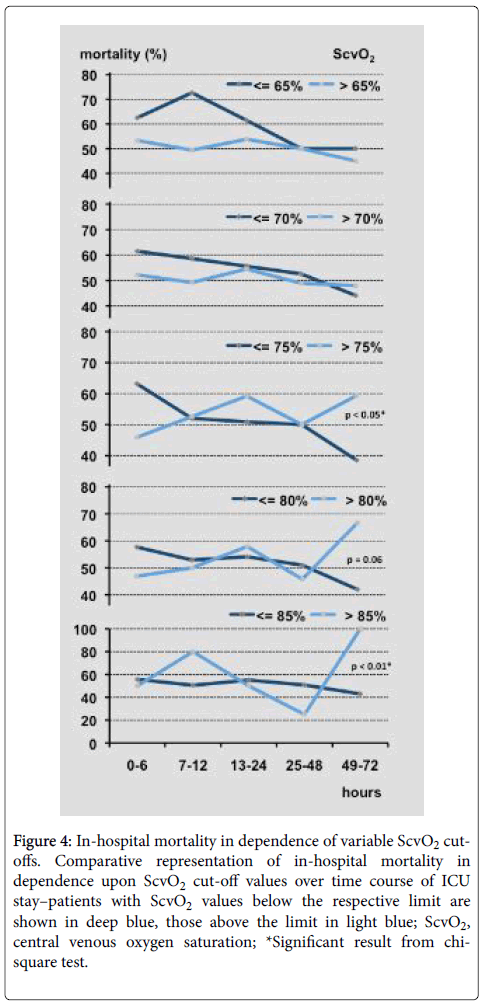
Figure 4 displays mortality rates in relation to increasing ScvO2 cut-off values in the period of the first 72 h. In early phases of septic shock mortality was higher in patients having lower ScvO2 values up to a cut-off of 80%. These differences were not statistically significant.
On day three ScvO2 cut-offs above 70% distinguished well between survival until patient’s discharge from hospital and intra-hospital mortality (Figure 4). A ROC analysis detected an optimal cut-off at 76.0% for third day’s ScvO2 (70.4% sensitivity, 60.6% specificity; area under the curve: 0.662). ScvO2 ≥ 84.0% (n=6) on day three identified non-survivors with a sensitivity of 100%.
Figure 4: In-hospital mortality in dependence of variable ScvO2 cut-offs. Comparative representation of in-hospital mortality in dependence upon ScvO2 cut-off values over time course of ICU stay–patients with ScvO2 values below the respective limit are shown in deep blue, those above the limit in light blue; ScvO2, central venous oxygen saturation; *Significant result from chi-square test.
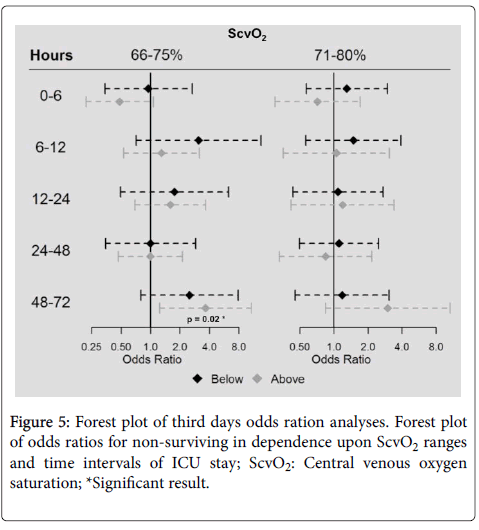
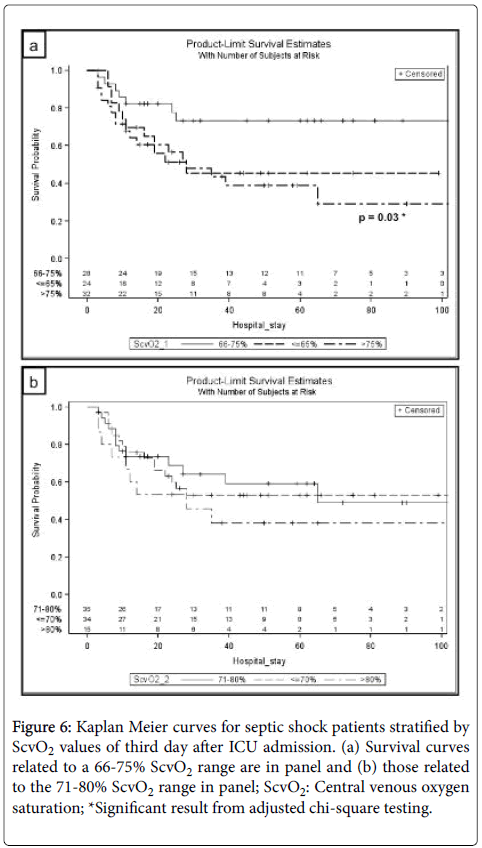
Odds ratios for in-hospital mortality of patients classified in defined ScvO2 corridors (66-75% and 71-80%) did not indicate any difference regarding mortality risk for patients with ScvO2 values below or above these reference limits at the times of measuring within 48 h after ICU admission (Figure 5). Mortality was higher in patients with ScvO2 values>75% (n=32) compared to those in the corridor of 66–75% (n=28) on day three with an odds ratio of 3.6 (95% confidence interval 1.2 to 10.8, p=0.02). Kaplan-Meier analyses of both mentioned corridors on day three of septic shock treatment are presented as Figures 6a and 6b.
Figure 6: Kaplan Meier curves for septic shock patients stratified by ScvO2 values of third day after ICU admission. (a) Survival curves related to a 66-75% ScvO2 range are in panel and (b) those related to the 71-80% ScvO2 range in panel; ScvO2: Central venous oxygen saturation; *Significant result from adjusted chi-square testing.
Discussion
Based on early EGDT studies [10,27] former treatment recommendations supported ScvO2 correction above a value of 70% within the first 6 h of sepsis [3,15]. In accordance Pope et al. [19] reported mortality rates to be significantly elevated if septic patients in the emergency department indicated maximum ScvO2 values below 70% (hypoxic range) compared to those being in normoxic range (ScvO2 71-89%). Park et al. confirmed these findings for septic shock patients exhibiting oxygen extraction ratios higher than 0.3, being approximately equivalent to calculated ScvO2 values lower than 70% [28] and Lee et al. also found reduced survival if ScvO2 could not be raised above 70% and lactate remained abnormal [29]. In line with these observations, our data showed diminished survival rates if ScvO2 values could not be increased above this threshold (<70%) during the first 72 h of ICU stay. About one third of our patients have had ScvO2 values below 70% and approximately in one fifth ScvO2 was below 65% when first measured in ICU. These results are supported by findings of Boulain et al. who prospectively showed that ScvO2 values<70% are frequent (27%) at ICU admission [30] and extended by van Beest et al., who demonstrated that even lower rates of ScvO2 (<60%) are still occurring (6%) [25]. Initial mean ScvO2 values (74% ± 11% [25] and 74 ± 10.4% [30]) were comparable to ours and there was also no difference detected between survivors and non-survivors [30].
On the other hand Park et al. [28] as well as Pope et al. [19] reported significantly elevated in-hospital mortality rates if ScvO2 values and oxygen extraction rates were in the hyperoxic range, i.e., above 89% or lower than 20%, respectively, with the latter most likely indicating ScvO2 values being above 80%. These observations were made within the first hour and after 6 h of ongoing goal directed therapy. The data reported on by Textoris et al. [20] confirmed and extended the period of increased mortality risk in septic patients by investigating maximum ScvO2 values taken during 72 h of treatment, although this group gave no information on the exact time of ScvO2 determination: Using a ScvO2 cut-off value of 80%, a 1.5 fold higher mortality rate for patients with maximum values above 80% was shown compared to those with values below this threshold (48% vs. 30%, p<0.05).
Our analysis of mortality rates associated with ScvO2 in the five time intervals using different cut-off values for ScvO2 starting from 65% up to 85% revealed significantly higher mortality rates with ScvO2 values above 75% on the third day. ROC analysis showed an optimal cut-off at 76.0% for third day’s ScvO2 (70.4% sensitivity, 60.6% specificity). Moreover, mortality was significantly higher in patients with ScvO2>75% (n=32) compared to those in the corridor of 66-75% (n=28) on day three with an odds ratio of 3.6 (confidence interval 1.2 to 10.8, p=0.02) (Figure 5). Kaplan-Meier analysis of mentioned ScvO2 corridor on day three confirmed lower survival rates for ScvO2 values below and above the corridor of 66-75%, reaching the level of statistical significance for ScvO2 values above 75% (Figure 6). Thus, patients whose ScvO2 values were in the corridor of 66-75% on third day of septic shock had threefold higher odds of survival compared to those whose ScvO2 was above 75%. This is a drastically lower limit than previously suggested (Pope et al. >90% [19], Textoris et al. >80% [20]) and within the previously recommended range of 70-80% [3,11]. Moreover, these recommendations have been extrapolated to nearly every kind of critical illness, perioperative situations [16,31] and decision-making concerning transfusion [32,33]. According to the present study ScvO2 ranges above 75% have to be considered as potentially deleterious for patients in later stages of septic shock. This is supported by the observation of no single individual, whose ScvO2 was above 85% on the third day of septic shock surviving. Unsurprisingly, specificity of ScvO2 values ≥ 84.0% on day three regarding in-hospital mortality was 100%.
Overall the most important finding was a noticeably increased mortality risk even if ScvO2 values were only slightly elevated (>75%) in later stages of septic shock, which until now have been considered as unsuspicious. They might be used as warning signs as recommended by some authors in the perioperative cardiosurgical setting [24,34].
Limitations
This is a retrospective study. Therefore the observed associations may not be causal and the results have to be confirmed by adequately powered prospective studies. Because the data are from a single center, particularities of local practice may have had an impact on measurement frequency, but also magnitude of ScvO2 values. In addition, ScvO2 measurements were determined for routine care leading to factors of uncertainty such as mix-up of patients and documentation errors. Considering predefined time corridors retrospectively leads to data gaps in some patients because measurements are taken in irregular, clinically necessary and practical intervals. In contrast, for other patients only a fraction of available measurements was used. Incomplete data also result from patients dying during the observation period. Nonetheless we analysed numerous ScvO2 values from septic patients with high mortality risk. In spite of these limitations, we consider it important to disseminate these results because they indicate that potentially harmful ScvO2 levels are apparently very close to formerly recommended treatment goals. In future prospective studies ScvO2 should ideally be measured regularly if not continuously in all patients irrespective of their clinical course. Specifically, in such studies the value of ScvO2 for mortality prediction by U-shaped models should be addressed in a time course dependent fashion.
Conclusion
We strengthened the hypothesis that ScvO2 is of potential value in predicting unfavourable outcome in septic shock. If ScvO2 values of at least 70% have not been achieved within 72 h of therapy, in-hospital mortality significantly increased. On the other hand ScvO2 values above 75% on day three were associated with a threefold mortality rate compared to values in a ScvO2 range of 66-75%. High values in early septic shock seem to be unsuspicious as well as lower ones in later stages. Further investigations have to confirm these findings in before they can be implemented in clinical decision-making.
Acknowledgements
The authors would like to thank Mr. Christoph Clauss for critical reading and editing the manuscript.
Funding
This work and the purchase of equipment and software were supported by the Department of Anaesthesiology and Surgical Intensive Care Medicine, University Medical Centre Mannheim, Germany and in part by the foundation Klaus Tschira Stiftung, Germany.
Competing Interests
The authors declare that they have no competing interests.
References
- Gu WJ, Wang F, Bakker J, Tang L, Liu JC (2014) The effect of goal-directed therapy on mortality in patients with sepsis - earlier is better: a meta-analysis of randomized controlled trials. Crit Care 18: 570.
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, et al. (2016) Assessment of Global Incidence and Mortality of Hospital-treated Sepsis - Current Estimates and Limitations. Am J Respir Crit Care Med 193: 259-272.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41: 580-637.
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, et al. (2017) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 43: 304-377.
- Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369: 840-851.
- He H, Long Y, Liu D, Wang X, Zhou X (2015) Clinical classification of tissue perfusion based on the central venous oxygen saturation and the peripheral perfusion index. Crit Care 19: 330.
- van Beest P, Wietasch G, Scheeren T, Spronk P, Kuiper M (2011) Clinical review: use of venous oxygen saturations as a goal-a yet unfinished puzzle. Crit Care 15: 232.
- Suetrong B, Walley KR (2016) Lactic acidosis in sepsis: It's not all anaerobic. Implications for diagnosis and management. Chest 149: 252-261.
- Marik PE (2014) Early management of severe sepsis: concepts and controversies. Chest 145: 1407-1418.
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, et al. (2011) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345: 1368-1377.
- Hartog C, Bloos F (2014) Venous oxygen saturation. Best Pract Res Clin Anaesthesiol 28: 419-428.
- Yealy DM, Kellum JA, Huang DT, Barnato AE (2014) A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med 370: 1683-1693.
- Peake SL, Delaney A, Bailey M (2014) Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371: 1496-1506.
- Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, et al. (2015) Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 372: 1301-1311.
- Gupta RG, Hartigan SM, Kashiouris MG, Sessler CN, Bearman GM (2015) Early goal-directed resuscitation of patients with septic shock: current evidence and future directions. Crit Care 19: 286.
- Chemtob RA, Eskesen TG, Moeller-Soerensen H, Perner A, Ravn HB (2016) Systematic review of the association of venous oxygenation and outcome in adult hospitalized patients. Acta Anaesthesiol Scand 60: 1367-1378.
- Rivers EP, Yataco AC, Jaehne AK, Gill J, Disselkamp M (2015) Oxygen extraction and perfusion markers in severe sepsis and septic shock: diagnostic, therapeutic and outcome implications. Curr Opin Crit Care 21: 381-387.
- Galley HF (2011) Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth 107: 57-64.
- Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, et al. (2010) Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med 55: 40-46.
- Textoris J, Fouche L, Wiramus S, Antonini F, Tho S, et al. (2011) High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care 15: R176.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707-710.
- Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270: 2957-2963.
- Hosking C, Wilander P, Goosen J, Jacobson H, Moeng M, et al. (2011) Low central venous oxygen saturation in haemodynamically stabilized trauma patients is associated with poor outcome. Acta anaesthesiologica Scandinavica 55: 713-721.
- Balzer F, Sander M, Simon M, Spies C, Habicher M, et al. (2015) High central venous saturation after cardiac surgery is associated with increased organ failure and long-term mortality: an observational cross-sectional study. Crit Care 19: 168.
- van Beest PA, Hofstra JJ, Schultz MJ, Boerma EC, Spronk PE, et al. (2008) The incidence of low venous oxygen saturation on admission to the intensive care unit: a multi-center observational study in The Netherlands. Crit Care 12: R33.
- Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, et al. (2010) Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 303: 739-746.
- Puskarich MA, Marchick MR, Kline JA, Steuerwald MT, Jones AE (2009) One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: a before and after study. Crit Care 13: R167.
- Park JS, Kim SJ, Lee SW, Lee EJ, Han KS, et al. (2015) Initial Low Oxygen Extraction Ratio Is Related to Severe Organ Dysfunction and High In-Hospital Mortality in Severe Sepsis and Septic Shock Patients. J Emerg Med 49: 261-267.
- Lee YK, Hwang SY, Shin TG, Jo IJ, Suh GY, et al. (2016) Prognostic Value of Lactate and Central Venous Oxygen Saturation after Early Resuscitation in Sepsis Patients. PLoS One 11: e0153305.
- Boulain T, Garot D, Vignon P, Lascarrou JB, Desachy A, et al. (2004) Prevalence of low central venous oxygen saturation in the first hours of intensive care unit admission and associated mortality in septic shock patients: a prospective multicentre study. Crit Care 18: 609.
- Shepherd SJ, Pearse RM (2009) Role of central and mixed venous oxygen saturation measurement in perioperative care. Anesthesiology 111: 649-656.
- Vallet B, Adamczyk S, Barreau O, Lebuffe G (2009) Physiologic transfusion triggers. Best Pract Res Clin Anaesthesiol 21: 173-181.
- Meybohm P, Shander A, Zacharowski K (2015) Should we restrict erythrocyte transfusion in early goal directed protocols? BMC anesthesiology 15: 75.
- Perz S, Uhlig T, Kohl M, Bredle DL, Reinhart K, et al. (2011) Low and "supranormal" central venous oxygen saturation and markers of tissue hypoxia in cardiac surgery patients: a prospective observational study. Intensive Care Med 37: 52-59.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 3904
- [From(publication date):
October-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 3219
- PDF downloads : 685