Research Article Open Access
Cdse/Zns Capped Thiolate for Application in Glucose Sensing
Samsulida Abd. Rahman1,2*, Nurhayati Ariffin1, Nor Azah Yusof2,3, Jaafar Abdullah2,3, Zuhana Ahmad Zubir4 and Nik Mohd Azmi Nik Abd Aziz41Industrial Biotechnology Research Centre (IBRC), SIRIM Berhad, No. 1, Persiaran Dato’ Menteri, Section 2, P.O. Box 7035, 40700 Shah Alam, Selangor, Malaysia
2Advanced Materials and Nanotechnology Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
3Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM, Serdang, Selangor, Malaysia
4Advance Material Research Centre (AMREC), SIRIM Berhad, Lot 34, Jalan Hi-Tech 2/3, Kulim Hi-Tech Park, 09000 Kulim, Kedah Darul Aman, Malaysia
- *Corresponding Author:
- Samsulida Abd. Rahman
Industrial Biotechnology Research Centre (IBRC)
SIRIM Berhad, No. 1, Persiaran Dato’ Menteri, Section 2
P.O. Box 7035, 40700 Shah Alam, Selangor, Malaysia
Tel: +60-355446963
E-mail: sulida@sirim.my
Received date: December 19, 2016; Accepted date: January 27, 2017; Published date: February 07, 2016
Citation: Rahman SA, Ariffin N, Yusof NA, Abdullah J, Zubir ZA, et al. (2017) Cdse/Zns Capped Thiolate for Application in Glucose Sensing. Biosens J 6:143. doi:10.4172/2090-4967.1000143
Copyright: © 2017 Rahman SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
A semiconducting water soluble core-shell quantum dot (QD) capped with thiolated ligand is used in this study for application in glucose sensing. These QDs were prepared in house based on hot injection technique. The ZnS shell at the outer surface of CdSe core QDs is made via specific process namely, SILAR (successive ionic layer adsorption and reaction). The distribution, morphology and optical characteristics of prepared core shell QDs have been assessed by transmission electron microscopy (TEM) and spectrofluorescence, respectively. The results show that the mean particle size of prepared QDs is in the range of 10-12 nm and the optimum emission condition was displayed at 620nm. The prepared CdSe/ZnS core shell QDs were modified by utilizing six organic ligands L-cysteine, L-histidine, thio-glycolic acid (TGA), mercapto-propionic acid (MPA), mercapto-succinic acid (MSA) and mercapto-undecanoic acid (MUA) at room temperature through a ligand-exchange procedure. This ligand exchange process was chosen in order to produce a very dense water solubilizing agent the QDs surrounding surface. The result shows that CdSe/ZnS capped with thioglycolic acid (CdSe/ZnS-TGA) exhibit the strongest fluorescence emission; therefore it was used in advanced sensing application for the detection of glucose. The highly active CdSe/ZnS-TGA was then interacted with Glucose Oxidase enzyme (GOx) and horseradish peroxidase enzyme (HRP). In this study, determination of glucose level is depending on the QDs fluorescence intensity quenching effect, which is correlated to reaction of the conjugated enzyme-QDs. In the presence of 0.1 mM glucose, fluorescence intensity of the bioconjugate QDs was quenched about 12, 000 a.u. This bioconjugated GOX/HRP/QDs-capped TGA was further analyzed with known concentrations of glucose. Quenched fluorescence intensity was proportionate with glucose concentration. The resultant GOX/HRP/QDs-capped TGA system can be a suitable platform for glucose determination in real samples.
Keywords
Semiconducting; Water-soluble; Core shell quantum dots; Modification; QDs-capped TGA; Conjugated
Introduction
Nanomaterials have special characteristics as reported in many article previously. Substantial surface area, superior reaction surface activity and higher catalytic efficiency [1] are some of their special features. Due to this reason, nanomaterials were selected as prospective transducers in enzyme based bioconjugated sensor. Substantial surface area of nanomaterials allows the absorption of enzyme happened quickly and efficiently. Besides, it also can minimizes the enzyme aggregation and protein unfolding, thus resulting in more stable nanomaterials-enzyme systems [2,3].
There are several nanomaterials that previously reported in biosensor application such as gold nanomaterials [4], carbon nanotubes [5], magnetic nanoparticles [6], titania nanoparticles [7], silica nanoparticles [8] and quantum dots (QDs) [9-14]. Among all of these nanomaterials, QDs are more favorable since they exhibit broad excitation and narrow emission wavelength. Besides, the QDs emission wavelength can be tunable. They also contain other special features such as extremely luminescent, photoresistant and potential utilization in biosensor. This is because their high ratio of surface-to-volume, higher catalytic efficiency and bigger reaction activity surface [1].
Water soluble QDs have fascinated an increasing passion in numerous research areas like biosensor and bioimaging because QDs have shown good compatibility characteristics to the physiological medium. Additionally, the development of biosensor utilizing bioconjugated system of QDs-enzyme serves for dual purpose; one is the immobilization of enzyme and second is application in biosensing based on changes in fluorescence intensity [15]. Fluorescence emission wavelength of the QDs is tunable via adjusting QDs size and customizing the nature of capping molecules, which are liable for surface charges modification for bimolecular coupling [16].
Many papers has been reported on the usage of QDs in diagnostic and sensoring system for healthcare monitoring such as labelling of cancer cell [17], cell imaging [18], drug delivery and virus detection [19]. Apart from that, QDs also are most familiar in the detection of glucose level. CdSe/ZnS is one of the types of core shell QDs that being prepared for biosensing applications [20-22]. It reported previously that CdSe/ZnS core shell QDs are extremely high fluorescent intensity in the visible spectrum and in addition, this ZnS outer layer increased their chemical and photostability.
Normally, CdSe/ZnS core-shell QDs has been prepared using high temperature in organic solvent and stabilized in hydrophobic solvents like trioctyl phosphine oxide (TOPO) and oleic acid. Since, this preparation method resulted in QDs soluble in solvents, several method have been studied to substitute TOPO and/or oleic acid with other organic ligands that soluble in water. However, the exchange of TOPO and/or oleic acid with other organic ligands generates several problems such as lessen quantum yield [23-26]. Gill et al. have been reported previously that the quantum yield of their synthesized QDs has been decreased by 50% as CdSe/ZnS QDs were modified with MPA [24]. The same situation has been reported by Stsiapura et al. (2006). In their studies, the quantum yield of their synthesized QDs has been depreciated two times when mixtures of MAA/MSA were used as ligand exchanger [25]. In addition, they also exhibit less colloidal stability where the QDs have low tendency to reside individual dispersing and more likely to form aggregates.
In this research, CdSe/ZnS core-shell QDs has been prepared using high temperature in organic solvent and stabilized with hydrophobic capping solvents such as TOPO and oleic acid [26]. Because of these QDs are not soluble in water, so ligand exchange methods have been utilized to modify the QDs surfaces for biosensing applications. The heterobifunctional ligands such as mercaptocarbonic acids and thiolated ligands will replace TOPO/oleic acid, where the mercapto or thiolated ends will be binds on the surface of the QDs while the carboxyl moiety offers water solubility [19,27]. Generally, thick shell restricts the usefulness of the core-shell QDs in biosensing application. This is due to the shell that limits the electron transfer to reach the core QDs. In this case, mercaptocarbonic or thiolated ligands will be used in order to prevent the limitation of electron transfer thus will prepare a best water solubilizing shell around the CdSe/ZnS QDs. So, these hydrophilic QDs are highly suitable for biosensing area.
As resulted in our previously studied [26], the core-shell CdSe/ZnS capped TGA, MPA and MSA displayed no signs of aggregation and these QDs are fully soluble in PBS buffer pH 7, thereby suggesting that high stability of the aqueous dispersions. Among of these three ligands, CdSe/ZnS-capped TGA shown highest fluorescent intensity response compared to other ligands. This results happen because of the ligand size where the smaller size of ligand will increased the aggregation level (TGA<MPA<MSA) because of the reducing of steric repulsion, thus mean intensity will be reduced. This rule is also agreed by Amit et al., [15]. The TGA ligand was smallest against MPA and MSA resulting in highest fluorescence intensity. The fluorescence intensity of prepared CdSe/ZnS core-shell QDs remains stable after being modified with TGA.
Due to this successful result, hereby we are reporting the use of CdSe/ZnS-capped TGA QDs in sensing application for glucose detection. We aim to prepare conjugated enzyme-QDs for glucose detection.
Results
Characterization of CdSe/ZnS core-shell QDs
Characterization of CdSe/ZnS QDs without and with capping ligand, TGA were shown and discussed thoroughly in our previous reported paper [26]. In short, the photoluminescent (PL) peak for CdSe core was occurred at 532.5 nm and full width at half maximum (FWHM) was 28.5 nm whereby, it indicated that good formation of monodisperse nanomaterials (results not shown). The PL peak of CdSe/ ZnS core shell was obtained at 572.5 nm The PL intensity was increased due to the encapsulation of ZnS shell onto the CdSe core (results not shown). The wavelength moved about 40 nm towards right between core and core shell. The CdSe and CdSe/ZnS have uniform sizes about 3 - 3.2 nm and 10 - 12 nm, respectively and it shows good colloidal nanoparticles. The distribution of QDs size was uniform, reasonable and monodisperse.
Afterwards, the prepared CdSe/ZnS core-shell QDs was capped via several ligands in order to make the QDs is water soluble. Since, CdSe/ ZnS-capped TGA exhibit highest fluorescence emission among the other ligands, this CdSe/ZnS-capping TGA will be used in this glucose detection studies.
Basic principle of glucose detection using CdSe/ZnS capped TGA
Basic principle for CdSe/ZnS-capped TGA nanomaterials for glucose detection can be viewed in Figure 1. The determination of glucose in this research is depending on the enzymatic reaction of glucose and the effect of presence of H2O2 on intensity of QDs fluorescence. In the presence of glucose, GOx have been catalyzed glucose to gluconic acid in the oxidation process via using the oxygen molecule as an electron acceptor and H2O2 will be produce simultaneously. The exchange of the electron happened at the outer surface of the core-shell QDs whereby H2O2 is reducing to oxygen and H2O is trap in electron holes at the surface of the QDs. These were resulted in non-fluorescent QDs anion and cause a reduction in fluorescence intensity. The higher glucose concentrations used, more H2O2 will be produced and resulted in big quenching effect.
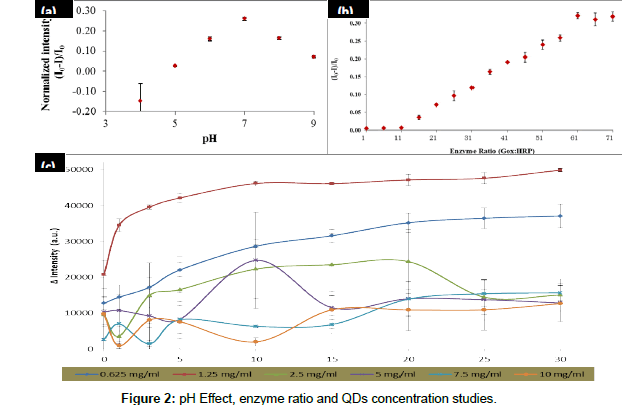
The parameter optimizations that have been studied were pH condition, enzyme ratio and QDs concentration. These parameter optimizations are important on the glucose biosensing and these optimizations were analyzed by using a mixture of GOx/HRP and TGAQDs as displayed in Figure 2. Firstly, the optimization of pH buffer on the fluorescence intensity quenching of the CdSe/ZnS-capped TGA was examined. Figure 2 shows that the quenching effect of the CdSe/ ZnScapped TGA was increased with the increasing of pH buffer value until it reached the optimum condition at pH 7. This is because glucose being in cyclic hemiacetal form at pH 7 where the β-D-glucopyranose and the α-D-glucopyranose is about 63.6% and 36.4%, respectively. The specific binding occurred between GOx and β-D-glucopyranose. However, this binding was not act on α-D-glucopyranose. The equilibrium state between the α-β anomers were pushed towards the β-side since it consumed in the reaction. Due to this equilibrium resulted in the ability of the glucose oxidase to oxidize all of the glucose in solution. The intensity was dropped after pH 7 because the glucose oxidase will slowly denature in base condition.
From the result shown in Figure 2, we can see that highest intensity was determined by using 1.25 mg/ml of QDs. However, the intensity of the QDs was decreased when highest QDs concentration was introduced into the reaction. This is due to the toxicity of the CdSe/ZnS at higher concentration resulted in the enzyme denaturation thus effect the decreasing of intensity of the QDs. Therefore, QDs concentration of 1.25 mg/ml will be used for next studies.
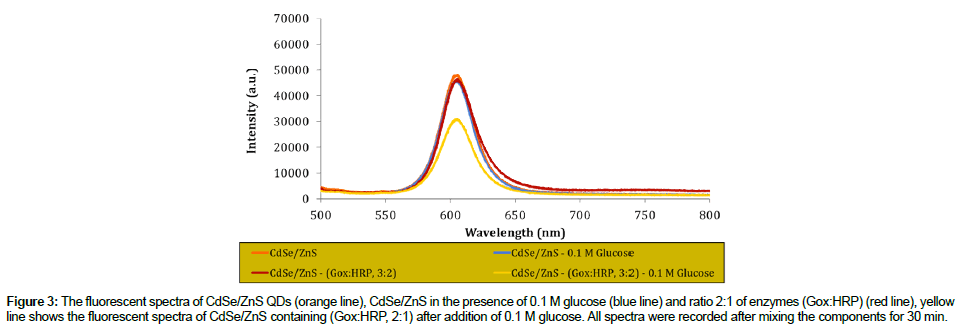
The reaction mechanism for glucose detection that depend on the effect of the CdSe/ZnS fluorescence intensity quenching has successfully proved and the result as shown in Figure 3. From this figure, it shows that the intensity of prepared QDs was maintained in the absence of GOx/HRP enzymes and glucose. However, in the presence of GOx/ HRP and glucose, the reaction occurred, resulted in the quenched of the QDs intensity.
Figure 3: The fluorescent spectra of CdSe/ZnS QDs (orange line), CdSe/ZnS in the presence of 0.1 M glucose (blue line) and ratio 2:1 of enzymes (Gox:HRP) (red line), yellow line shows the fluorescent spectra of CdSe/ZnS containing (Gox:HRP, 2:1) after addition of 0.1 M glucose. All spectra were recorded after mixing the components for 30 min.
The calibrations were performed for a several time and assay were done trice for each time assay as shown in Figure 4. Furthermore, the inset of Figure 4 showed that there was a good linearity between quenched intensity of the CdSe/ZnS and glucose level are ranging from 0 to 10 mM. The corresponding regression coefficient was about 0.998. The limit of detection (LOD) was obtained at 0.045. The LOD was calculated through 3σ/s; with the s is the slope of calibration graph while the σ is the standard deviation of corrected blank from the signals of fluorescent of the CdSe/ZnS-capped TGA QDs.
Materials and Methods
Preparation of core, shell, core-shell and surface modification of core-shell QDs has been discussed previously in our proceeding journal, Samsulida Abd. Rahman et al. [26]. So, it will not be discussed further in this paper.
Materials and Apparatus
CdSe/ZnS-capped TGA core-shell QDs was made in house. N-ethyl-3- (3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxy sulfosuccinimide (sulfo-NHS), glucose oxidase (GOx) and horseradish peroxidase (HRP) were purchased from Sigma, respectively. Solution of PBS buffer, pH 7 has been prepared in house by adding disodium hydrogen diphosphate (NaH2PO4) and sodium dihydrogen phosphate (NaH2PO4) until reach pH 7. Both Na2HPO4 and NaH2PO4 were purchased from Scharlau. All the starting materials for enzyme conjugation and glucose detection were directly used without any further purification.
The experiment was performed from wavelength 300-800 nm with absorbance less than 0.1 at wavelength 480 nm. The assay solution for fluorescence measurements was place into a cuvette. The spectrums of emission for the entire sample were determined by utilizing Novacure spectrophotometer, EFOS and mercury lamp was act as their source of light. Excitation wavelength for the entire assay was specific at 375 nm.
Study of conjugation of CdSe/ZnS QDs with glucose oxidase enzyme in aqueous system
Add 2.3 μl of EDC and 1 mg of NHS into the beaker that contain of 5 ml of CdSe/Zn-TGA. Mix the solutions for 30 min and simultaneously add 25 μl (GOx) and 10 μl (HRP), respectively. Continue stirring for another 2 hours before centrifugation process at 12000 rpm for 10 min. Re-dissolved the solution with PBS pH 7 and stored at 4 °C for the next usage. All the characterization of the conjugated CdSe/ZnS-QDs with glucose oxidase enzyme will be determined using spectrofluorescence technique. Characterization of the enzyme conjugation and interaction will also be evaluated by mixing different ratio of QDs-enzyme (1/1, 1/2, 1/3, 1/4) in the assay system. For analytical performance of the bioconjugation QDs-enzyme system, the stability, interference study, repeatibility and reproducibility of the system will be evaluated.
Conclusions
In summary, we have demonstrated a CdSe/ZnS capped TGA water soluble core-shell QDs for determination of glucose. The spectrofluorescence signals of the prepared CdSe/ZnS-capped TGA QDs were quenched by glucose successively. The parameter optimization of the reaction optimum conditions includes effect of pH, enzyme ratio and concentrations of QDs have been discussed. An extremely good linearity for glucose determination in range between 0 - 10 mM and obtained the LOD at 0.045 mM. The fluorescence nanosensor for glucose detection with sensitive, selective and convenient assay procedure has been established and this procedure will be analysing using real samples.
References
- Narayanan SS, Sarkar R, Pal SK (2007) Structural and functional characterization of enzyme-quantum dot conjugates: covalent attachment of CdS nanocrystal to r-chymotrypsin. J Phys Chem 111: 11539-11543.
- Gole A, Dash C, Ramakrishnan V, Sainkar S.R, Mandale A.B et al. (2001) Pepsin-gold colloid conjugates: preparation, characterization, and enzymatic activit. Langmuir17: 1674-1679.
- Ohara TJ, Rajagopalan R, Heller A (1994) “Wired” enzyme electrodes for amperometric determination of glucose or lactate in the presence of interfering substances. Anal. Chem 66: 2451-2457.
- Yu A, Liang Z, Cho J, Caruso F (2003) Nanostructured electrochemical sensor based on dense gold nanoparticle films. Nano Lett 3: 1203-1207.
- Xianbo QL, Li LJ, Yao X, Li J (2007) Direct electrochemistry of glucose oxidase and electrochemical biosensing of glucose on quantum dots/carbon nanotubes electrodes. Biosens. Bioelectron 22: 3203-3209.
- Huang SH, Liao MH, Chen DH (2003) Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnol. Prog 19:1095-1100
- Zhang Y, He P, Hu N (2004) Horseradish peroxidase immobilized in TiO2nanoparticle films on pyrolytic graphite electrodes: direct electrochemistry and bioelectrocatalysis. Electrochim. Acta 49: 1981-1988.
- Hilliard LR, Zhao X, Tan W (2002) Immobilization of oligonucleotides onto silica nanoparticles for DNA hybridization studies, Anal. Chim. Acta 470: 51-56.
- Cao L, Ye J, Tong L, Tang B (2008) A new route to the considerable enhancement of glucose oxidase (GOx) activity: the simple assembly of a complex from CdTe quantum dots and GOx, and its glucose sensing. Chem. Eur. J 14: 9633-9640.
- Hua M, Tiana J, Lua HT, Weng LX, Wang LH (2010) H2O2-sensitive quantum dots for the label-free detection of glucose. Talanta 82: 997-1002.
- Jaricot SC, Darbandi M, Kucur E, Nann T (2008) Silica coated quantum dots: a new tool for electrochemical and optical glucose detection. Microchim. Acta 160: 375-383.
- Huang CP, Liu SW, Chen TM, Li YK (2008) A new approach for quantitative determination of glucose by using CdSe/ZnS quantum dots. Sens. Actuators B 130: 338-342.
- Wu W, Zhou T, Shen J, Zhou S (2009) Optical detection of glucose by CdS quantum dots immobilized in smart microgels. Chem. Commun 4390-4392.
- Wu P, He Y, Wang HF, Yan XP (2010) Conjugation of glucose oxidase onto Mn doped ZnS quantum dots for phosphorescent sensing of glucose in biological fluids. Anal. Chem 82: 1427–1433.
- Saran AD, Sadawana MM, Srivastava R, Bellare JR (2011) An optimized quantum dot ligand system for biosensing applications: Evaluation as a glucose biosensor. Colloids and Surfaces A: Physicochem. Eng. Aspects 384: 393-400.
- Huang CP, Li YK, Chen TM (2007) An investigation of urea detection by using CdSe/ZnS quantum dots. Biosens. and Bioelectrons 22: 1835-1838.
- Xie RG, Kolb U, Li JX, Basche T, Mews A (2005) Synthesis and characterization of highly luminescent CdSe-Core CdS/Zn and CdS/ZnS multishell nanocrystals. J. Am. Chem. Soc 127: 7480-7488.
- Willard DM, Carillo LL, Jung J, Van Orden A (2001) CdSe-ZnS quantum dots as resonance energy transfer donors in a model protein binding assay. Nano Lett 1: 469-474.
- Chan WCW, Maxwell DJ, Gao XH, Bailey RE, Han MY, et al. (2002) Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol 13: 40-46.
- Sapsford KE, Pons T, Medintz IL, Mattoussi H (2006) Biosensing with Luminescent Semiconductor Quantum Dots. Sensors 6: 925-953.
- Hines MA, Guyot-Sionnest P (1996) Synthesis and characterization of strongly luminescing ZnS-Capped CdSe nanocrystals. J. Phys. Chem 100: 468-471.
- Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, et al. (1997) (CdSe)ZnS Core-Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B 101: 9463-9475.
- Kloepfer JA, Bradforth SE, Nadeau J.L (2005) Photo-physical Properties of Biologically Compatible CdSe Quantum Dot Structures. J. Phys. Chem. B109: 9996-10003.
- Gill R, Willner I, Shweky I, Banin U (2005) Fluorescence Resonance Energy Transfer in CdSe/ZnS-DNA Conjugates: Probing hybridization and DNA Cleavage. J. Phys. Chem. B 109: 23715-23719.
- Stsiapura V, Sukhanova A, Baranov A, Artemyev M, Kulakovich O, et al. (2006) DNA-Assisted Formation of Quasi-Nanowires from Fluorescent CdSe/ZnS Nanocrystals. Nanotechnology 17: 581-587.
- Samsulida AR, Nurhayati A, Nor AY, Jaafar A, Zuhana AZ, et al. (2014) Synthesis and Surface Modification of Biocompatible Water Soluble Core-Shell Quantum Dots Adv. Mat. Research 879: 184-190.
- Chan WCW, Nie SM (1998) Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science281: 2016-2018.
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 3034
- [From(publication date):
June-2017 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 2164
- PDF downloads : 870