Research Article Open Access
CD4+T Cells Expansion in P. berghei (NK-65) Infected and Immunized BALB/C Mice
Vineet Kumar1, Aruna Rakha2, Ruchika Saroa1 and Upma Bagai1*1Parasitology Laboratory, Department of Zoology, Panjab University, Chandigarh160014, India
2Department of Translational and Regenerative Medicine, PGIMER, Chandigarh 160012, India
- *Corresponding Author:
- Bagai U
Associate Professor
Department of Zoology
Panjab University
Chandigarh-160014, India
Tel: 919876669632
E-mail: upmabagai@yahoo.co.in
Received Date: March 30, 2015; Accepted Date: May 21, 2015; Published Date: May 25, 2015
Citation: Kumar V, Rakha A, Saroa R, Bagai U (2015) CD4+T Cells Expansion in P. berghei (NK-65) Infected and Immunized BALB/C Mice. J Clin Exp Pathol 5:229. doi:10.4172/2161-0681.1000229
Copyright: ©2015, Vineet Kumar et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
The immune system of malaria infected host undergoes both activation and suppression during different phases of parasite’s life cycle. In Plasmodium chabaudi chabaudi (AS), T helper cells of host have been reported to be necessary for inducing a protective immune response against the blood stages of parasite [1]. T cells provide protection to infection by cytokine-mediated mechanisms or through production of antibodies. Experiments with B cell deficient mice have demonstrated that B cells and antibodies are essential for complete clearance of parasites from blood of the host [2]. Depending upon the cytokines secreted by the Helper T cells, they are classified into two subpopulations: Th1 and Th2 cells. Th1 cells primarily release IFN-γ, IL-2, whereas, Th2 cells secrete IL-4, IL-5, IL-6, IL-10 and IL-13.
Keywords
Plasmodium berghei; T cell response; Humoral response; Cytokines
Introduction
The immune system of malaria infected host undergoes both activation and suppression during different phases of parasite’s life cycle. In Plasmodium chabaudi chabaudi (AS), T helper cells of host have been reported to be necessary for inducing a protective immune response against the blood stages of parasite [1]. T cells provide protection to infection by cytokine-mediated mechanisms or through production of antibodies. Experiments with B cell deficient mice have demonstrated that B cells and antibodies are essential for complete clearance of parasites from blood of the host [2]. Depending upon the cytokines secreted by the Helper T cells, they are classified into two subpopulations: Th1 and Th2 cells. Th1 cells primarily release IFN-γ, IL-2, whereas, Th2 cells secrete IL-4, IL-5, IL-6, IL-10 and IL-13.
CD4+ T cells play an important role in conferring protective immunity towards the liver stages of the malaria parasite [3]. CD4+ T cells stimulate B cells to induce significant level of antimalarial antibody response and also help in the induction of CD8+ T-cell responses which help in arresting the growth of liver-stage parasites in the host. It has also been reported that protective immunity can be transferred to naive hosts by transfer of immune CD4+ T cells [4].
CD4+ CD25+ FoxP3+ regulatory T cells (Tregs) are also involved in the mechanism of immune regulation against Plasmodium infection. This subset of T cells play critical role in maintaining immune homeostasis and controlling excessive immune responses [5]. Natural Treg cells during primary exposure to malaria enhanced Th1 memory responses and increased disease severity during re-infection. It also resulted in improved control of parasite burden by the host [6].
CD8+ T cells have been reported to be essential for protective immunity against liver-stage malaria [7]. People living in a malaria-endemic area have been reported to have CD8+ T-cell clones that proliferate and produce IFN-γ in response to blood-stage malarial antigens in an HLA-restricted manner [8]. IFN-γ produced by the T cells as well as NK cells plays a critical role in regulating the protective immune response against blood stage malaria infection [9]. Splenomegaly is common symptom of malaria in both humans and murine models. The spleen removes parasitized red cells from the circulation along with providing a strong hematopoietic response during acute infection.
Plasmodium berghei is a lethal rodent malaria parasite recognized as a valuable model due to its homology with P. falciparum. Morphology, physiology, life cycle and genome content of P. berghei shows high similarity with the human malaria parasite. Therefore, the present study has been undertaken to investigate the alterations in various splenic T cells population (CD3+, CD4+, CD8+ and CD4+Treg cells) and to differentiate between the Th1/Th2 immune responses induced in mice immunized with total parasite antigens (TPA) as compared to lethal P. berghei infected animals
Materials and Methods
Experimental design
Four groups of mice are used. Group–I: Normal BALB/c mice-15; Group-II and III: 1 × 106 P. berghei infected BALB/c mice/ Placebo control BALB/c mice-35; Group-IV: Immunized BALB/c mice-35. Total animals equals to 85.
| Groups | Name of groups | Animals used |
|---|---|---|
| GP-I | Normal BALB/c mice | 15 |
| GP-II/GP-III | 1×106 P. berghei infected BALB/c mice/ Placebo control BALB/c mice | 35 |
| GP-IV | Immunized BALB/c mice | 35 |
| Total animals | 85 |
Table 1: Experimental design.
Animals and parasite infection
Inbred BALB/c mice of either sex (5to 6weeks old), weighing 20-25 g procured from IMTECH (Institute of Microbial Technology), Chandigarh, have been used as experimental model. Animals were given pellet feed and water. Guidelines approved by the Institutional Animal Ethics Committee (45/99/CPCSEA) of the Panjab University, Chandigarh have been followed for care and handling of animals. P. berghei (NK-65) was maintained in BALB/c mice by intra-peritoneal (i.p) inoculation of 1 × 106 infected erythrocytes from parasitized to normal mice. Giemsa- stained thin blood smears were studied to check course of parasitemia [10].
Isolation of cell free parasite
Cell free parasites were obtained from infected blood after saponin lysis and repeated washing in phosphate buffered saline, PBS, pH 7.2 [potassium dihydrogen orthophosphate, 0.043% (w/v)] to remove red cell membranes [11]. Saponin lysis yielded grey colour pellet of cell-free parasite. It was suspended in 0.25 M sucrose followed by homogenization to obtain Total parasite homogenate (TPH). Total parasite antigen (TPA) was obtained by centrifugation of TPH at 800 g for 15 min in cold centrifuge [12]. It was suspended in 1 ml of phosphate buffered saline and sonicated for 30 s on ice. Concentration of protein in TPA (770.9 ± 27.6 g/ml) was measured using BSA (bovine serum albumin) as standard [13].
Immunization and challenge of mice
On D0, mice of GP-IV were injected sub-cutaneously (s.c.) with 100 µg of TPA emulsified in Freund’s complete adjuvant (FCA). Two booster doses were administered with similar amount of protein in Freund’s incomplete adjuvant (FIA) on D14 and D28.
Placebo control mice (GP-III) were administered PBS (0.01M, pH 7.2) along with Freund’s adjuvant (FCA/FIA) on similar days [14]. On D35, mice of GP-III and GP-IV groups were challenged with 1 × 106 P. berghei infected erythrocytes. The post challenge course of parasitemia was studied by Giemsa stained blood smears.
Immunophenotyping of spleen Mononuclear cells (MNCs)
BALB/c mice were sacrificed on D1, D3, D5, D7 and D9 in infected control group and on D0, D1, D3, D6, D9 and D20 in immunized group. Spleen was dissected out after anaesthetising mice in a tissue culture dish and teased it into a single cell suspension by passing through plunger. Mononuclear cells (MNCs) obtained after repeated washing with FACS buffer were counted by Neubauer’s haemocytometer [15].
Anti-mouse CD3 (FITC conjugated, clone 17A2 mAb, 0.5 mg/ml), CD4 (PE conjugated, clone H129.19 mAb, 0.2 mg/ml), CD8 (APC conjugated, clone 53-6.7 mAb, 0.2 mg/ml) were used for immunophenotyping. These antibodies were purchased from BD BIOSCIENCES, SINGAPORE. Samples were analyzed on FACS CALIBAR (B.D) after collecting 10,000 events in CSIF at PGIMER, Chandigarh. The results were analyzed using BD FACS DIVA software [16].
Regulatory T cell analysis
Mononuclear cells were extracted from spleen of control and mice immunized with total parasite antigens were processed for T-regulatory cell analysis. Antibodies viz; anti mouse CD4+ (FITC conjugated, 0.5 mg/ml), CD25+ (APC conjugated, 0.2 mg/ml), FoxP3+ (PE conjugated, 0.2 mg/ml) were purchased from BD BIOSCIENCES, SINGAPORE. Samples were analyzed using BD FACS DIVA software on flow cytometer (FACS CALIBAR BD) after collecting 10,000 events [17].
Indirect fluorescent antibody (IFA) test
Level of anti-malarial antibody was tested in sera of immunized mice by IFA. Serum samples were diluted by two fold serial dilutions using PBS (0.15 m, pH 7.2). Slides were counter stained in 0.5% w/v Evan’s blue in PBS, pH 7.2 to remove any non-specific fluorescence. The highest serum dilution that gave detectable fluorescence (LEICA DMLS, GERMANY) was termed as end point [18].
Quantification of Th1 immune response
For Th1 immune response, level of IgG2a was determined by ELISA. Goat anti-mouse IgG2a isotype specific HRP conjugated secondary antibody was added to the plate.
IL-2 and IFN-γ levels were assayed using commercial cytokine ELISA kits [DIACLONE, FRANCE]. Primary monoclonal antibodies (DIACLONE, FRANCE) specific to respective cytokine and secondary biotin-conjugated anti-mouse cytokine specific monoclonal antibodies (BENDER MEDSYSTEMS, AUSTRIA) were used. Streptavidin peroxidase and tetramethylbenzidine (TMB) have been used as substrate. The absorbance was read at 450 nm [19,20].
Quantification of Th2 immune response
For Th2 immune response, level of IgG1 was determined by ELISA. Goat anti-mouse IgG1 isotype specific HRP conjugated secondary antibody was added to the plate.
IL-4 and IL-10 levels were assayed using commercial cytokine ELISA kits [DIACLONE, FRANCE]. Primary monoclonal antibodies (DIACLONE, FRANCE) specific to respective cytokine and secondary biotin-conjugated anti-mouse cytokine specific monoclonal antibodies (BENDER MEDSYSTEMS, AUSTRIA) were used. Streptavidin peroxidase and tetramethylbenzidine (TMB) have been used as substrate. The absorbance was read at 450 nm [19,20].
Statistical Analysis of Data
Data collected from various experiments was subjected to statistical analysis. Mean ± SD of five mice has been used to obtain data for each experiment. Student’s t test has been applied to determine the statistical significance and inter group difference of various parameters. Data of the immunized and control groups have been compared and the p value<0.005 has been termed as statistically significant value.
Results
Post challenge course of parasitemia in immunized mice
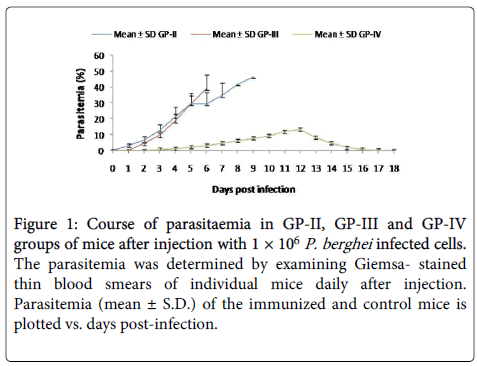
In GP-IV group parasite appeared on D3 post challenge (PC), maximum infection (10.28 ± 1.01%) was observed on D12 (PC). No parasite was observed in blood smear after D18 and all the animals of immunized group survived the challenge, whereas, in Placebo control group (GP-III), parasite appeared on D2 (PI) and typical P. berghei infection pattern was observed as in GP-II. Maximum infection of 43.56 ± 2.21% was recorded on D8 (PI). All the animals of control group died by D9 (Figure 1).
Figure 1: Course of parasitaemia in GP-II, GP-III and GP-IV groups of mice after injection with 1 × 106 P. berghei infected cells. The parasitemia was determined by examining Giemsa- stained thin blood smears of individual mice daily after injection. Parasitemia (mean ± S.D.) of the immunized and control mice is plotted vs. days post-infection.
Flow cytometry analysis of control and immunized spleen MNCs
The frequencies of CD3+, CD4+ and CD8+T cells were found to be increased in immunized mice as compared to controls.
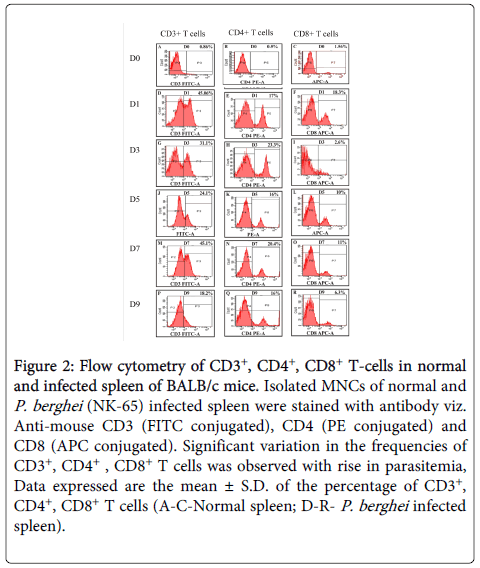
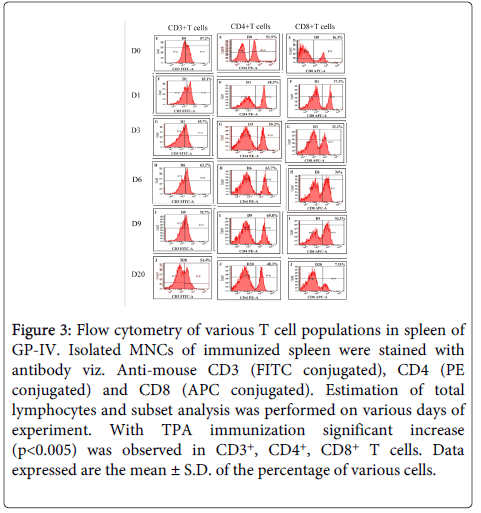
CD3+ cells: The percentage of CD3+ T cells was found to be significantly higher (p<0.005) in mice immunized with TPA as compared to controls. There was a two fold increase in CD3+ T cells in the spleen of immunized mice after challenge on D1 followed by decline on D3 (PC) and remained higher after that till D9 (PC). Whereas, in placebo controls, higher frequencies of CD3+ T cells was observed on D1 (PC), which declined on D3 (PC) followed by increase on D7 (PC) before declining sharply on D9 (PC) after which all the mice of control group died (Figures 2 and 3).
Figure 2: Flow cytometry of CD3+, CD4+, CD8+ T-cells in normal and infected spleen of BALB/c mice. Isolated MNCs of normal and P. berghei (NK-65) infected spleen were stained with antibody viz. Anti-mouse CD3 (FITC conjugated), CD4 (PE conjugated) and CD8 (APC conjugated). Significant variation in the frequencies of CD3+, CD4+ , CD8+ T cells was observed with rise in parasitemia, Data expressed are the mean ± S.D. of the percentage of CD3+, CD4+, CD8+ T cells (A-C-Normal spleen; D-R- P. berghei infected spleen)
Figure 3: Flow cytometry of various T cell populations in spleen of GP-IV. Isolated MNCs of immunized spleen were stained with antibody viz. Anti-mouse CD3 (FITC conjugated), CD4 (PE conjugated) and CD8 (APC conjugated). Estimation of total lymphocytes and subset analysis was performed on various days of experiment. With TPA immunization significant increase (p<0.005) was observed in CD3+, CD4+, CD8+ T cells. Data expressed are the mean ± S.D. of the percentage of various cells.
CD4+ cells: Spleen of TPA immunized mice exhibited statistically significant (p<0.005) higher percentage of CD4+ T cells as compared to controls. In immunized mice, CD4+ T cells were higher (p<0.005) till D9 (PC) as compared to controls. Due to the weak Th2 immune response, all the controls died, whereas, immunized mice which had higher frequencies of CD4+ T cells generated strong Th2 immune response leading to complete survival in this group. Increase in the CD4 T cells in controls was also observed on D3 (PC) and D9 (PC) but it was not significant enough to provide protection (Figures 2 and 3).
CD8+ cells: In TPA immunized mice statistically significant (p<0.005) higher percentage of CD8+ T cells was recorded as compared to controls. CD8+ T cells were found to be significantly high (p<0.005) till D9 (PC), whereas, in infected controls CD8+ T cells were found to be high on D1 (PC) and D7 (PC) which gradually declined on D9 (PC). After parasite clearance (D20) the percentage of CD8+T cells returned back to the normal in immunized group also (Figures 2 and 3).
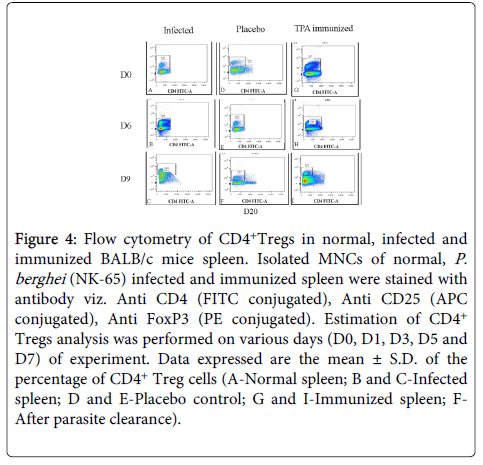
CD4+ T regulatory cell analysis: In TPA immunized spleen, the frequencies of CD4+ Treg cells were found to be significantly (p<0.005) declined as compared to controls.
In immunized group percentage of CD4+Treg cells decreased on D6 (PC), whereas, in controls the percentage of CD4+ Treg cells increased on D1 (PC) and remained high. All the mice of this group died due to D9 (PI). However, lower frequencies of CD4+ Treg cells were observed in GP-IV on D6 (PC) as compared to controls, indicating towards the stimulation of humoral immune response evident from increased CD4+ T cells also. After parasite clearance (D20), CD4+ Treg cell were observed to be increased, whereas, number of CD4+ T cells reduced (Figure 4).
Figure 4: Flow cytometry of CD4+Tregs in normal, infected and immunized BALB/c mice spleen. Isolated MNCs of normal, P. berghei (NK-65) infected and immunized spleen were stained with antibody viz. Anti CD4 (FITC conjugated), Anti CD25 (APC conjugated), Anti FoxP3 (PE conjugated). Estimation of CD4+ Tregs analysis was performed on various days (D0, D1, D3, D5 and D7) of experiment. Data expressed are the mean ± S.D. of the percentage of CD4+ Treg cells (A-Normal spleen; B and C-Infected spleen; D and E-Placebo control; G and I-Immunized spleen; FAfter parasite clearance).
Imunofluorescence Assay (IFA)
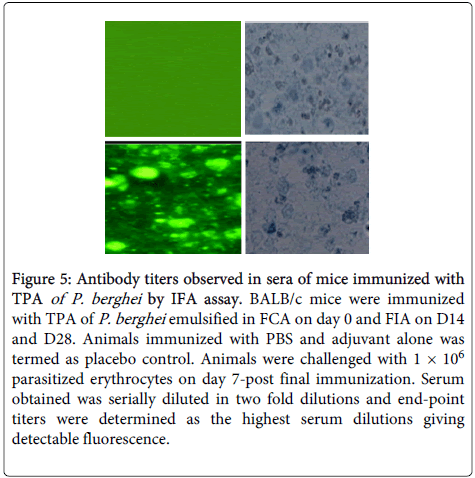
Antimalarial antibodies raised against TPA were found to be specific for parasite because fluorescing structure corresponded to P. berghei infected cells as observed under phase contrast microscope (Figure 5).
Figure 5: Antibody titers observed in sera of mice immunized with TPA of P. berghei by IFA assay. BALB/c mice were immunized with TPA of P. berghei emulsified in FCA on day 0 and FIA on D14 and D28. Animals immunized with PBS and adjuvant alone was termed as placebo control. Animals were challenged with 1 × 106 parasitized erythrocytes on day 7-post final immunization. Serum obtained was serially diluted in two fold dilutions and end-point titers were determined as the highest serum dilutions giving detectable fluorescence.
Antibody titre of 1:2048 was observed in pre challenge immune serum obtained from GP-IV mice. It increased to 1:4096 after challenge of immunized mice with live parasite on D6 and D12 (PC). After clearance of parasite on D20 (PC), 1:2048 antibody titre was recorded in sera of immunized animals.
Quantification of Th1 Immune Response
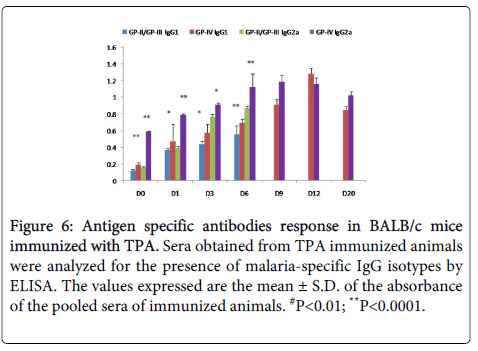
Statistically significant (p<0.005) higher level of IgG2a was observed on D6 (PC) in mice immunized with TPA as compared to controls. IgG2a was found to be increased till D9 (PC) and declined after parasite clearance (Figure 6).
Figure 6: Antigen specific antibodies response in BALB/c mice immunized with TPA. Sera obtained from TPA immunized animals were analyzed for the presence of malaria-specific IgG isotypes by ELISA. The values expressed are the mean ± S.D. of the absorbance of the pooled sera of immunized animals. #P<0.01; **P<0.0001.
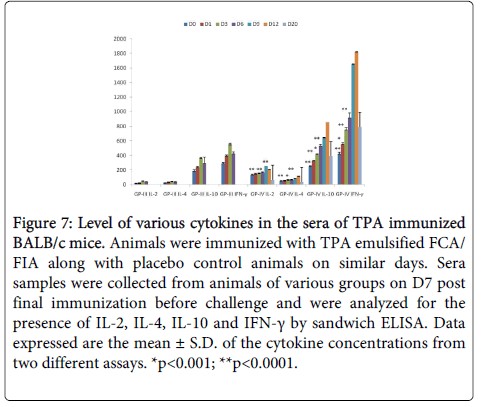
TPA immunized mice showed significantly higher (p<0.005) levels of IL-2 and IFN- γ as compared to controls (Figure 7). There was a two to three fold increase in the level of IL-2 and IFN- γ on D9 (PC). Increased levels of IFN-γ and IL-2 indicate towards Th1 type of immune response in immunized mice. On D12 (PC) IL-2 level was significantly declined due to the switch over from Th1 to Th2 immune response.
Figure 7: Level of various cytokines in the sera of TPA immunized BALB/c mice. Animals were immunized with TPA emulsified FCA/ FIA along with placebo control animals on similar days. Sera samples were collected from animals of various groups on D7 post final immunization before challenge and were analyzed for the presence of IL-2, IL-4, IL-10 and IFN-γ by sandwich ELISA. Data expressed are the mean ± S.D. of the cytokine concentrations from two different assays. *p<0.001; **p<0.0001.
Quantification of Th2 Immune Response
Statistically significant (p<0.005) increase was also observed in the levels of IgG1 in immunized mice as compared to controls (Figure 6). The level of IgG1 was found to be significantly high till the complete clearance of parasite was observed in blood of immunized mice. After parasite clearance (D20) the level of IgG1 declined (Fig.6). Elevated levels of IgG1 in immunized sera point towards the generation of strong Th2 immune response.
TPA immunized mice showed significantly higher (p<0.005) levels of IL-4 and IL-10 as compared to controls (Fig. 7). Statistically significant increase in the levels of IL-4 and IL-10 in immune sera as compared to controls point towards the generation of Th2 response.
Discussion
T-cells play an important role in protecting host and for disease pathogenesis during infection with Plasmodium. T cells are required to eliminate parasite which is hidden inside host cells and thereby evade host’s defence. In response to infection, antigen-specific T and B cells are generated in spleen which contains a large amount of immune cells [21].
B-cells plays significant role in inducing Th2 response and shutting down Th1 response. In self-clearing P. chabaudi strain, efficient clearance of erythrocytic stages is dependent on the presence of B-cells or antibodies [22]. CD4+T cells have been reported to mediate protective immunity to control blood stage parasites especially in parasite clearance [23]. In P. chabaudi infected spleen the frequencies of CD4+ and CD8+ T cells were reported to be significantly increased on D6 (post infection) which dropped on D12 (post infection) and then increased again after parasite clearance (D18), thereafter the percentages of CD4+T cells approached normal levels [22].
In present study also, spleen of infected mice showed a significant increase in the frequencies of CD4+ T cells indicating the generation of humoral response in host but it was not significant enough to impart protection, whereas, in immunized animals the significant increase was observed in the frequencies of CD4+ T cells and all mice of this group survived. After parasite clearance (GP-IV) the frequencies of CD4+ T cells returned to normal.
CD8+ T cells have been reported to protect the host from blood-stage malaria primarily via secretion of IL-12, IFN-γ, Tumor necrosis factor. In present data, CD8+ T cells in spleen of infected control mice significantly increased on D1 (PI) followed by decline. However, in TPA immunized spleen CD8+ T cells were significantly higher and remained higher till D9 (PC). It has been also observed that TPA immunized mice had significantly (p<0.005) lower parasitemia as compared to control animals indicating a cell mediated immune response. Moreover, patency was longer in TPA immunized mice as compared to control mice.
Treg cells have been reported to be immunosuppressive through the secretion of suppressive cytokines such as TGF-β and IL-10 [24]. Depletion of Tregs, resulted in alleviated malaria-induced inhibition of T-cell proliferation and IL-2 secretion and correlated with significantly reduced IL-10 responses, suggesting that Treg cells could be mediating their suppressive activity during infection through the production of this cytokine.
Earlier it has been reported that in P. chabaudi AS infection in spleen showed a significant increase in CD4+CD25+Foxp3+ T cells, which was followed by a significant and sustained decrease in their number due to reduced proliferation and apoptosis of CD4+Foxp3+ T cells [25]. In present study also, significant increase was observed in frequencies of Treg cells in control groups, whereas, in immunized mice, lower frequencies of Treg was recorded. In ANKA strain of Plasmodium berghei, Treg depletion resulted in a higher frequency of memory cells developing in the absence of Treg cells [26]. Present study also reports higher frequencies of CD4+ T cells and lower frequencies of CD4+ Tregs in immunized group.
Elevated frequencies of CD4+ T cells in immunized group were evaluated for induction of Th1 and Th2 response by cytokine profiling. IgG2a has been reported to be involved in antibody mediated protection against P. berghei [27]. In previous study of our laboratory, significant higher levels of IgG1 isotype along with increased level of IgG2a antibody in 43 and 48kDa immunized group are correlated with the mixed Th1/Th2 cytokine profile [28]. Results of present study demonstrate that immunized animals has significant (p<0.005) rise in IgG1 level whereas, IgG2a levels significantly (p<0.005) increased on D1 (PC) till D9 (PC), then declined on D12 (PC) which points towards the mixed Th1/Th2 immune response. This indicates that immunization activated both Th1/Th2 subpopulations of T helper cells.
Further cytokines such as IFN-γ, IL-2, IL-4 and IL-10 were analyzed to differentiate between the Th1 and Th2 immune response. In our previous laboratory studies, significant higher level of IL-4 in ML-I (55, 64, 66 and 74 kDa) immunized sera, correlated towards the protective immune response [11]. Significant (p<0.005) elevation in serum level of IL-4 in TPA immunized animals on D1 (PC) till D9 (PC) as compared to infected control mice in present study also indicate towards the higher antibody titres which provide complete protection to the host.
IL-10 induces B cell proliferation, plasma cell differentiation, development and maturation of antimalarial antibodies [29]. In our studies, significantly high (p<0.005) level of IL-10 was observed in TPA immunized mice on D9 (PC) which significantly declined on D20.
In previous studies, significantly higher levels of IFN-γ in ML-I immunized mice have been reported to be involved in protection of mice against P. berghei challenge [11]. In accordance with this study, significantly (p<0.005) higher level of IFN-γ on D9 (PC) in immune sera of TPA immunized mice point towards the complete protection and survival of mice. IL-2 helps in expansion of antigen-specific T cells and also mediates multiple immune responses on a variety of cell types including thymocytes, activated B cells, monocytes, natural killer cells and oligodendrocytes [29].
It has also been reported that IL-2 in 10,000 g immunized mice is involved in lowering the parasitemia, in P. berghei infection [11]. Significant elevated level of IL-2 on D9 (PC). In our study also indicate towards the complete protection in TPA immunized mice. In P. chabaudi infection the switch in T helper cells response have been reported during peak parasitemia and correlates with symptoms of anaemia [22]. In another study, in P. chabaudi infected serum, secretion of IL-2 has been reported to be declined on D9 (post infection) [25], in our study also, the elevated level of IL-2 declined on D12 (PC) in immunized sera, pointing towards the switch from Th1 to Th2 immune response.
In conclusion, our data showed that weak Th1 and Th2 immune responses are generated in P. berghei infected mice. Weak cell mediated immune response lead to the death of all control animals. However, in immunized mice, a strong humoral as well as cell mediated immune response was observed leading to the complete protection of the mice confirmed by the increased frequencies of CD4+ and CD8+ T cells and their respective cytokines. Treg cells which are involved in pathogenesis of disease are also higher in control groups as compared to immunized.
Acknowledgment
It is a pleasure to acknowledge the technical work of CSIF for immunophenotyping by flow cytometer (FACS Calibar B.D.) at PGIMER, Chandigarh. Authors are also thankful to University Grant Commission, New Delhi for providing financial assistance under UGC-Major Research Project.
References
- Langhorne J, Quin SJ, Sanni LA (2002) Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. ChemImmunol 80: 204-228.
- Langhorne J, Cross C, Seixas E, Li C, von der Weid T (1998) A role for B cells in the development of T cell helper function in a malaria infection in mice. ProcNatlAcadSci U S A 95: 1730-1734.
- Overstreet MG, Cockburn IA, Chen YC, Zavala F (2008) Protective CD8 T cells against Plasmodium liver stages: immunobiology of an 'unnatural' immune response. Immunol Rev 225: 272-283.
- Taylor-Robinson AW, Phillips RS, Severn A, Moncada S, Liew FY (1993) The role of TH1 and TH2 cells in a rodent malaria infection. Science 260: 1931-1934.
- Brown H, Turner G, Rogerson S, Tembo M, Mwenechanya J, et al. (1999) Cytokine expression in the brain in human cerebral malaria. J Infect Dis 180: 1742-1746.
- Hansen DS, Schofield L (2010) Natural regulatory T cells in malaria: host or parasite allies? PLoSPathog 6: e1000771.
- Hafalla JC, Cockburn IA, Zavala F (2006) Protective and pathogenic roles of CD8+ T cells during malaria infection. Parasite Immunol 28: 15-24.
- Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, et al. (2002) Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360: 610-617.
- Good MF, Xu H, Wykes M, Engwerda CR (2005) Development and regulation of cell-mediated immune responses to the blood stages of malaria: implications for vaccine research. Annu Rev Immunol 23: 69-99.
- Santiyanont R, Panyim S, Wilairat P, Yuthavong Y, (1985) Parasite identification, counting and staining. In: Application of genetic engineering techniques in tropical diseases, pathogens with special reference to plasmodia. A laboratory manual of selected techniques. UNDP/WorldBank/WHO special program for research and techniques in tropical diseases, Bangkok, Thailand. 413-448.
- Bagai U, Pawar A (2013) A blood stage fraction of Plasmodium berghei induces protective and long lasting immune response in BALB/c mice. ParasitolInt 62: 329-336.
- Banyal HS, Pandey VC, Dutta GP (1979) Subcellular fractionation and localization of marker enzymes of erythrocytic stages of Plasmodium knowlesi. Ind J Parasitol 3: 9-14.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ (1951) Protein measurement with the Folin phenol reagent. J BiolChem 193: 265-275.
- Freeman RR, Holder AA (1983) Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoeliischizont antigen. ClinExpImmunol 54: 609-616.
- Lyons AB, Parish CR (1994) Determination of lymphocyte division by flow cytometry. J Immunol Methods 171: 131-137.
- Bierer BE, Sleckman BP, Ratnofsky SE, Burakoff SJ (1989) The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol 7: 579-599.
- Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057-1061.
- Collins WE, Skinner JC (1972) The indirect fluorescent antibody test for malaria. Am J Trop Med Hyg 21: 690-695.
- Zhang Q, Xue X, Qu L, Pan W (2007) Construction and evaluation of a multistage combination vaccine against malaria. Vaccine 25: 2112-2119.
- Snapper CM, Paul WE (1987) Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Igisotype production. Science 236: 944-947.
- Engwerda CR, Beattie L, Amante FH (2005) The importance of the spleen in malaria. Trends Parasitol 21: 75-80.
- Helmby H, Jönsson G, Troye-Blomberg M (2000) Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudichabaudi AS. Infect Immun 68: 1485-1490.
- Langeveld M, Gamadia LE, ten Berge IJ (2006) T-lymphocyte subset distribution in human spleen. Eur J Clin Invest 36: 250-256.
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F (1999) An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190: 995-1004.
- Berretta F, St-Pierre J, Piccirillo CA, Stevenson MM (2011) IL-2 contributes to maintaining a balance between CD4+Foxp3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection. J Immunol 186: 4862-4871.
- Nie CQ, Bernard NJ, Schofield L, Hansen DS (2007) CD4+ CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infect Immun 75: 2275-2282.
- Akanmori BD, Waki S, Suzuki M (1994) Immunoglobulin G2a isotype may have a protective role in Plasmodium berghei NK65 infection in immunised mice. Parasitol Res 80: 638-641.
- Bagai U, Pawar A, Kumar V (2010) Antibody responses to 43 and 48 kDa antigens of blood-stage Plasmodium berghei in Balb/c mice. J Parasit Dis 34: 68-74.
- Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR (1993) Interleukin-10. Annu Rev Immunol 11: 165-190.
- Taylor-Robinson AW1 (1995) Regulation of immunity to malaria: valuable lessons learned from murine models. Parasitol Today 11: 334-342.
- Ang KK, Holmes MJ, Kara UA (2001) Immune-mediated parasite clearance in mice infected with Plasmodium berghei following treatment with manzamine A. Parasitol Res 87: 715-721.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14426
- [From(publication date):
June-2015 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 9823
- PDF downloads : 4603