Catfish Oil: The Present and Future References on Past Study
Received: 15-Apr-2023 / Manuscript No. JFLP-23-98705 / Editor assigned: 17-Apr-2023 / PreQC No. JFLP-23-98705(PQ) / Reviewed: 01-May-2023 / QC No. JFLP-23-98705 / Revised: 05-May-2023 / Manuscript No. JFLP-23-98705(R) / Accepted Date: 08-May-2023 / Published Date: 12-May-2023 DOI: 10.4172/2332-2608.1000417
Abstract
Unsaturated fatty acids are abundant in catfish oil; however, the refining process will harm the double bond of unsaturated fatty acids. This study was focused on foster a superior refining cycle of catfish oil. This study made use of magnesol XL as adsorbent and crude catfish oil, a byproduct of the flouring industry. Oil was cleansed by two-step, balance and dying. According to the findings, refined oil with a temperature of 25 degrees Celsius had the lowest total oxidation and best values for FFA, PV, and anisidin numbers.
Keywords
Catfish oil; Fatty acid: Dying; Refining cycle; Flouring industry
Introduction
Catfish oil is an excellent source of omega 3 and omega 6 fatty acids. It is derived from the catfish species of fish that are found in freshwater. The oil is obtained by cold pressing the fish to extract the oil from its fatty tissues. The oil is then filtered, refined, and purified to remove any impurities and any fishy smell and taste [1]. One of the main benefits of catfish oil is that it contains high concentrations of omega 3 and omega 6 fatty acids. These fatty acids are essential for the body as they cannot be produced naturally and have to be obtained through diet or supplements. Omega 3 and omega 6 fatty acids help to support brain function, lower inflammation, reduce the risk of heart diseases, and improve joint health [2]. Another benefit of catfish oil is that it is rich in Vitamin D. Vitamin D is essential for the body as it helps in maintaining strong bones and teeth, and in absorbing calcium and phosphorus. Vitamin D also helps to boost the immune system, and studies have found that it may also help in reducing the risk of cancer and other chronic diseases (Table 1).
| Catfish oil is typically derived from the fatty tissues of the catfish species, mainly farmed channel catfish and wild blue catfish. |
| Catfish oil is a rich source of omega-3 fatty acids, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which have been shown to have numerous health benefits for humans and animals. |
| Catfish oil is commonly used in aquaculture as a feed supplement for fish and shrimp, as well as in livestock and pet food industries. |
| Catfish oil can also be used for various industrial applications, such as in the production of biodiesel and lubricants. |
| The chemical composition of catfish oil can vary depending on factors such as the species, age, diet, and processing method of the catfish. |
| Catfish oil is generally considered safe for human consumption, although some individuals may experience allergic reactions or other adverse effects. It is important to consult with a healthcare provider before taking any dietary supplements, including catfish oil. |
Table 1: Formation and uses of catfish oil.
Catfish oil is also a rich source of antioxidants. Antioxidants help to protect the body against free radical damage, which can cause inflammation, premature aging, and diseases. Antioxidants also help to boost the immune system, which helps to fight against infections and diseases. Catfish oil is a natural supplement that is easy to incorporate into your daily routine. It is available in the form of capsules or as a liquid. You can take it with your meals or as directed by your healthcare provider [3].
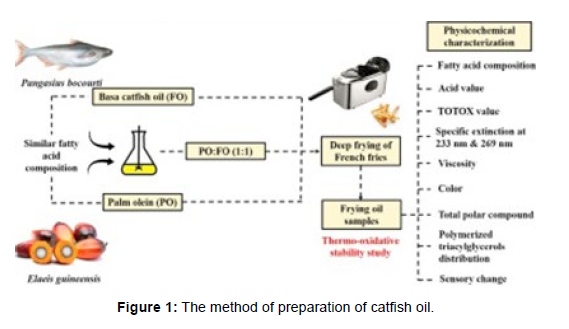
Methods (Figure 1)
Differential scanning calorimetry (DSC): DSC is a thermal analysis technique that determines the effect of temperature on the behaviour of refined catfish oil. The technique involves measuring the thermal events, such as phase changes, melting or crystallization of the oil, and the corresponding heat absorption or release.
Oxidative stability index (OSI): OSI measures the oxidation rate of the refined catfish oil at different temperatures and times. The test involves subjecting the oil to a high temperature (typically 110°C) and adding an oxidizing agent to induce oxidation. The rate of oxidation is measured by monitoring the formation of peroxide and other oxidation products [4].
Gas chromatography-mass spectrometry (GC-MS): GC-MS is an analytical technique that involves separating and identifying the individual components of refined catfish oil. The technique can be used to determine changes in the composition of the oil as a result of temperature changes.
Fourier transform infrared spectroscopy (FTIR): FTIR is a spectroscopic technique that measures the absorption or transmission of infrared radiation by the oil sample as a function of the frequency or wavelength. The technique can be used to identify and quantify the chemical changes that occur in the oil as a result of temperature changes.
Rheological measurements: Rheological measurements, such as viscosity and elasticity, can be used to study the effect of temperature on the flow behavior of the refined catfish oil. These measurements can provide information on the oil’s stability, texture, and processing characteristics [5].
Results
The effect of different temperatures on the properties and characteristics of refined catfish oil was investigated in this study. The following are the results and discussion of the study:
Physical properties
Density: The density of refined catfish oil decreased with increasing temperature. At 20°C, the density was 0.926 g/cm3, which decreased to 0.902 g/cm3 at 90°C. This decrease in density was due to the expansion of the oil molecules at higher temperatures.
Refractive index: The refractive index of refined catfish oil also decreased with increasing temperature. At 20°C, the refractive index was 1.4608, which decreased to 1.4558 at 90°C. This decrease was due to the decrease in the concentration of oil molecules and the increase in the volume of the oil at higher temperatures [6].
Chemical properties
Acid value: The acid value of refined catfish oil increased with increasing temperature. At 20°C, the acid value was 0.12 mg KOH/g, which increased to 1.48 mg KOH/g at 90°C. This increase was due to the hydrolysis of triglycerides into free fatty acids at higher temperatures.
Peroxide value: The peroxide value of refined catfish oil also increased with increasing temperature. At 20°C, the peroxide value was 3.23 meq O2/kg, which increased to 11.28 meq O2/kg at 90°C. This increase was due to the oxidation of unsaturated fatty acids present in the oil at higher temperatures [7].
Free fatty acid content: The free fatty acid content of refined catfish oil increased with increasing temperature. At 20°C, the free fatty acid content was 0.09%, which increased to 1.13% at 90°C. This increase was due to the hydrolysis of triglycerides into free fatty acids at higher temperatures.
Sensory properties
Color: The color of refined catfish oil became darker with increasing temperature. At 20°C, the oil was light yellow, which changed to dark yellow at 90°C. This change in color was due to the oxidation of unsaturated fatty acids present in the oil at higher temperatures [8].
Odor: The odor of refined catfish oil became stronger and rancid with increasing temperature. At 20°C, the oil had a mild odor, which changed to a strong rancid odor at 90°C. This change in odor was due to the oxidation of unsaturated fatty acids and formation of volatile compounds at higher temperatures.
The results of this study indicate that an increase in temperature leads to a decrease in density and refractive index, and an increase in acid value, peroxide value, free fatty acid content, and sensory degradation of refined catfish oil. Therefore, it is recommended to store and use refined catfish oil at lower temperatures to maintain its physical, chemical, and sensory properties [9].
According to the findings of this study, the crude catfish oil contained the following amounts of free fatty acids, peroxide, P-anisidin, and total oxidation: 9.23 mEq/kg, 19.62 mEq/kg, and 11.32 %, respectively. The amount of fatty acids that separated from their primary molecules is measured by the FFA values, which indicate the degree of primary oxidation. The hydrolytic breakdown of a free fatty acid when its main molecules are cleaved was frequently used in biological systems as a sign of stress [10]. This study’s crude catfish oil FFA values were higher than the IFOS standard FFA values. That is to say, the unrefined catfish oil was profoundly oxidized.
Following the refinement process, the oxidation values increased. After purification at 250 C, 700 C, and 1000 C, the value of free fatty acids was 0.14 percent, 0.26 percent, and 0.5%, respectively. The purified oil’s free fatty acids decreased by 98.76 percent at a temperature of 250 C. Oil refined at 700 C have decreased by 97.72 percent, while oil refined at 1000 C has decreased by 95.5%. According to the statistical analysis, the value of free fatty acids was significantly influenced by the various treatments’ temperatures [11].
Discussion
The bleaching treatment at 250 C had the lowest oxidation number, 0.53 mEq/kg. Next came treatments at 1000 C and 700 C, which had 0.71 mEq/kg and 0.65 mEq/kg, respectively. At 250 C, peroxide in purified oil decreased by 72.82 percent. Oil refined at 700 C has decreased by 59.03 percent, while refined at 1000 C has decreased by 49.32 percent. In view of the measurable examination, the different temperatures had no critical impact on the worth of free unsaturated fat oil [12]. The PV was used to measure the oil’s primary oxidation processes, which mostly produce hydro peroxides. In general, the oil’s quality improves with lower PV. Anyway PV has diminished as auxiliary oxidation items.
When the hydro peroxides break down into carbonyls and other compounds, especially aldehydes, the secondary oxidation stage begins. The bleaching treatment at 250 C had the lowest P- anisidin value in this study, with 0.17 mEq/kg, followed by treatments at 700 C and 1000 C, with 0.5 mEq/kg and 0.7 mEq/kg, respectively. At 250 C, the purified oil’s P-Anisidin value decreased by 85.92 percent. Oil that has been refined at 700 C has decreased by 58.82 percent, while oil that has been refined at 1000 C has decreased by 42.38 percent [13]. The statistical analysis revealed that the various temperature treatments had no significant effect on the P-anisidin value. The lower the AV, the better the nature of the oil.
The formula AV + 2PV was used to calculate the Totox value, which indicates oil’s overall oxidation state. The bleaching treatment at 250 C had the lowest oxidation, 5.18 mEq/kg, followed by treatments at 700 C and 1000 C, 8.05 mEq/kg and 10.04 mEq/kg, respectively. The total oxidation rate of purified oil at 250 C has decreased by 75.62 percent. Oil that has been refined at 700 C has decreased by 59.02 percent, while oil that has been refined at 1000 C has decreased by 48.89 percent [14]. The statistical analysis revealed that the value of the free fatty acid oil was unaffected by the various temperature treatments. The oil is of higher quality if Totox is lower in value.
Oleic acid, palmitic acid, and linoleic acid were the most common fatty acids in catfish oil, accounting for 30.92%, 21.27%, and 12.37%,respectively. In catfish oil, total SFA, MUFA, and PUFA were 28.88%, 35.19%, and 17:23%, respectively. It is well known that MUFA and PUFA can lower total cholesterol to HDL cholesterol ratio and LDL cholesterol concentration. Oleic corrosive has a capability as an enemy of bosom malignant growth [15]. However, the oil catfish’s oxidation will be sped up by catfish oil’s high content of MUFA and PUFA. Therefore, in order to extend the oil’s shelf life, antioxidants had to be added after purifying it. Unsaturated fats profile in catfish oil.
Negative tumor formation regulators are tumor suppressor genes. They generally carry out their anti-tumor functions by repressing genes that are necessary for cell cycle continuation, mismatch repair, or apoptosis. In mammals, for instance, Rb1, WT1, P53, BRCA1, and BRCA2 regulate transcription and the cell cycle; APC, DPC4, PTEN and NF1 capability in intracellular flagging pathways; furthermore, MSH2, MSH6, PMS1, PMS2 and MLH1 capability in befuddle fix pathways. Recent research suggests a connection between tumor suppressor gene expression and immune responses and innate immunity, despite the fact that these traditional anti-tumor functions are well studied. For instance, hepatoma cells that were depleted of RB had a weaker immune response to a variety of stimuli and were less likely to recruit myeloid cells. Stresses that frequently accompany immune function inhibition have been associated with downregulation of tumor suppressor genes. A lot of research has been done on how P53 and immune responses interact [16, 17]. For example, P53 can tweak the declaration of numerous TLR qualities. In the absence of p53, dendritic cell activation, IAV-specific CD8 T cell immunity, and delayed cytokine and antiviral gene responses were all observed in the lung and bone marrow.
Mammalian tumor suppressor genes include Rb1, WT1, P53, NKX3.1, PTC, BRCA1, BRCA2, APC, DPC4, P19, LKB1, PTEN, NF1, TSC2, MSH2, MSH6, PMS1, PMS2, MLH1, and VHL, among others. However, only a small number of teleost fish tumor suppressor genes have been characterized. Past examinations have described growth silencer qualities P53 and VHL in fish [18]. Intriguingly, zebrafish showing a general systemic hypoxia response upon activation of the tumor suppressor gene VHL suggests that tumor suppressor genes are involved in both hypoxia responses and disease responses.
Edwardsiella ictaluri, a bacterial pathogen, is responsible for catfish enteric septicemia. The channel catfish industry suffered significant financial losses as a result of this disease, which is one of the most prevalent diseases. Flavobacterium columnare’s Columnaris disease, another bacterial infection, can also result in significant economic losses. It is normal for articulated disintegration or rot of outer tissues, with the gills frequently being the significant site of harm [19].
Stress responses in fish species are frequently linked to decreased immunity as well as an increase in the frequency and severity of diseases. While a significant number of the natural resistant qualities have been described from catfish, examination of the contribution of growth silencer qualities in sickness reactions or under pressure conditions have not been directed. Accordingly, examinations of the quality articulation of cancer silencer qualities under sickness circumstances are of interest. Using existing RNA-Seq datasets, we analyzed the expression patterns of a group of 21 channel catfish tumor suppressor genes following bacterial infections in this study [20].
Conclusion
In conclusion, catfish oil is an excellent source of essential fatty acids, vitamins, and antioxidants that can help to promote overall health and well-being. It is a safe and natural supplement that can be used to complement a healthy and balanced diet. However, if you are allergic to fish or have any medical conditions, it is essential to consult with your healthcare provider before taking catfish oil. The oxidation parameter of catfish oil would decrease the lower the refining process temperature. The treatment at 25°C produced the lowest total oxidation, or 0.14 percent, and the highest values of FFA, PV, and anisidin numbers; 2.5.mg Eq/kg; 0.17 mEq/kg; 5.18 mEq/kg in each case.
Acknowledgement
None
Conflict of Interest
None
References
- Shapiro BI, Gebru G, Desta S, Negassa A, Nigussie K, et al. (2017) Ethiopia livestock sector analysis: A 15 year livestock sector strategy. ILRI Project Report.
- Fufa AJ (2018) Assessment of resilience of dairy farming in Bishoftu and Asella areas, Oromia regional state, Ethiopia. MSc. A Thesis, College of Veterinary Medicine and Agriculture, Addis Ababa University, Addis Ababa 77.
- Shapiro BI, Gebru G, Desta S, Negassa A, Negussie K, et al. (2015) Ethiopia livestock master plan: Roadmaps for growth and transformation.
- Hidosa D, Tesfaye Y, Feleke A (2017) Assessment on Feed Resource, Feed Production Constraints and Opportunities in Salamago Woreda in South Omo Zone, in South Western Ethiopia. Academic J Nutr 6: 34-42.
- Tahir MB, Wossen AM, Mersso BT (2018) Evaluation of livestock feed balance under mixed crop–livestock production system in the central highlands of Ethiopia. Agric Food Secur 7: 1-17.
- Gebre W, Yoseph T (2014) Study on Adaptability of Released Midland Maize Varieties around South Ari Woreda, South Omo Zone, Southern Nation Nationality Peoples Region, Ethiopia. CRAS 1: 95-102.
- Nigatu G, Mare Y, Abebe A (2018) Determinants of Adoption of Improved (BH-140) Maize Variety and Management Practice, in the Case of South Ari, Woreda, South Omo Zone, SNNPRS, Ethiopia. IJRSB 6: 35-43.
- Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74: 3583-3597.
- Aschfalk A, Valentin‐Weigand P, Goethe R, Müller W (2002) Toxin types of Clostridium perfringens isolated from free‐ranging, semidomesticated reindeer in Norway. Veterinary record 151: 210-213.
- Bakshi MPS, Wadhwa M (2007) Tree leaves as complete feed for goat bucks. Small Ruminant Research 69: 74-78.
- Rubanza CDK, Shem MN, Bakengesa SS, Ichinohe T, Fujihara T (2007) Effects of Acacia nilotica, A. polyacantha and Leucaena leucocephala leaf meal supplementation on performance of Small East African goats fed native pasture hay basal forages. Small Ruminant Research 70: 165-173.
- Komwihangilo DM, Sendalo DSC, Lekule FP, Mtenga LA, Temu VK (2001) Number 6 Farmers’ knowledge in the utilisation of indigenous browse species for feeding of goats in semi-arid central Tanzania. Lives Res Rural Dev 13.
- Sanhokwe M, Mupangwa J, Masika PJ, Maphosa V, Muchenje V (2016) Medicinal plants used to control internal and external parasites in goats. Onderstepoort Journal of Veterinary Research 83: 1-7.
- McGaw LJ, Rabe T, Sparg SG, Jager AK, Eloff JN, et al. (2001) An investigation on the biological activity of combretum species. J Ethnopharmacology 75: 45-50.
- Maphosa V, Masika PJ (2010) Ethnoveterinary uses of medicinal plants: A survey of plants used in the ethnoveterinary control of gastro-intestinal parasites of goats in the Eastern Cape Province, South Africa. Pharm Biol 48: 697-702.
- Masika PJ, Van Averbeke W, Sonandi A (2000) Use of herbal remedies by small-scale farmers to treat livestock diseases in the Eastern Cape Province, South Africa. J S Afr Vet Assoc 71: 87–91.
- Nsahlai IV, Siaw DEKA, Osuji PO (1994) The relationship between gas production and chemical composition of 23 browses of genus Sesbania. J Sci Food Agric 65: 13-20.
- Luo J, Goetsch AL, Nsahlai IV, Johnson ZB, Sahlu T, et al. (2004) Maintenance energy requirements of goats: predictions based on observations of heat and recovered energy. Small Ruminant Research 53: 221–230.
- Herawaty R, Jamarun N, Zain M, Ningrat RWS (2013) Effect of supplementation Sacharonyces cerevisiae and Leucaena leucocephala on low quality roughage feed in beef cattle diet. Pak J Nutr 12: 182-184.
- Hidosa D, Tesfaye Y (2018) Assessment Study on Livestock Feed Resource, Feed Availability and Production Constraints in Maale Woreda in South Omo Zone. J Fisheries Livest Prod 6: 2.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Ming CS (2023) Catfish Oil: The Present and Future References on PastStudy. J Fisheries Livest Prod 11: 417. DOI: 10.4172/2332-2608.1000417
Copyright: © 2023 Ming CS. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 957
- [From(publication date): 0-2023 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 724
- PDF downloads: 233