Research Article Open Access
Celecoxib 2% Cream in Acute Soft Tissue Injuries: Randomized, Double-blind, Placebo-controlled Clinical Trial
Villegas-Rivera Geannyne1*, Covarrubias-Pinedo Amador1, Romero-Medina Silvia2, Solorza-Camacho Karim Saul2, Alatorre-Carranza María del Pilar1, Rodríguez-Herrera Lourdes Yolotzin1, Jaime Islas-Nilssa Graciela1 and Galaviz-Muro Adriana11Instituto de Investigación Clínica de Occidente, Clinical Research, Mexico
- *Corresponding Author:
- Geannyne VR
Instituto de Investigación Clínica de Occidente, Clinical Research
5674 Ludwig Van Beethoven Street, Zapopan, Jalisco, 45030, México
Tel: +52 (01) 33 36298485
E-mail: gvillegas.md@gmail.com
Received date: January 25, 2017; Accepted date: February 17, 2017; Published date: February 24, 2017
Citation: Geannyne VR, Amador CP, Silvia RM, Karim Saul SC, María del Pilar AC, et al. (2017) Celecoxib 2% Cream in Acute Soft Tissue Injuries: Randomized, Double-blind, Placebo-controlled Clinical Trial. Clin Res Foot Ankle 5:226. doi:10.4172/2329-910X.1000226
Copyright: © 2017 Geannyne VR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Research on Foot & Ankle
Abstract
Objective: The aim of the study was to evaluate the efficacy of pain reduction and tolerability of topical administration of Celecoxib 2% cream compared to Celecoxib 1% cream and placebo cream in Mexican patients who had acute soft tissue injury in lower limbs.
Methods: A randomized, double-blind, placebo control trial with 3 parallel groups was conducted. We include Mexicans patients older than 18 years with diagnosis of acute soft tissue injury in lower limbs. They were randomly assigned to Celecoxib 2% cream (CEL-2), Celecoxib 1% cream (CEL-1) or placebo cream (PLA). All treatments should be applied 3 times a day for a period of 7 days. Every day the pain was assessed with a Visual Analogue Scale (VAS). Secondary, we evaluate inflammation and adverse events.
Results: A total of 95 patients were included. VAS on day 1 and 7 in group CEL-2 were 57.41 ± 10.39 mm and 4.34 ± 7.02 mm, in CEL-1 59.38 ± 9.37 mm and 10.41 ± 12.78 mm, and in PLA 55.61 ± 8.09 mm and 9.32 ± 9.93 mm. CEL-2 showed greater pain decrease compared to CEL-1 and PLA, p<0.05. CEL-1 group significantly decreased inflammation more than PLA, p<0.05. 15 adverse events were reported in 9 patients, none was severe.
Conclusion: The results shown in the present study demonstrate that topical administration of Celecoxib cream 2%, TID for 7 days was effective in pain relief in patients with acute soft tissue injury.
Keywords
Soft tissue injuries; Lower limb; Celecoxib 2% cream; Celecoxib 1% cream; Placebo cream.
Introduction
Soft Tissue Injuries (STI) includes all injuries to muscles, ligaments, tendons and skin. STI and especially ankle injuries are the most common locations for trauma, it is estimated that 20% of all sports injuries present in the ankles, and that 85% of these are due to twisted ankles [1,2].
Soft tissue responds to trauma in three phases that can overlap: inflammation, tissue formation and tissue remodeling [3]. The prostaglandin pathway is primarily responsible for the release of mediators of inflammation so the treatment is focused on the inhibition of Cyclooxygenase (COX); thus Nonsteroidal Anti- Inflammatory Drugs (NSAIDs) block the COX-1 and COX-2 isoforms, and Celecoxib exclusively COX-2 [4]. Both groups are ideal for controlling sports injuries because they decrease excessive inflammation and pain [5].
Because of the adverse gastrointestinal effects of NSAIDs, effective topical formulations have been successfully used in pain relief of muscle injuries such as sprains and bruises compared to placebo [6-8].
Experience with topical NSAIDs in the management of sports injuries show that there are no significant differences in pain relief when compared to oral formulations [9]. Although in some cases it has been shown that topical formulations can reach higher concentrations in the inflamed tissues than the oral ones [10]. Likewise, topical application may limit systemic adverse effects by increasing local effects and minimizing systemic drug concentrations [11-13].
There are no clinical trial publications evaluating the analgesic and anti-inflammatory effects of topical COX-2 inhibitors in soft tissue injuries. However, other studies comparing the efficacy of COX-2 vs oral NSAIDs formulations have been published [14-16].
On the other hand, topical formulations of analgesics in which microemulsions are incorporated facilitate the availability of the drug at the desired site. Specifically with Celecoxib, we have the antecedent that incorporating microemulsions increases the absorption and with it the local inhibition of COX-2 [17].
Thus, the aim of the study was to evaluate the efficacy of pain reduction and tolerability of topical administration of Celecoxib 2% cream compared to Celecoxib 1% cream and placebo cream in Mexican patients who had acute soft tissue injury in lower limbs.
Methodology
A total of 95 Mexican patients, older than 18 years with diagnosis of acute soft tissue injury, located in the calf, shin, ankle, heel or foot were included. This could be acute injury of ligaments, tendons or muscles (including sprain or twist grade 1 or 2) occurred 48 hours before the baseline visit. The Visual Analogue Scale (VAS) should be ≥ 40 mm and all signed informed consent before any intervention.
Pregnant women, in the nursing period or who did not have any method of contraception, patients with active skin injuries or diseases at the intended site of application, pharmacological and nonpharmacological treatment for the injury 24 hours before entering the study, use of corticosteroids orally or parenterally 30 days prior to injury, as well as hepatic, renal or cardiovascular alterations, were not included.
Design Study and Intervention
A randomized, double-blind, placebo control trial with 3 parallel groups was conducted at the Instituto de Investigación Clínica de Occidente in the city of Guadalajara, Mexico. The Institute is a private research center authorized by COFEPRIS to conduct phase I, II and III clinical trials. The study was conducted between June 2015 and March 2016.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference for Harmonization of Good Clinical Practice (ICH-GCP), was evaluated by the Research Ethics Committee of the centre and authorized by the Federal Commission for the Protection against Health Risks (COFEPRIS) with registration number: 143301912 × 2347/2015.
The study consisted of 2 clinical visits and a telephone call in a period of 7 days. A clinical history, physical examination, anthropometry, vital signs, laboratory tests including a pregnancy test and assessments of pain and inflammation were performed: Visual Analog Scale (VAS), Categorical Scale (CS), and circumference of the injury.
Screening was performed and patients were randomized to one of three study treatments: Celecoxib 2% cream (CEL-2), Celecoxib 1% cream (CEL-1) or placebo cream (PLA). All treatments should be applied 3 times a day for a period of 7 days. Patients were advised to maintain relative rest and elevate the limb above the level of the heart. The use of cold/hot dressings or compression was not recommended in order to allow the correct absorption of the study creams. Also, they were given a patient diary in which they had to write down every day the number of applications, hour, VAS and adverse events.
At day 3 they were telephoned and asked how they rated their pain in a CS and whether they had any adverse events. On day 7, patients were reassessed using a medical history, physical examination, physical examination, vital signs, laboratory tests including a pregnancy test, and evaluations of pain and inflammation: VAS, CS and circumference of the injury.
For the evaluation of pain, a VAS was used in which the patients marked their pain on a scale of 0 mm (none) to 100 mm (maximum). For CS, a 4-point Linkert scale was used (none, mild, moderate or severe). The circumference of the injury was measured in cm with a tape measure.
Safety measures included the monitoring of adverse events throughout the study and changes with clinical significance of laboratory tests. Also, Paracetamol until 4 g per day was allowed as rescue therapy.
Variables
The primary response variable was pain reduction, assessed by VAS at day 7 in relation to baseline. Secondary response variables were the measurement of the circumference of the injury and the measurement of pain on a categorical scale. Likewise, we evaluated the adverse effects and any alteration with clinical significance of the laboratory tests.
Statistics
The hypothesis of the study was the superiority of Celecoxib cream over placebo cream. Since there is no literature on the clinical efficacy of Celecoxib topical in soft tissue injuries, the data from the Predel HG study, which evaluated the efficacy of a Diclofenac Gel vs. placebo, was taken [1]. The Jeyaseelan clinical trial formula was used [18] by taking the change in the VAS score. An α 5% level, β of 10% with a statistical power of 90% was considered, to find a difference of at least 16.2 mm with a standard deviation of 21.5 mm for a total of 111 patients.
The allocation of treatments was performed by the Sponsor using the Randomized Blocks procedure. They randomized the three study treatments into 10 blocks of 12 patients each and every block had 4 treatments of each group. The research products were identical and treatments were blinded using alphanumeric codes. To standardize the application of the cream, each patient was given a plastic dispenser (similar to a credit card with a straight line equivalent to 4 g of cream). In each application they should fill it with the cream and then apply it to the injured site.
For safety analysis we included all randomized patients who applied at least once the study products. Per protocol analysis was performed for efficacy. For the quantitative variables, analysis of variance (ANOVA) and the Student’s t-test were used, and for the qualitative X2 tests. It was considered that there were significant differences when the p value was <0.05.
Results
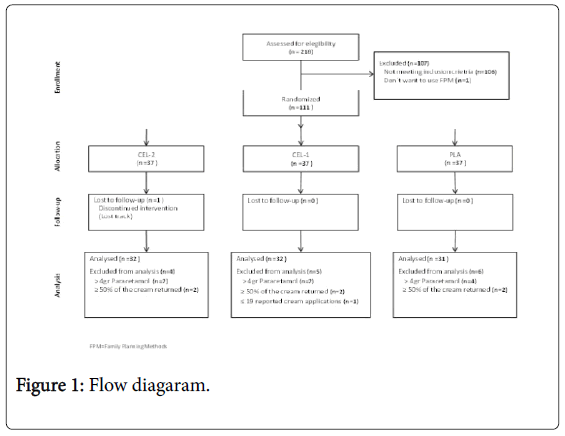
A total of 218 patients were invited to participate, however 107 were excluded. Of the 111 who entered the study, 37 were randomized to each group. The population analyzed per protocol was 95 patients (Figure 1).
A sum of 58% (n=55) of the patients were women and the mean age was 30.45 ± 11.11 years. The weight was 73.9 ± 15.14 kg, the height was 1.67 ± 0.09 m and the BMI was 26.4 ± 4.78 kg/m2.
A sum of 61% (n=58) had a bachelor´s degree, 99% (n=94) were laborally active and in all three groups the intensity of physical activity was moderate or low (Table 1).
| DEMOGRAPHICS | CEL-2 | CEL-1 | PLA |
|---|---|---|---|
| (n=32) | (n=32) | (n=31) | |
| Gender | |||
| Female, n (%) | 18 (56) | 16 (50) | 21 (68) |
| Male, n (%) | 14 (44) | 16 (50) | 10 (32) |
| Age (years) | 25.5 ± 10.40 | 26.5 ± 11.03 | 32.2 ± 11.9 |
| Scholarship | |||
| Primary, n (%) | 4 (13) | 4 (13) | 6 (19) |
| Secondary school, n (%) | 3 (9) | 7 (22) | 5 (16) |
| High school, n (%) | 4 (13) | 2 (6) | 2 (6) |
| Bachelor´s degree, n (%) | 21 (66) | 19 (59) | 18 (58) |
| Laborally active | 31 (97) | 32 (100) | 32 (100) |
| Physical activity | |||
| Low intensity, n (%) | 16 (50) | 18 (56) | 17 (55) |
| Moderate intensity, n (%) | 16 (50) | 14 (44) | 14 (45) |
Table 1: Demographic characteristics of study groups.
The main mechanism of injury was direct blow in 51% (n=48), fall from their own height in 35% (n=33) and stumble in 7% (n=7), Table 2. The anatomical region affected was the ankle in the lateral retromalleolar region with 39% (n=37) and the posterior leg region in 18% (n=17), Table 3. Also, by grouping the regions we observed that ankle injury was present in 78% (n=25) for CEL-2, 75% (n=24) for CEL-1 and 68% (n=21) for PLA. While the toes and legs were injured less frequently.
| Mechanism | CEL-2 | CEL-1 | PLA |
|---|---|---|---|
| (n=32) | (n=32) | (n=31) | |
| Jump and fall up, n (%) | 1 (3) | 0 (0) | 0 (0) |
| Fall from own height, n (%) | 5 (16) | 15 (47) | 13 (42) |
| Direct blow, n (%) | 16 (50) | 16 (50) | 16 (52) |
| Ankle sprained, n (%) | 6 (19) | 0 (0) | 0 (0) |
| Stumble, n (%) | 4 (13) | 1 (3) | 2 (6) |
Table 2: Mechanism of injury.
| Anatomical Region Affected | CEL-2 | CEL-1 | PLA |
|---|---|---|---|
| (n=32) | (n=32) | (n=31) | |
| Toes, n (%) | 0 (0) | 2 (6) | 3 (10) |
| Anterior leg region, n (%) | 1 (3) | 1 (3) | 1 (3) |
| Anterior region of the ankle, n (%) | 6 (19) | 4 (13) | 1 (3) |
| Calcaneal region, n (%) | 1 (3) | 3 (9) | 0 (0) |
| Dorsal region of the foot, n (%) | 2 (6) | 3 (9) | 6 (19) |
| Plantar region, n (%) | 2 (6) | 0 (0) | 0 (0) |
| Posterior leg region, n (%) | 6 (19) | 5 (16) | 6 (19) |
| Posterior ankle region, n (%) | 2 (6) | 0 (0) | 0 (0) |
| Lateral retromalolar region, n (%) | 11 (34) | 13 (41) | 13 (42) |
| Medial retromalolar region, n (%) | 1 (3) | 1 (3) | 1 (3) |
Table 3: Anatomical region affected.
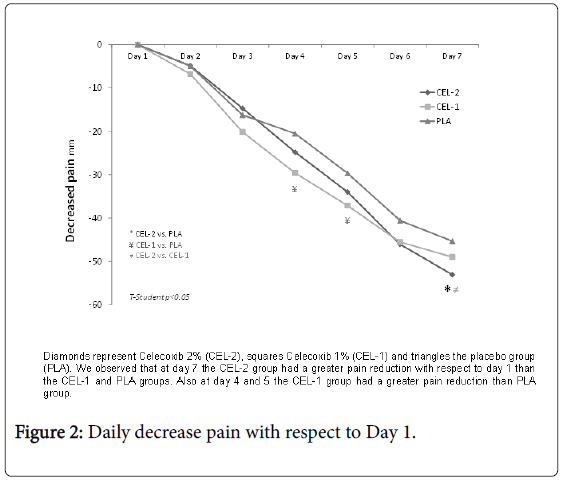
VAS on day 1 and 7 in group CEL-2 were 57.41 ± 10.39 mm and 4.34 ± 7.02 mm, in CEL-1 59.38 ± 9.37 mm and 10.41 ± 12.78 mm, and in PLA 55.61 ± 8.09 mm and 9.32 ± 9.93 mm. In all three groups the pain at the end of the baseline decreased significantly p<0.001, however, only the CEL-2 group showed greater pain decrease compared to CEL-1 and PLA, p<0.05 (Table 4). We also observed that the reduction of pain for day 4 and 5 of the CEL-1 group was greater in comparison to PLA (Figure 2).
| CEL-2 | CEL-1 | PLA | ||||
|---|---|---|---|---|---|---|
| (n=32) | (n=32) | (n=31) | ||||
| Day 0 | Day 7 | Day 0 | Day 7 | Day 0 | Day 7 | |
| VAS, mm | 57.41 (10.39) | 4.34*† (7.02) | 59.38 (9.37) | 10.41* (12.78) | 55.61 (8.09) | 9.32* (9.93) |
| Circumference, cm | 27.75 (7.83) | 27.10* (7.66) | 25.73 (6.9) | 25.38*‡ (6.87) | 24.11 (6.32) | 23.98* (6.34) |
† CEL-2 vs . PLA y CEL-2 vs. CEL-1, T-Student p>0.05
‡ CEL-1 vs. PLA, T-Student p>0.05
Table 4: Visual analogue scale and circumference of the affected region.
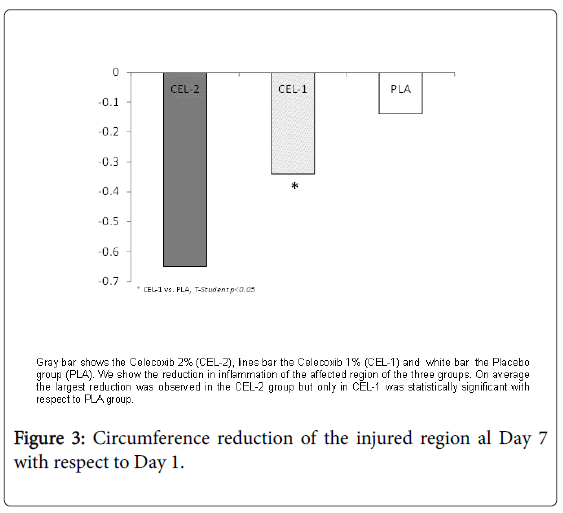
The circumference of the injury on day 1 and day 7 in group CEL-2 were 27.75 ± 7.83 cm and 27.10 ± 7.66 cm, in CEL-1 25.73 ± 6.9 cm and 25.38 ± 6.87 cm, and in PLA 24.11 ± 6.32 cm and 23.98 ± 6.34 cm. Also in these variables the three groups showed significant improvement at the end, p<0.05. The CEL-1 group significantly decreased inflammation more than PLA (Figure 3).
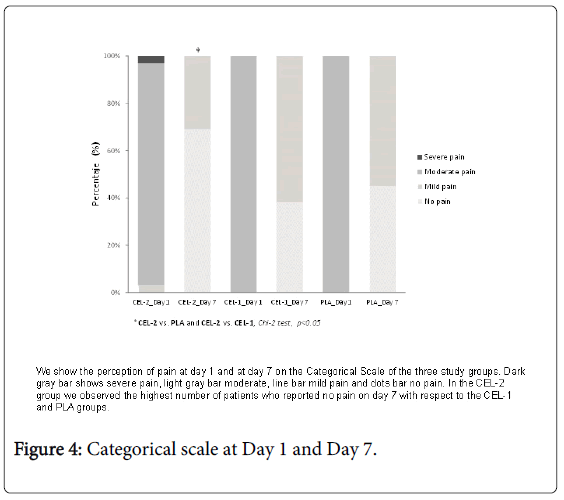
As for the pain reported in the Categorical Scale, at the end of the treatment the three groups reported decreased pain and it was observed that there were significant differences between them. Thus, the largest proportion of patients who described pain in the categorical scale as “no pain” corresponded to the CEL-2 group with 69% (n=22) and the remaining 31% (n=10) as “mild pain”, p<0.05 (Figure 4).
Laboratory tests showed no abnormalities at baseline and at the end of the study; all three groups were within normal ranges and were not modified at day 7. The study products were well tolerated by the patients and only 15 adverse events were reported in 9 patients: the two main ones were cream weast 47% (n=7) and pruritus 40% (n=6), not being different between groups (Table 5).
| Adverse Event | CEL-2 | CEL-1 | PLA |
|---|---|---|---|
| (n=7) | (n=6) | (n=2) | |
| Pruritus / itch | 3 | 3 | 0 |
| Cream waste | 3 | 2 | 2 |
| Erythema | 0 | 1 | 0 |
| Rhinitis | 1 | 0 | 0 |
Number of adverse events Exact Fisher test, p=0.720
Table 5: Adverse events.
Discussion
The results shown in the present study demonstrate how topical administration of Celecoxib cream 2%, TID for 7 days was effective in pain relief in patients with acute soft tissue injury.
At the end of the study, all patients in the three formulations had improvement in pain and inflammation, which was expected by the natural evolution of the injuries [19]. Likewise, the results obtained were similar to those reported in previous studies with oral Celecoxib. For example, in a study with a population similar to ours in which the efficacy of Celecoxib 200 mg BID and NSAIDs was compared, it was shown that Celecoxib significantly reduced pain at day 7 when it was evaluated with a VAS score [20].
In another study with Asian population the analgesic efficacy of Celecoxib 200 mg BID was similar to that of Diclofenac SR 75 mg BID in patients with ankle sprain when VAS scores were purchased at day 4 [21].
In the patients of our study it was also observed as the antiinflammatory effect of Celecoxib topical was superior to placebo. Although no clinical trials have been published, this effect was previously demonstrated experimentally when arachidonic acid was applied to the ear of mice in which edema decreased after the topical application of Celecoxib with microemulsions [22].
Regarding safety we can compare the results with those presented in other studies in which it was observed that topical and placebo NSAIDs were well tolerated and there were no statistically significant differences between them [7,23]. Even though we did not determine the concentrations reached in plasma, indirectly we can assume that these were minimal since no systemic adverse effects related to Celecoxib were reported.
With the information presented, we can conclude that the analgesic and anti-inflammatory properties of the selective COX-2 inhibitors make them an ideal pharmacological group for the management of acute musculoskeletal injuries [24,25]. In addition to the above, evidence that topical formulations may reach higher concentrations in inflamed tissues than oral ones [10], Celecoxib 2% cream may be a good alternative for pain management in patients with acute soft tissue injury.
Since there are no publications of clinical studies of topical COX-2 inhibitor in the management of pain in patients with soft tissue injuries, this may be a baseline study for future comparisons against oral formulations or against other NSAIDs.
Funding
This study obtained grants from the 2012 Stimulus to Innovation Program of the National Council of Science and Technology (CONACyT) of Mexico with number 184858.
Statement of financial disclosure and conflict of interest
The content of this report is solely the responsibility of the authors and does not represent the official view of Productos MAVER.
Productos MAVER has given unrestricted financial support to initiate and perform this study. Furthermore they offered the celecoxib and placebo cream aimed at the intervention. We fulfilled this study without any influence or interference of the sponsor.
Grants
Sponsored by Productos MAVER S.A. de CV.
Grants from the 2012 Stimulus to Innovation Program of the National Council of Science and Technology (CONACyT) of Mexico with number 184858.
References
- Predel HG, Hamelsky S, Gold M, Giannetti B (2012) Efficacy and safety of diclofenac diethylamine 2.32% gel in acute ankle sprain. Med Sci Sports Exerc 44: 1629-36.
- Smith A, Sloan J, Wass A, Draycott S (2009) Soft tissue injury commissioned series: 6 Lower leg, ankle and foot. Emerg Med J 26: 193-200.
- Sexton J (2002) Managing soft tissue injuries. Emergency Nurse 10: 11-16.
- Chen MR, Dragoo JL (2013) The effect of nonsteroidal anti-inflammatory drugs on tissue healing. Knee Surg Sports Traumatol Arthrosc 21: 540-549.
- Buvanendran A (2006) COX-2 inhibitors in sports medicine: utility and controversy Nonsteroidal Br J Sports Med 40: 895-96.
- Lionberger DR, Joussellin E, Lanzarotti A, Yanchick J, Magelli M (2011) Diclofenac epolamine topical patch relieves pain associated with ankle sprain. J Pain Res 4: 47-53.
- Li C, Frangione V, Rovati S, Zheng Q (2013) Diclofenac epolamine medicated plaster in the treatment of minor soft tissue injuries: a multicenter randomized controlled trial. Curr Med Res Opin 29: 1137-1146.
- Predel HG, Giannetti B, Seigfried B, Novellini R, Menke G (2013) A randomized, double-blind, placebo-controlled multicentre study to evaluate the efficacy and safety of diclofenac 4% spray gel in the treatment of acute uncomplicated ankle sprain. J Int Med Res 41: 1187-1202.
- Vinciguerra G, Belcaro G, Cesarone MR, Errichi BM, Di Renzo A, et al. (2008) Management of uncomplicated ankle sprains with topical or oral ketoprofen treatment. A registry study. Minerva Cardioangiol 56: 47-53.
- Kai S, Kondo E, Kawaguchi Y, Kitamura N, Yasuda K (2013) Flurbiprofen concentration in soft tissues is higher after topical application than after oral administration. Br J Clin Pharmacol 75: 799-804.
- van den Bekerom MP, Sjer A, Somford MP, Bulstra GH, Struijs PA, et al. (2015) Non-steroidal anti-inflammatory drugs (NSAIDs) for treating acute ankle sprains in adults: benefits outweigh adverse events. Knee Surg Sports Traumatol Arthrosc 23: 2390-2399.
- Carter D, Amblum-Almer J (2015) Analgesia for people with acute ankle sprain. Emerg Nurse 23: 24-31.
- Buvanendran A (2015) Nonsteroidal Anti-inflammatory Drugs: Deer TR. Treatment of Chronic Pain by Medical Approaches: the AMERICAN ACADEMY of PAIN MEDICINE Textbook on Patient Management. Springer, New York 33-42.
- Jones P, Lamdin R (2010) Oral cyclo-oxygenase 2 inhibitors versus other oral analgesics for acute soft tissue injury: systematic review and meta-analysis. Clin Drug Investig 30: 419-437.
- Ekman EF, Fiechtner JJ, Levy S, Fort JG (2002) Efficacy of celecoxib versus ibuprofen in the treatment of acute pain: a multicenter, double-blind, randomized controlled trial in acute ankle sprain. Am J Orthop 31: 445-451.
- Petrella R, Ekman EF, Schuller R, Fort JG (2004) Efficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clin J Sport Med 14: 225-231.
- Baboota S, Shakeel F, Ahuja A, Ali J, Shafiq S (2007) Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm 57: 315-332.
- Jeyaseelan L (1989) Methods of determining sample sizes in clinical trials. Indian Pediatrics 26: 115-121.
- Fong DT, Chan YY, Mok KM, Yung PSh, Chan KM (2009) Understanding acute ankle ligamentous sprain injury in sports. Sports Med Arthrosc Rehabil Ther Technol 30: 14.
- Cardenas-Estrada E, Oliveira LG, Abad HL, Elayan F, Khalifa N, et al. (2009) Efficacy and safety of celecoxib in the treatment of acute pain due to ankle sprain in a Latin American and Middle Eastern population. J Int Med Res 37: 1937-1951.
- Nadarajah A, Abrahan L, Lau FL, Hwang LJ, Fakir-Bolte C (2006) Efficacy and tolerability of celecoxib compared with diclofenac slow release in the treatment of acute ankle sprain in an Asian population. Singapore Med J 47: 534-542.
- Subramanian N, Ghosal SK, Moulik SP (2005) Enhanced in vitro percutaneous absorption and in vivo anti-inflammatory effect of a selective cyclooxygenase inhibitor using microemulsion. Drug Dev Ind Pharm 31: 405-416.
- Derry S, Wiffen P, Moore A (2016) Topical Nonsteroidal Anti-inflammatory Drugs for Acute Musculoskeletal Pain. JAMA 315: 813-814.
- Warden SJ (2005) Cyclo-oxygenase-2 inhibitors: beneficial or detrimental for athletes with acute musculoskeletal injuries? Sports Med 35: 271-283.
- Polzer H, Kanz KG, Prall WC, Haasters F, Ockert B, et al. (2012) Diagnosis and treatment of acute ankle injuries: development of an evidence-based algorithm. Orthop Rev (Pavia) 24: e5.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 7672
- [From(publication date):
March-2017 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 6499
- PDF downloads : 1173