Review Article Open Access
Catechol Tetrahydroisoquinolines Enhance a-Synuclein Aggregation and Specify the Neurotoxicity to Dopaminergic Neurons
Zixuan Chen1, Jianqing Lu1, Jinyan Duan1, Xiaotong Zheng2, Yanyan Zhang1, Chao Han1 and Yulin Deng1*1School of Life Science, Beijing Institute of Technology, Beijing, China
2College of Life Science and Bioengineering, Beijing University of Technology, Beijing, China
- Corresponding Author:
- Yulin Deng
School of Life Science
Beijing Institute of Technology
5 South Zhongguancun Street, Haidian District
Beijing, 100081, People’s Republic of China
Tel: 86-010-6891-5996
E-mail: deng@bit.edu.cn
Received date: February 09, 2017; Accepted date: February 25, 2017; Published date: February 28, 2017
Citation: Chen Z, Lu J, Duan J, Zheng X, Zhang Y, et al. (2017) Catechol Tetrahydroisoquinolines Enhance a-Synuclein Aggregation and Specify the Neurotoxicity to Dopaminergic Neurons. J Alzheimers Dis Parkinsonism 7:308. doi:10.4172/2161-0460.1000308
Copyright: © 2017 Chen Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease with no definitive neuroprotective therapies. Previous studies have demonstrated that Catechol tetra hydroisoquinolines (CTIQs) are toxic to dopaminergic neurons. These toxins can induce mitochondrial dysfunction, which consequently contributes to the pathogenesis of PD. Unlike external neurotoxins, such as agrochemicals and MPTP, CTIQs can be synthesized in the brains of human based on the dopamine and the particular aldehyde. Besides, aggregated α-synuclein (α-syn), one of the hallmarks of PD, has been proved to be a key contributor in the development of PD and can be affected by many neurotoxins. Some studies have presented that CTIQs enhanced the aggregation of α-syn and increased the neurotoxin of α-syn, which might be the pathological mechanism of PD. Therefore, this chapter will reveal the role of CTIQs and α-syn and try to clarify the relationship between them.
Keywords
Parkinson disease; Catechol tetrahydroisoquinolines; α-synuclein; Neurotoxin
Abbreviations
HPLC/ESI-MS/MS: High-Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometer;LCMS/ MS: Liquid Chromatography Tandem-Mass Spectrometry; HPLCECD: High-Performance Liquid Chromatography Combined with Electrochemical Detection; HPLC-QQQ-MS: High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry; HPLC–MS; High-Performance Liquid Chromatography- Mass Spectrometry
Introduction
Parkinson disease (PD) is the second most common multisystem neurodegenerative disease after Alzheimer disease, whose incidence rates are around 1% and 4% of the population above the age of 60 and 80 years, respectively [1]. It is a progressive movement disorder, which is clinically manifested by resting tremor, rigidity, postural instability and akinesia/bradykinesia usually along with cognitive impairment. The major hallmarks of PD are the loss of dopaminergic neurons and formation of Lewy Bodies (LBs) in the substantia nigra pars compacta (SNpc). The dopamine(DA) replacement therapy with levodopa(LDOPA) is a milestone in the treatment of PD. However, L-DOPA can induce motor fluctuation and dyskinesias after several years of treatment [2,3]. So it is of important realistic significance to explore effective approaches for prevention, diagnosis and treatment of PD through clarifying its pathological mechanism.
α-syn was the first reported gene, the mutation of which was described in a family with dominantly inherited PD in 1997 [4]. α-syn was believed as the key protein involved in PD, overexpression of which has been wildly used as PD models [5]. Although increasing studies on the physiological and pathological roles of α-syn popped up in recent years, the exact mechanism of α-syn aggregation in PD is still elusive. Approximately 5%-10% cases of PD are familial, while the majority cases of PD are sporadic. The sporadic cases of PD are believed to result from the combination of genetic differences and environmental factors. And the studies have proven that many neurotoxins can facilitate the formation of abnormal α-syn [6,7]. So it is important to understand how the environment can influence the function of α-syn to explore the exact mechanism of abnormal aggregation of α-syn. Various epidemiologic elements have been proposed as risk factors in PD, including genetic mutations, head trauma, bacterial or viral infections and environmental toxins. Since exogenous neurotoxin, 1-Methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP), was discovered to induce PD-like syndrome in peoples, neurotoxins have been paid more attention and extensively investigated. In recent years, endogenous neurotoxins, CTIQs, were considered as the risk factors potentially involved in the pathogenesis of PD [8,9]. Unlike exogenous neurotoxins, CTIQs is a group of products which could be synthesized in the brain of human based on the condensation of DA and some certain aldehydes. So it is proposed that they might be the key factors involved in α-syn aggregation [10-12] In this review, we will describe the relationship between CTIQs and the abnormal aggregation of α-syn and try to provide a helpful insight into therapeutic approaches for PD.
Endogenous Catechol Tetrahydroisoquinolines
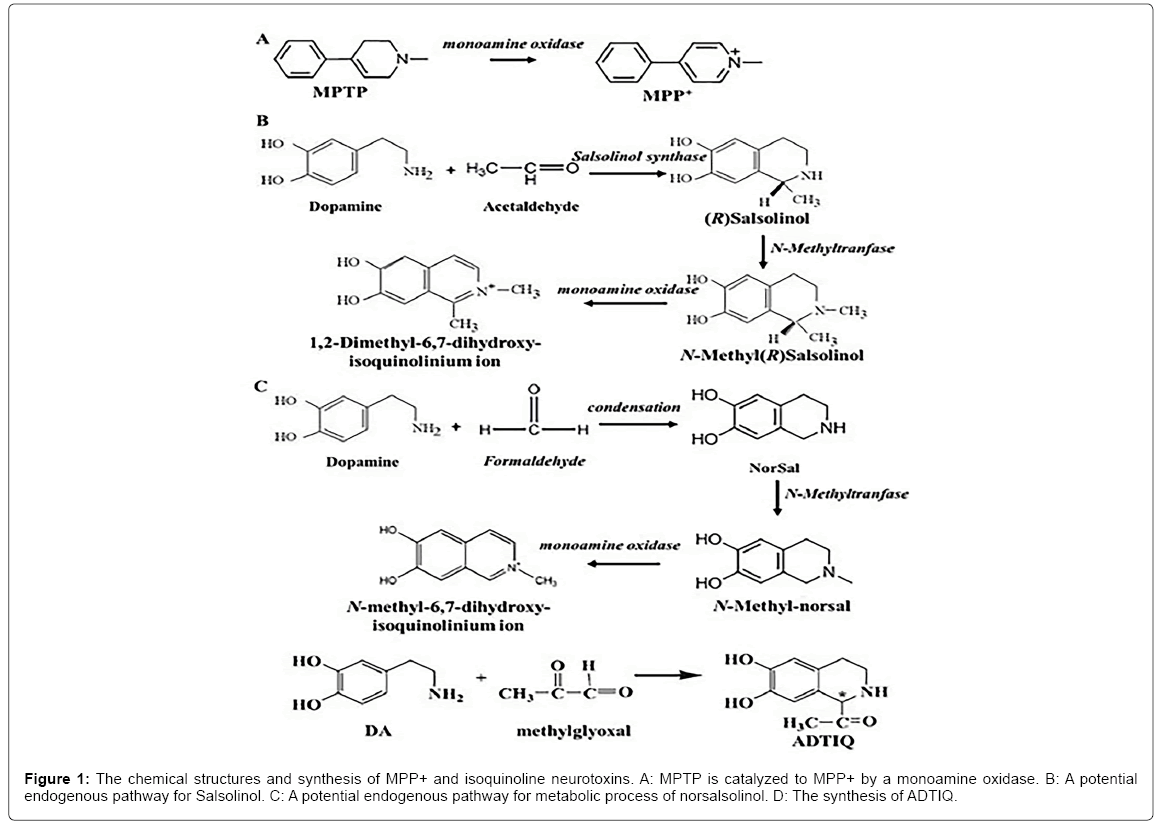
A body of evidences proved that the CTIQs are a group of important endogenous compounds that are discovered in the mammalian brain especially in PD patients [11,13,14]. CTIQs and their derivatives can be synthesized based on different active aldehydic derivatives and DA product or its metabolites, such as 1-methyl-6,7-dihydroxyl- 1,2,3,4-tetrahydroisoquinoline(Sasolinol,Sal),1-acetyl-6,7-dihydroxyl- 1,2,3,4-tetrahydroisoqu-inoline (ADTIQ) and 6,7-dihydroxyl-1,2,3,4- tetrahydro- isoquinoline (norsalsolinol,norsal) [12,15,16]. These CTIQs listed above could be reacted into N-methylnorsalsolinol (NM-NorSal), N-methylsalsolinol (NM-Sal) and N-methyl-ADTIQ (NM-ADTIQ) by nitrogen methyltransferase respectively, and then generated to ion oxides of MeDHIQ+, DiMeDHIQ+ and MeAcDHIQ+ by MAO enzyme respectively [17]. CTIQs together with their nitrogen methylation and oxidation products are the structure analogs of MPTP and MPP+. So we have known that these CTIQs are the products of DA that would be a reasonable explanation for the specific degeneration of dopaminergic neurons in PD patients [10]. Also, there is a chiral c enantiomer in the C1 location of CTIQ, which is the reason of existence of (R) and (S)- enantiomer of CTIQ. In the mammalian brain, they were synthesized by an enzymatic and non-enzymatic Pictet-Spengler condensation of neuroamines with aldehydes [12]. The chemical formulae, synthesis and metabolism of common CTIQs are shown in Figure 1.
It is known that the aldehydic compounds are usually derived from lipid peroxidation in which the polyunsaturated fatty acid is peroxidized under the condition of oxidative stress [18]. And oxidative stress has been deemed as one of the important contributors to the death of nigral neurons in PD patients [19,20]. In general, oxidative stress can generate much more reactive oxygen species (ROS) and further induce mitochondrial damage. In turn, damaged mitochondria can cause the generation of excessive superoxide, which further results in the mitochondrial dysfunction and finally cell death. This progress makes a vicious cycle [21]. However, the exact pathogenesis mechanism of this vicious cycle in PD is still not clear. Recently, Deng’s group confirmed the existence of Sal synthetase, that is a thermostable enzyme, and the higher enzymatic condensation reaction of DA and acetaldehyde was detected in the striatum and substantia nigra of rat brains [22,23]. Of CTIQs, Sal is a representative neurotoxin, which presents a high level in brains of PD patients and has been shown to increase ROS, inhibit mitochondrial function and cell injury [24-26]. Therefore, we have a hypothesis of CTIQs induced PD that when oxidative stress occurs, the aldehydic product of lipid peroxidation condenses with DA to form a series of CTIQs such as Sal in DA neurons. These CTIQs can cause the mitochondrial toxicity, which in turn induces the generation of excessive oxidative stresses in DA neurons. This process makes a vicious cycle, finally resulting in the death of DA neurons. Although some researchers found that there was an increase of CTIQs in the cerebrospinal fluid(CSF) of PD patients [27,28], the CTIQs may not be much higher in PD patients than that in the normal people since they do not stay at some stage and are always in the processes of metabolism.
Neurotoxins of CTIQs Potentially Involved in PD
In recent years, environmental toxins are considered as important factors in the pathogenesis of PD. The neurotoxins are divided into two groups: exogenous neurotoxins and endogenous neurotoxins. Exogenous neurotoxins primarily include agrochemicals such as MPTP, rotenone, and paraquat, while endogenous neurotoxins mainly include tetrahydroisoquinoline (TIQ) compounds and β-carboline derivatives. Although exogenous neurotoxins have been proven to be involved in the development of PD, there is still small chance that general population are exposed to them. Therefore, endogenous neurotoxins have caused widespread concern.
The major neurotoxic CTIQs
1-methyl-4-phenyl-2,3-dihydropyridium(MPTP) is a well-known exogenous neurotoxin, which has been widely used in animal models of PD [29-32]. MPTP was first discovered in the drugs, which made many addicts show signs of parkinsonism-like symptoms after they smoked them [33]. When MPTP enters to our blood system, it can easily cross the BBB and then be converted to the dihydropyridinium ion (MPDP+), which further oxidizes into 1-methyl-4-phenylpyridinium (MPP+) by astrocytic monoamine oxidase B(MAOB) [34]. MPP+ can increase the synthesis of ROS by inducing the mitochondrial damage including decreased mitochondrial membrane potential, impaired respiratory chain complexes and release of cytochrome C [35-37]. The symptoms induced by MPTP are very similar to that of PD [29], suggesting that a long-term accumulation of certain endogenous neurotoxins, which have similar structure or function with MPTP, will lead to PD. Therefore, dopamine-derived alkaloids have been proposed as potential endogenous neurotoxins. So far, some naturally generated neurotoxins, similar to MPTP, have been discovered, which are divided into two main categories: the tetrahydroisoquinolines (TIQs) and the β-carbolines. The most attractive endogenous neurotoxins are TIQs. Among TIQs, CTIQ compounds are members of isoquinoline derivatives. Their nitrogen methylation and oxidation products are the structure analogs of MPTP and MPP+. All of them have been proposed as endogenous neurotoxin candidates, which are intensively investigated both in vitro and in vivo experiments and they perform severe toxicity on cellular and animal models of PD [8,9,38].
Among the CTIQs, 1-methyl-6,7-phenyl-1,2,3,4 – tetrahydroisoquinoline (Salsolinol, Sal) and its derivatives can cause the prominent toxic effect on the nervous system [24] Sal derivatives were the first isoquinoline compounds found in the urine and brain of PD patients who received the treatment of L-DOPA [13]. Sal is known to be synthesized from DA and acetaldehyde (AcH) and has two enantiomers, (S)-Sal and (R)-Sal. (R)-Sal is dominant, which can be further catalyzed to N-methyl-(R)-salsolinol (NM(R)Sal) by N-methyltransferase and then oxidize into 1,2-dimethyl-6,7-dihydroxyisoquinolinium ion (DMDHIQ+) [39]. Sal can be detected in various regions of human brain, but it is highly concentrated in the striatum and substantia nigra [40,41]. Several studies have confirmed that Sal is toxic to dopaminergic neurons in particular. As demonstrated by in vivo studies, chronic administration of Sal can cause the degeneration of striatal dopaminergic neurons [42]. And Sal can decrease the DA levels in these regions possibly through inhibiting the rate-limiting enzyme of dopamine synthesis, tyrosine hydroxylase (TH) [43,44]. In vitro experiments, Sal has been shown to reduce the viability of SH-SY5Y cells in a dose-dependent manner by activating caspase-3 [45,46]. Moreover, Sal has also been demonstrated to cause the morphological changes, mitochondrial impairment and apoptotic cell death in neural stem cells (NSCs) [47]. The neurotoxicity of Sal has been believed to associate with the generation of oxidative stress. Recently, Kang observed that Sal induced the oligomerization of cytochrome c possibly through increasing oxidative stress, indicating that Sal might participate in the pathogenesis of PD by blocking energy supply from mitochondria [38]. Later on, Kang et al. found that Sal could induce human ceruloplasmin(hCP) aggregation by increasing free radical generation, which might be involved in the pathogenesis of PD [48]. Several data indicated that the link between Sal and oxidative stress could be the mitochondrial impairment, of which the manifestations include the inhibition of ATP levels, mitochondrial membrane potential loss, cristae disruption and the release of cytochrome c [25,46,47,49,50]. However, in another study, Sal was considered to play a two-phase opposing action in the central nervous system. Sal in the low concentrations (50 and 100 μM) activated as a neuroprotective factor; however, at its tenfold concentration (500 μM) it activated as a neurotoxic factor, which significantly enhanced the glutamate-induced neurotoxicity [25]. Similar data of Sal were observed in MTT assay [51]. The opposite effects of Sal suggest that its toxic role might be exerted along with its accumulation of mitochondrial damage after the vicious cycle we proposed occurs.
Norsal is one of Sal derivatives, which and its derivatives have also been detected in the urines and CSFs of PD patients [52,53]. Musshoff et al. discovered the norsal in DA-rich areas of the basal ganglia [54]. Recently, norsal has also been found in the Drosophila head using Micellar Electrokinetic Capillary Chromatography [55]. Several evidences showed that norsal had the cytotoxic effects [56,57] and induced the apoptosis of SH-SY5Y cells by increasing cytochrome c release and activating caspase3, in which ROS was a significant contributor [58]. In addition, Nagasawa et al. isolated the Sal and norsal from the Marine Sponge Xestospongia cf. vansoesti and demonstrated that they were proteasome inhibitors, which may be the mechanism of DA neuron death [53]. N-methyl-norsal (NM-norSal) is the N-methylated product of norsal, which inhibited TH, the key enzyme in dopamine synthesis and therefore was possibly contributed to the depletion of DA in PD [59,60]. However, the data from HPLCECD showed that NM-norSal was located in the widespread neuronal regions of several mammalian species, which revealed that NM-norSal might be a week neurotoxin and its contribution in the pathobiology of PD could come out after years of its accumulation [16].
NM-Sal is another crucial neurotoxin which can be catalyzed by N-methyltransferase based on Sal. It is reported that there was an increase of N-methyltransferase activity in the peripheral lymphocytes and the brain in the unilateral 6-hydroxydopamine lesion rat model compared with that in control [61]. NM-Sal, other than Sal, is located selectively in the nigrostriatum [62,63], which has also been determined in the human intraventricular fluid [64]. And NM-Sal is considered to be a stronger neurotoxin than Sal and is capable of inhibiting mitochondrial functions and thus inducing apoptosis of dopaminergic cells [65,66]. And the (R)-enantiomer of NM-Sal can cause more severe DNA damage than the (S)-enantiomer [67]. So far, NM-Sal was expected to be the most toxic CTIQ.SH-SY5Y cells treated with NM-Sal exhibited significant apoptosis, while the cells treated with Sal or MPP+ exhibited moderate apoptosis. Also, MTT assays indicated that the 50% cell death rate of MPP+, Sal and NM-Sal were 250, 1000 and 750 uM, respectively [68]. Another report demonstrated that both Sal and NM-Sal were responsible for mitochondrial membrane potential loss, cytochrome c release and cristae disruption in parkin-deficient PC12 cells after H2O2 exposure [50]. Otherwise, in some oxidative stress conditions, the levels of Sal and NM-Sal could be synthesized in vivo. For instance, after ethanol exposure, Sal and NM-Sal were generated in fetal brains of rats probably by increasing the level of AcH [69]. And Sal and NM-Sal were also upregulated in the dopamine and Fe2+ impaired SH-SY5Y cells, which could be served as the oxidative stress model [70]. Consistent with these, the CTIQs were also generated under the oxidative stress condition induced by manganese in PC12 cells [71].
ADTIQ is a link between PD and diabetes
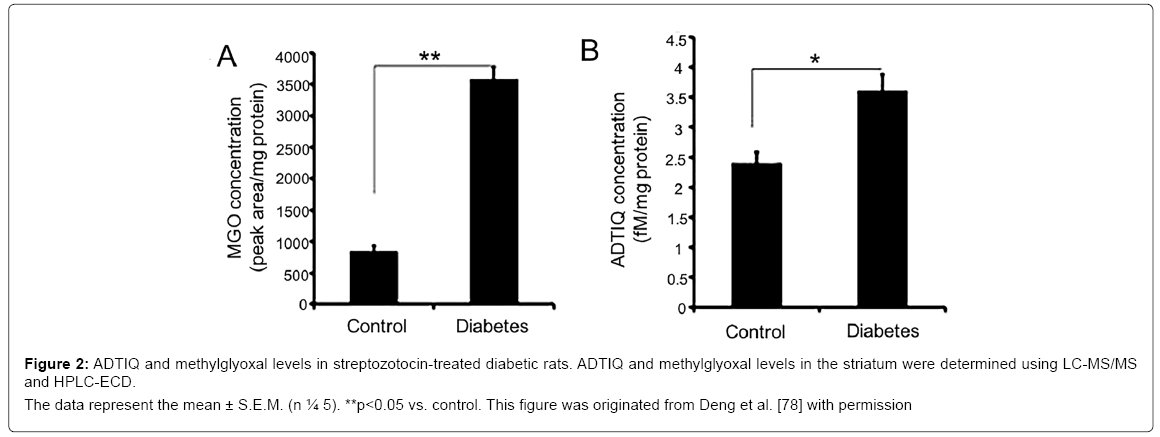
1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydro-isoquinoline(ADTIQ) is a Sal-like compound which was detected in the putamen, caudate nucleus, frontal cortex, substantia nigra and cerebellum of PD patients [72]. In vivo study, HPLC-ESI-MS/MS results showed that the concentration of ADTIQ was 63.6 ± 4.6 fM/mg wet tissue in the substantia nigra of rat [73]. In putamen of PD patients, the level of ADTIQ was higher than that in controls, which suggested that this novel compound might be related to PD [15]. And ADTIQ is endogenously synthesized from the condensation of dopamine and methylglyoxal (MGO). MGO is a well-known toxic compound in diabetic neuropathy and can increase ROS and inhibit mitochondrial respiration in SH-SY5Y cells [74,75]. MGO increased the levels of DA and Sal, contributing to neuronal cell death [76]. Moreover, the elevated levels of ADTIQ and MGO were observed in the cell model of hyperglycemia and the brains of rats with diabetes [77]. Figure 2 showed significantly increased levels of ADTIQ and MGO in the striatum of SD rats with type2 diabetes. Otherwise, ADTIQ induced mitochondrial damage and reduced cell viability in SH-SY5Y cells, indicating that ADTIQ is involved in the pathogenesis of PD [78].These results implicate that ADTIQ may be a link between diabetes and PD.
Figure 2: ADTIQ and methylglyoxal levels in streptozotocin-treated diabetic rats. ADTIQ and methylglyoxal levels in the striatum were determined using LC-MS/MS
and HPLC-ECD.
The data represent the mean ± S.E.M. (n ¼ 5). **p<0.05 vs. control. This figure was originated from Deng et al. [78] with permission
TIQs-like candidates of endogenous neurotoxins
There are still other TIQs-like candidates for the endogenous neurotoxins that contributed to the pathogenesis of PD. 1-benzyl-1,2,3,4- tetrahydroisoquinoline (1BnTIQ) has been reported in vivo and in vitro studies and proved to possess the neurotoxic effects [79-81]. However, the neurotoxic effect of 1BnTIQ is dependent on its concentration:1BnTIQ in a low concentration(50 μM) played a neuroprotective role, whereas in 10 times higher concentration (500 μM) it played a neurotoxic role by increasing apoptosis markers [81]. Recently, Kotake et al. detected a novel metabolite of 1BnTIQ, 1-benzyl-3,4-dihydroisoquinoline (1BnDIQ) in rat liver S9 and demonstrated that 1BnDIQ is more toxic than 1BnTIQ [82,83]. In addition, the analog of 1BnTIQ, 1-(3’,4’-dihydroxybenzyl)-1,2,3,4- tetrahydroisoquinoline (3’,4’DHBn TIQ) also exerted the DA neurotoxic effect and reduced cell viability as a potential endogenous parkinsonism inducer [84]. Importantly, the mechanism of these TIQ derivatives induced neurotoxicity may be related to the inhibition of mitochondrial function [82].
α-synuclein Aggregation and Parkinson Disease
For many neurodegenerative diseases including PD, it is a typical characteristic that protein aggregate from monomer to β-sheet-rich amyloid fibrils.α-syn, known as a central component of LBs, is a synaptic protein with the tendency of misfolding and aggregation [85]. Although α-syn was first discovered in patients with familial forms of PD and its A53T, E46K and A30P mutations had also been shown to be related to the familial form of PD [4,86], α-syn appeared to be a vital genetic factor of PD pathology both in familial and sporadic cases. Its expression level was positively correlated with the severity of PD [87]. Accumulating evidences proved that the abnormal deposit of α-syn could result in neurotoxicity through a gain-of-function manner [88-90]. The research carried out by Recasens et al. [91] showed that the nigrostriatal neurons in mice and monkeys appeared to degenerate slowly after injection of Lewy bodies extracted from patients with PD. And the endogenous α-syn had also been prone to aggregate triggered by exogenous human α-syn. Besides, slight loss of dopaminergic neurons and significant reduction of DA were observed in the SNpc of α-syn transgenic mice. And the transgenic mice exhibited progressive PDlike motor deficits and anxiety, in the brain of which there exist α-syn aggregates [92]. Our previous studies indicated that overexpression of α-syn induced the production of oxidative stress, intracytoplasmic DA and endogenous neurotoxins, which may further cause cell death [49].
Based on the impact of LBs in the development of PD, the inclination for α-syn to aggregation has been explored intensely. Multiple lines of proofs have shown that α-syn can be transformed from its normal structure into an unusual disease-associated structure influenced by many factors containing inhesion factors and external factors [6,7,88,93,94]. On the one hand, within α-syn itself, some of its mutations can induce a more toxic effect on DA neurons and were more vulnerable to aggregation [88,95,96]. For example, A53T mutant-type α-syn protein had the higher propensity to aggregate compared with the wild-type α-syn protein, as a result of the increased β-sheet formation and lack of strong intramolecular long-range interactions in the N-terminal region [96]. On the other hand, a lot of evidences indicated that aggregated α-syn could be facilitated by many environmental neurotoxins. Agrochemicals such as paraquat(PQ), rotenone, maneb were believed to be important factors that gave rise to the accumulation and aggregation of α-syn and could be the contributors to the occurrence and development of PD [97-100]. Moreover, heavy metals have also been paid more attention, because α-syn has multiple binding sites of metal [101]. Many types of research showed that the high metal ions could aggravate the formation of α-syn fibrillation and aggregation, result in the cell injury and consequently contribute to the progressive of PD [7,102-106]. And MPTP, a well-known neurotoxin, is also associated with the aggregation of α-syn, which was confirmed by a study that the oligomers of SNCA (51 kD) increased in the SNpc of monkeys administrated intravenously with MPTP (0.3 mg/kg) twice a week for three months [107]. Moreover, there are other environmental factors including solvents and nanoparticles that have been proved to be associated with α-syn aggregation. For example, when the animals were exposed to Trichloroethylene (TCE), one kind of solvents, they showed the nigrostriatal degeneration, the onset of parkinsonian features, a loss of nigral dopaminergic neurons and increased nigral accumulation of α-syn [108,109]. Now, nanoparticles (NPs) have been used as new approaches for the treatment of PD [110,111]. However, in some conditions, the NPs can induce the adverse effect on the DA neurons and may accelerate the development of PD possibly through decreasing DA concentration and TH levels, increasing oxidative stress, changing mitochondrial metabolism, inhibiting cell proliferation and enhancing cell apoptosis [112-114]. It is worthy to note that NPs can also regulate the expression and aggregation of α-syn [115-117]. These data above implicated that the neurotoxicity induced by internal and external factors was ascribed to the formation of aberrant α-syn. Therefore, growing α-syn aggregation is a crucial procedure in the development of PD.
The evidences above have demonstrated that soluble a-syn monomers can be triggered by multiple environmental factors to transform into fibrillation and aggregation. But the reason for this convention induced by these factors needs to be addressed. In recent years, the epigenetic mechanism has been thought as a potential mechanism involved in PD and could be a link between environmental factors and α-syn aggregation. Epigenetic mechanism is a mechanism that regulates gene expression but not affects DNA sequence, including DNA methylation, histone modification and noncoding RNA regulation. Firstly, the effect of DNA methylation on α-syn aggregation mainly focuses on the regulation of its gene SNCA. There is evidence that human SNCA intron 1 is hypomethylated in sporadic PD patients’ brain including substantia nigra, putamen, and cortex, resulting in the activation of SNCA expression [118]. Later on, Tan et al. detected the methylation of SNCA CpG-2 including the 2nd, 4th and 9th CpG sites. They found that the methylation levels of each CpG2 sites were all significantly reduced in leukocytes of PD patients compared with that in healthy controls, especially in the 4th CpG site [119]. Also, Desplats et al. found that the accumulation and/or aggregation of α-syn may shift DNMT1 subcellular location from the nuclear to the cytoplasm, which might directly cause a decay for DNA methylation of PD related genes such as SNCA, SEPW1 and PRKAR2A [120]. These evidences provide the possibility that reduced methylation of SNCA promoter induced by certain factors can activate the α-syn expression and then the increased α-syn can decrease its DNA methylation, finally resulting in α-syn aggregation. Secondly, histone modification is also an important mechanism of α-syn aggregation, and its effect mainly focuses on the histone acetylation [121]. Recently, the team from Freed, presented that long-term administration of a histone deacetylase (HDAC) inhibitor, phenylbutyrate, reduced α-syn aggregation in the brain and improved motor and cognitive function in aged transgenic mice expressing a tyrosine to cysteine mutant human α-syn [122]. Similarly, Valproic acid, an inhibitor of histone deacetylase, counteracted the alteration of α-syn, the death of nigral neurons and the declined levels of striatal DA in the rotenone-treated rats [123]. Nevertheless, there were some evidences showing that the inhibition of HDAC6 exerted the detrimental effects including the accumulation of α-syn oligomers, the aggravated degeneration of nigrostriatal DA neurons and behavioral deficits [124- 126]. In this line, HDAC or its inhibitors have been shown to exert effective roles in the inhibition of α-syn misfolding and treatment of PD. Thirdly, the physiologic and physiopathologic roles of noncoding RNAs (ncRNAs), especially microRNAs (miRNAs), have been investigated intensively because of their diagnosis and therapeutic features of the neurodegenerative diseases including PD [127-129]. Accumulating evidences demonstrated that several miRNAs could regulate the expression of α-syn, which is involved in the neurotoxin of α-syn to DA neurons [130-136]. For example, overexpression of miR-21 promoted the expression of α-syn protein, while inhibition of miR-21 effectively decreased the expression of α-syn [135]. Also, Niu et al. observed that miR-133b ameliorated the axon degeneration in PC12 cells treated with MPP+ by decreasing α-syn expression and increasing Bcl-2/Bax ratio, indicating that miR-133b is implicated in the pathogenesis of PD [134]. Therefore, miRNAs could be another mechanism of α-syn aggregation and provide new diagnostic and therapeutic avenues for PD.
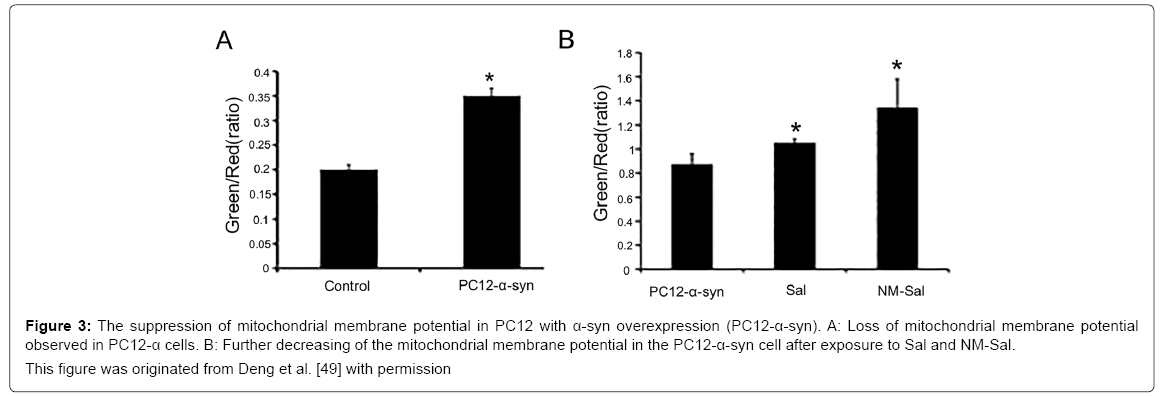
Although it is known that aggregated α-syn is a clinicopathological feature of PD, its exact mechanism for DA neuron degeneration and death in Parkinson disease has not yet been clarified. One of the popular hypotheses for the pathogenic mechanism of α-syn is that aggregated α-syn can inhibit the function of mitochondria, which further leads to the death of dopaminergic neurons. Many mitochondria exist in the synapses of Dopaminergic neurons, the impairment of which is an initial step for cell apoptosis. In this mechanism, aggregated α-syn can interact with mitochondrial membranes and further induce the release of respiratory protein cytochrome c, an increase of mitochondrial calcium, complex I dysfunction, a decrease of membrane potential and mitochondrial swelling, finally leading to the mitochondrial dysfunction [137-140]. And mitochondrial dysfunction can generate the excessive ROS and eventually induce death of dopaminergic neuron [141]. Figure 3 showed a decreased mitochondrial membrane potential in PC12 cells with α-syn overexpression compared to that in controls, and this tendency was intensified after exposure to Sal and NM-Sal [49].These findings suggested that the mitochondrial dysfunction is the key link between the neurotoxin of α-syn and PD.
Figure 3: The suppression of mitochondrial membrane potential in PC12 with α-syn overexpression (PC12-α-syn). A: Loss of mitochondrial membrane potential
observed in PC12-α cells. B: Further decreasing of the mitochondrial membrane potential in the PC12-α-syn cell after exposure to Sal and NM-Sal.
This figure was originated from Deng et al. [49] with permission
CTIQs and α-syn Aggregation
Although exogenous neurotoxins exert the multiple effects in the pathogenesis of PD, most of people have less chance to contact with them. For this reason, endogenous neurotoxins might be the central hub in the pathogenesis of PD. However, it is still not clear that how these CTIQs perform their neurotoxic role in PD patients. According to the information in part one, the groups of CTIQs can be biosynthesized in human brain by the certain synthetase, which has been evidenced by the fact that Deng’s team confirmed the existence of Sal synthetase [22].
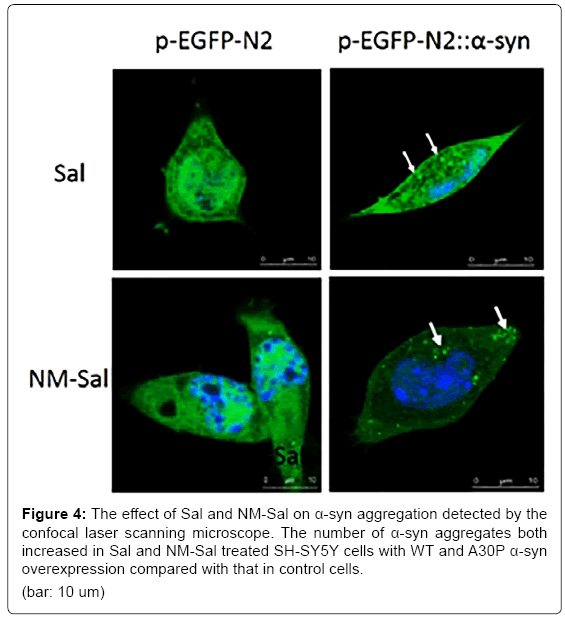
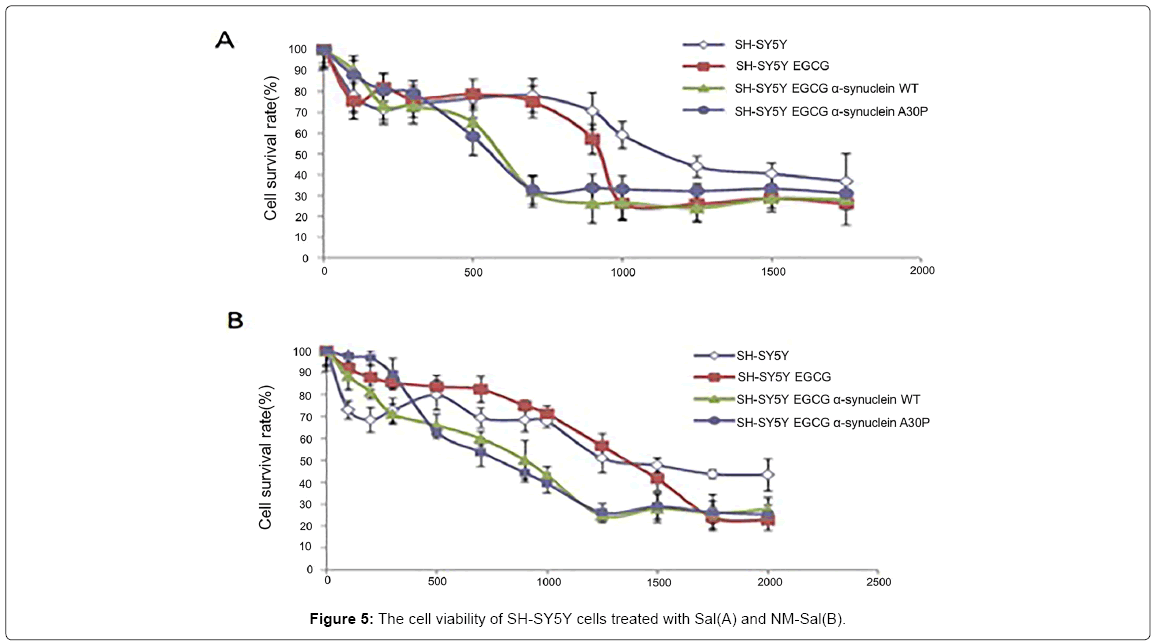
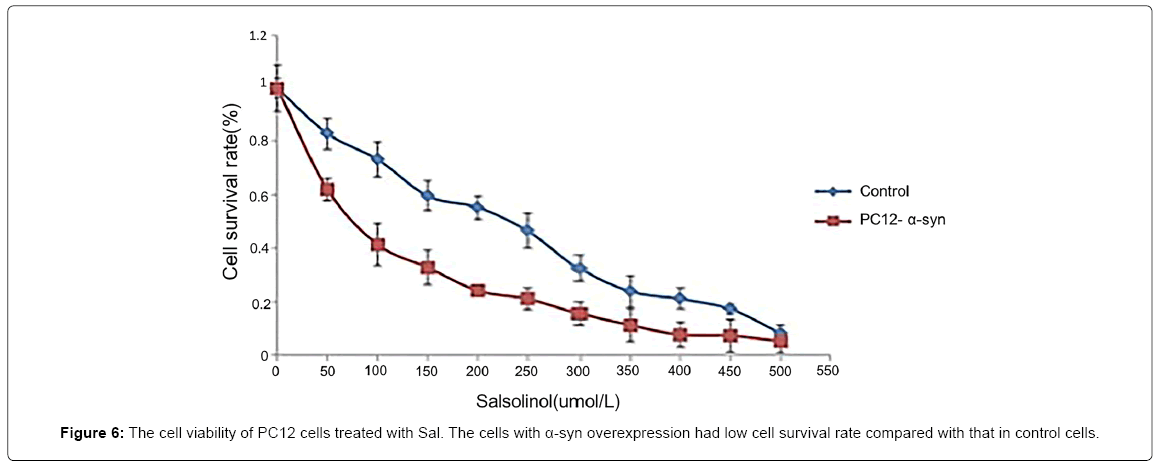
Since the CTIQs and α-syn are both crucial elements in the pathogenesis of PD, we anticipate that there is a relationship between them. Recently, Kurnik et al. demonstrated a similar phenomenon. They discovered that Sal led to the generation of pathological aggregates of α-syn and initiated cell apoptosis in the myenteric neurons [142]. A few years ago, Kheradpezhouh et al. indicated that the expression of α-syn also increased in human dopaminergic SK-N-SH cells treated with different doses of Sal for 24 h [143]. In this line, we observed that Sal and NM-Sal could also enhance the aggregation of α-syn in SHSY5Y cells with α-syn overexpression. The pictures from confocal laser scanning microscope showed that the number of the α-syn aggregates increased both in Sal and NM-Sal treated SH-SY5Y cells with α-syn WT and A30P overexpression compared with that in control cells (Figure 4). And MTT assay displayed that the cell viabilities were significantly inhibited in SH-SYBY cells with α-syn WT and A30P overexpression compared with that in control cells after treatment with different doses of Sal and NM-Sal (Figure 5). Similar results were also observed in PC12 cells with α-syn overexpression after treatment with Sal (Figure 6). These results above suggest that CTIQs enhanced the neurotoxin of α-syn.
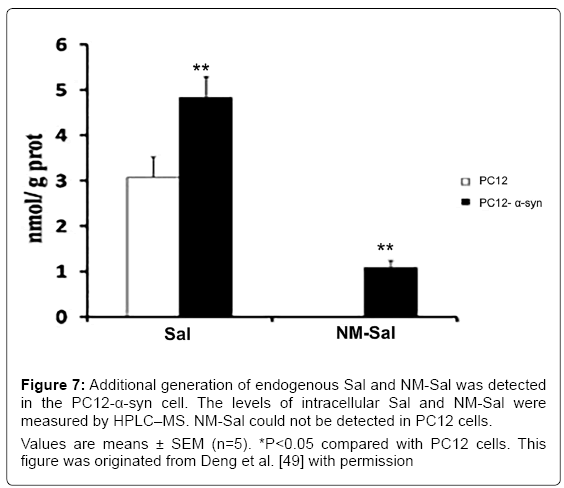
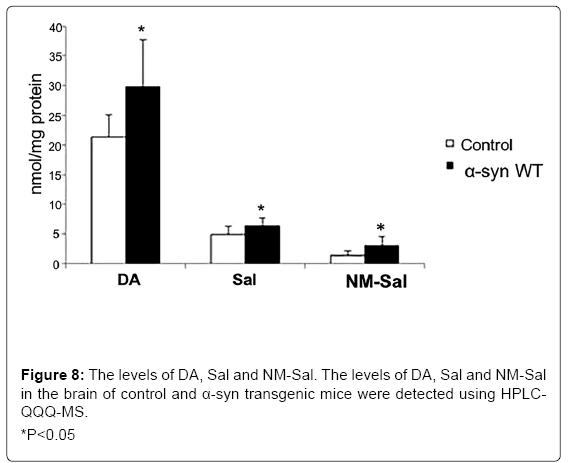
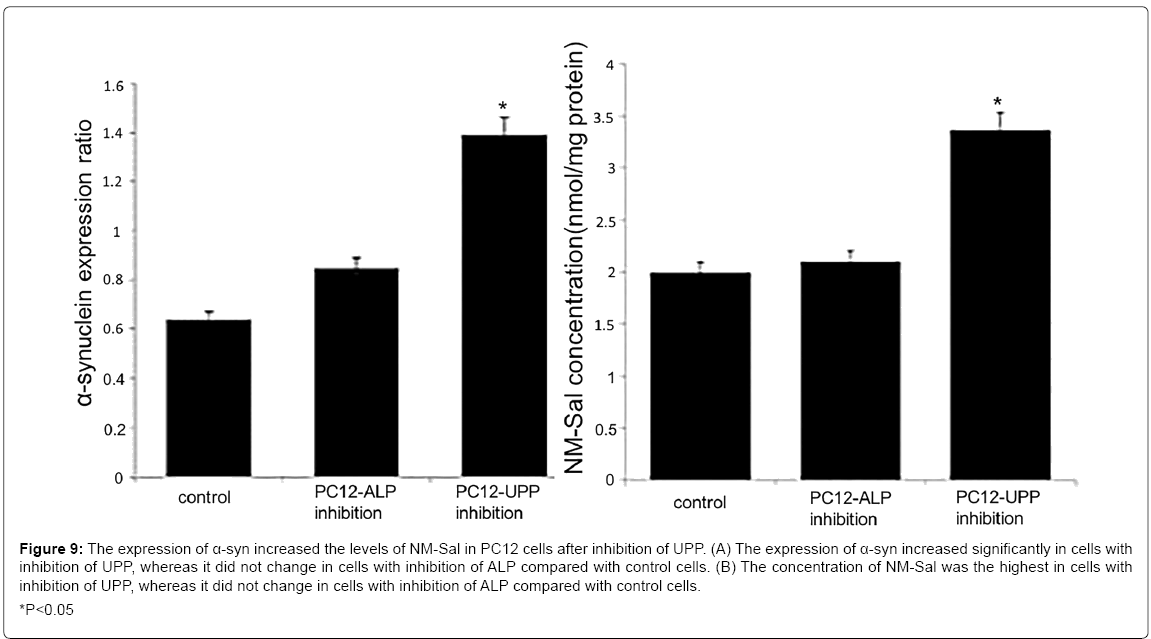
On the other hand, our group also found that the overexpression of a-syn induced the production of Sal and NM-Sal [49] (Figure 7). It may be a bridge between α-syn and cell death. Similar results were also observed in a transgenic mice model. The levels of DA, Sal and NM-Sal were all elevated in the brain of α-syn transgenic mice compared with that of control mice (Figure 8). It is reported that the approaches for the degradation of α-syn mainly contain autophagy-lysosomal pathway (ALP) and ubiquitin-proteasome pathway (UPP) [144]. Therefore, after inhibition of both pathways, we detected the levels of NM-Sal by HPLCQQQ- MS. The results indicated that the levels of NM-Sal increased in UPP inhibition cells compared with that in control cells, suggesting that the elevated expression of α-syn due to the inhibition of UPP increased the levels of NM-Sal in PC12 cells (Figure 9). Also, there is a report that the concentration of ADTIQ was upregulated significantly in the brain of A53T or A30P α-syn transgenic mice. And in vitro, SHSY5Y cells treated with ADTIQ displayed a decreased viability with mitochondrial damage. Both data implicated the interaction between ADTIQ and α-syn [78]. These data above give us insight that the neurotoxins induced by the elevated expression of α-syn may ascribe to the generation of CTIQs.
Figure 7: Additional generation of endogenous Sal and NM-Sal was detected
in the PC12-α-syn cell. The levels of intracellular Sal and NM-Sal were
measured by HPLC–MS. NM-Sal could not be detected in PC12 cells.
Values are means ± SEM (n=5). *P<0.05 compared with PC12 cells. This
figure was originated from Deng et al. [49] with permission
Figure 9: The expression of α-syn increased the levels of NM-Sal in PC12 cells after inhibition of UPP. (A) The expression of α-syn increased significantly in cells with
inhibition of UPP, whereas it did not change in cells with inhibition of ALP compared with control cells. (B) The concentration of NM-Sal was the highest in cells with
inhibition of UPP, whereas it did not change in cells with inhibition of ALP compared with control cells.
*P<0.05
Deeply, according to the information above, the mitochondrial dysfunction exists both in α-syn overexpression models and in CTIQs treated models [25,38,46,47,49,50,65,66,78,82,137-140]. Therefore, the contribution of α-syn and CTIQs to the pathogenesis of PD could both ascribe to the damaged mitochondria, which further verified the interrelation between CTIQs and α-syn.
Conclusion
The two pathological hallmarks of PD are the selective loss of DA neurons in the SNpc and accumulation of LBs and Lewy neurites [145]. Since the first literary description of PD in 1817 [146], the investigations on pathological mechanisms of PD keep emerging in an endless stream, however, its exact mechanisms are still unclear. PD is a kind of multifactor diseases, the etiology of which cannot be entirely explained by any single factor. It is generally accepted that PD results from the combination of genetic and environmental factors.
It is evident that the combined impact of genetic and environmental factors on PD was far beyond that of either alone. Since α-syn aggregation has been shown to be one of the hallmarks in PD, the efforts on the pathological role of α-syn sprung up. Although many data revealed that environmental factors such as agrochemicals, MPTP were involved in the misfolding of α-syn, few people have a chance of exposure to them. Recently, endogenous neurotoxins such as Sal and NM-Sal are believed to play a crucial role in the pathogenesis of PD, especially in the aggregation of α-syn, as result of their biosynthetic property in vivo.
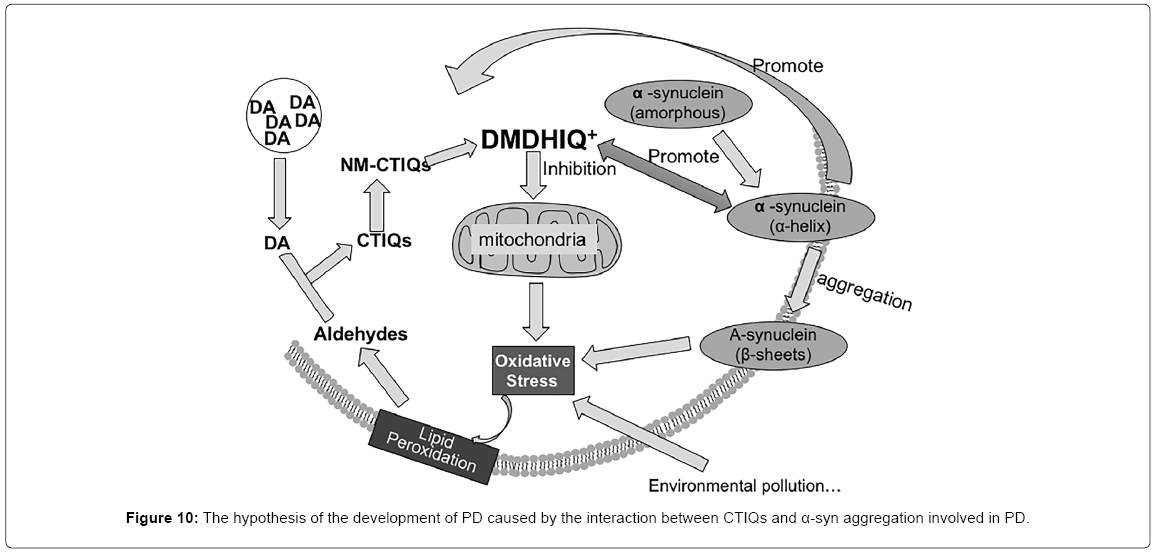
According to the previous studies, we developed a hypothesis that when oxidative stress occurs, the aldehydic product of lipid peroxidation condenses with DA to form a series of CTIQs such as Sal in DA neurons. The production of CTIQs induced by the oxidative stress enhances the misfolding of α-syn, in turn, overexpression of α-syn further upregulates the levels of CTIQs. This progress makes a vicious cycle, which induces the mitochondrial damage, finally leading to the death of dopaminergic neurons (Figure 10). Therefore, these continuing explorations provide the possibility of discovering new biomarkers for early diagnosis and establishing a novel interventional therapy for PD.
Acknowledgement
This manuscript was supported by grants 2016M591089 from the China Postdoctoral Science Foundation. The authors have no conflicts of interest.
References
- Lees AJ, Hardy J, Revesz T (2009) Parkinson's disease. Lancet 373: 2055-2066.
- Calabresi P, Ghiglieri V, Mazzocchetti P, Corbelli I, Picconi B (2015) Levodopa-induced plasticity: A double-edged sword in Parkinson's disease? Philos Trans R Soc Lond B Biol Sci 370.
- Iderberg H, McCreary AC, Varney MA, Kleven MS, Koek W, et al. (2015) NLX-112, a novel 5-HT1A receptor agonist for the treatment of L-DOPA-induced dyskinesia: Behavioral and neurochemical profile in rat. Exp Neurol 271: 335-350.
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with parkinson's disease. Science 276: 2045-2047.
- Dehay B, Fernagut PO (2016) Alpha-synuclein-based models of Parkinson’s disease. Rev Neurol (Paris) 172: 371-378.
- Chen M, Wang T, Yue F, Li X, Wang P, et al. (2015) Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury and cerebral alpha-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience 286: 383-392.
- Li WJ, Jiang H, Song N, Xie JX (2010) Dose- and time-dependent alpha-synuclein aggregation induced by ferric iron in SK-N-SH cells. Neurosci Bull 26: 205-210.
- Tharakan B, Dhanasekaran M, Manyam BV (2012) Differential effects of dopaminergic neurotoxins on DNA cleavage. Life Sci 91: 1-4.
- Kang JH (2012) Salsolinol, a tetrahydroisoquinoline-derived neurotoxin, induces oxidative modification of neurofilament-L protection by histidyl dipeptides. BMB Rep 45: 114-119.
- Deng Y, Zhang Y, Duan J, Xiong Y, Qing H (2012) An Overview of endogenous catechol-isoquinolines and their related enzymes: Possible biomarkers for Parkinson’s disease. Curr Transl Geriatr Exp Gerontol Rep 1: 59-67.
- Naoi M, Maruyama W, Nagy GM (2004) Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: Occurrence, metabolism and function in human brains. Neurotoxicology 25: 193-204.
- Melchior C, Collins MA (1982) The route and significance of endogenous synthesis of alkaloids in animals. Crit Rev Toxicol 9: 313-356.
- Sandler M, Carter SB, Hunter KR, Stern GM (1973) Tetrahydroisoquinoline alkaloids: In vivo metabolites of L-dopa in man. Nature 241: 439-443.
- Scholz J, Klingemann I, Moser A (2004) Increased systemic levels of norsalsolinol derivatives are induced by levodopa treatment and do not represent biological markers of parkinson's disease. J Neurol Neurosurg Psychiatry 75: 634-636.
- Deng Y, Zhang Y, Li Y, Xiao S, Song D, et al. (2012) Occurrence and distribution of salsolinol-like compound, 1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (ADTIQ) in parkinsonian brains. J Neural Transm (Vienna) 119: 435-441.
- DeCuypere M, Kalabokis VN, Hao R, Schroeder D, Miller DD, et al. (2008) Localization of N-methyl-norsalsolinol within rodent and human brain. J Neurosci Res 86: 2543-2552.
- Kotake Y, Tasaki Y, Hirobe M, Ohta S (1998) Deprenyl decreases an endogenous parkinsonism-inducing compound, 1-benzyl-1,2,3,3-tetrahydroisoquinoline in mice: In vivo and in vitro studies. Brain Research 787: 341-343.
- Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R (2008) Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol 153: 6-20.
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, et al. (1996) Immunohistochemical detection of 4-hydroxynonenal protein adducts in parkinson disease. Proc Natl Acad Sci U S A 93:2696-2701.
- Bolner A, Micciolo R, Bosello O, Nordera GP (2016) A panel of oxidative stress markers in parkinson's disease. Clin Lab 62: 105-112.
- Bolisetty S, Jaimes EA (2013) Mitochondria and reactive oxygen species: Physiology and pathophysiology. Int J Mol Sci 14: 6306-6344.
- Chen XC, Chen Y, Wu GS, Lu JQ, Iqbal J, et al. (2013) Existence and characterization of salsolinol synthase in neuronal cells and rat brain. Neurochemical Journal 7: 192-197.
- Chen XC, Arshad A, Qing H, Wang R, Lu JQ, et al. (2011) Enzymatic condensation of dopamine and acetaldehyde: A salsolinol synthase from rat brain. Biologia 66: 1183-1188.
- DeCuypere M, Lu Y, Miller DD, LeDoux MS (2008) Regional distribution of tetrahydroisoquinoline derivatives in rodent, human and parkinson's disease brain. J Neurochem 107: 1398-1413.
- Mozdzen E, Kajta M, Wasik A, Lenda T, Antkiewicz-Michaluk L (2015) Salsolinol, an endogenous compound triggers a two-phase opposing action in the central nervous system. Neurotox Res 27: 300-313.
- Wanpen S, Kooncumchoo P, Shavali S, Govitrapong P, Ebadi M (2007) Salsolinol, an endogenous neurotoxin, activates JNK and NF-kappaB signaling pathways in human neuroblastoma cells. Neurochem Res 32: 443-450.
- Moser A, Kompf D (1992) Presence of Methyl-6,7-Dihydroxy-1,2,3,4-Tetrahydroisoquinolines, derivatives of the neurotoxin isoquinoline, in parkinsonian lumbar Csf. Life Sci 50: 1885-1891.
- Maruyama W, Abe T, Tohgi H, Dostert P, Naoi M (1996) A dopaminergic neurotoxin, (R)-N-methylsalsolinol, increases in parkinsonian cerebrospinal fluid. Ann Neurol 40: 119-122.
- Lin X, Shi M, Masilamoni JG, Dator R, Movius J, et al. (2015) Proteomic profiling in MPTP monkey model for early Parkinson disease biomarker discovery. Biochim Biophys Acta 1854: 779-787.
- Yabuki Y, Ohizumi Y, Yokosuka A, Mimaki Y, Fukunaga K (2014) Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience 259: 126-141.
- Philippens IH, Joosen MJ, Ahnaou A, Andres I, Drinkenburg WP (2014) Anti-Parkinson effects of a selective alpha2C-adrenoceptor antagonist in the MPTP marmoset model. Behav Brain Res 269: 81-86.
- Steidinger TU, Slone SR, Ding H, Standaert DG, Yacoubian TA (2013) Angiogenin in Parkinson disease models: Role of Akt phosphorylation and evaluation of AAV-mediated angiogenin expression in MPTP treated mice. PLoS ONE 8: e56092.
- Langston JW, Ballard PA Jr (1983) Parkinson's disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N Engl J Med 309: 310.
- Hare DJ, Adlard PA, Doble PA, Finkelstein DI (2013) Metallobiology of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Metallomics 5: 91-109.
- Han Y, Gao P, Qiu S, Zhang L, Yang L, et al. (2016) MTERF2 contributes to MPP(+)-induced mitochondrial dysfunction and cell damage. Biochem Biophys Res Commun 471: 177-183.
- Richardson JR, Caudle WM, Guillot TS, Watson JL, Nakamaru-Ogiso E, et al. (2007) Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicol Sci 95: 196-204.
- Rekha KR, Selvakumar GP (2014) Gene expression regulation of Bcl2, Bax and cytochrome-C by geraniol on chronic MPTP/probenecid induced C57BL/6 mice model of Parkinson's disease. Chem Biol Interact 217: 57-66.
- Kang JH (2013) Salsolinol, a catechol neurotoxin, induces oxidative modification of cytochrome c. BMB Rep 46: 119-123.
- Zhu W, Wang D, Zheng J, An Y, Wang Q, et al. (2008) Effect of (R)-salsolinol and N-methyl-(R)-salsolinol on the balance impairment between dopamine and acetylcholine in rat brain: Involvement in pathogenesis of Parkinson disease. Clin Chem 54: 705-712.
- Musshoff F, Lachenmeier DW, Kroener L, Schmidt P, Dettmeyer R, et al. (2003) Simultaneous gas chromatographic-mass spectrometric determination of dopamine, norsalsolinol and salsolinol enantiomers in brain samples of a large human collective. Cell Mol Biol (Noisy-le-grand) 49: 837-849.
- Musshoff F, Schmidt P, Dettmeyer R, Priemer F, Jachau K, et al. (2000) Determination of dopamine and dopamine-derived (R)-/(S)-salsolinol and norsalsolinol in various human brain areas using solid-phase extraction and gas chromatography/mass spectrometry. Forensic Sci Int 113: 359-366.
- Antkiewicz-Michaluk L, Romañska I, Papla I, Michaluk J, Bakalarz M, et al. (2000) Neurochemical changes induced by acute and chronic administration of 1,2,3,4-tetrahydroisoquinoline and salsolinol in dopaminergic structures of rat brain. Neuroscience 96: 59-64.
- Briggs GD, Nagy GM, Dickson PW (2013) Mechanism of action of salsolinol on tyrosine hydroxylase. Neurochem Int 63: 726-731.
- Misztal T, Hasiec M, Tomaszewska-Zaremba D, Dobek E, Fulop F, et al. (2011) The influence of salsolinol on dopaminergic system activity within the mediobasal hypothalamus of anestrous sheep: A model for studies on the salsolinol-dopamine relationship. Acta Neurobiol Exp (Wars) 71: 305-312.
- Brown D, Tamas A, Reglödi D, Tizabi Y (2013) PACAP protects against salsolinol-induced toxicity in dopaminergic SH-SY5Y cells: Implication for Parkinson's disease. J Mol Neurosci 50: 600-607.
- Wszelaki N, Melzig MF (2012) Low level of glutathione can intensify the toxic effect of salsolinol in SH-SY5Y neuroblastoma cell line. Neurotoxicology 33: 424-428.
- Shukla A, Mohapatra TM, Agrawal AK, Parmar D, Seth K (2013) Salsolinol induced apoptotic changes in neural stem cells: Amelioration by neurotrophin support. Neurotoxicology 35: 50-61.
- Kim SS, Kang JY, Kang JH (2016) Oxidative modification of human ceruloplasmin induced by a catechol neurotoxin, salsolinol. BMB Rep 49: 45-50.
- Zhang Y, Ma H, Xie B, Han C, Wang C, et al. (2013) Alpha-synuclein overexpression induced mitochondrial damage by the generation of endogenous neurotoxins in PC12 cells. Neurosci Lett, 547: 65-69.
- Su Y, Duan J, Ying Z, Hou Y, Zhang Y, et al. (2013) Increased vulnerability of parkin knock down PC12 cells to hydrogen peroxide toxicity: The role of salsolinol and NM-salsolinol. Neuroscience 233: 72-85.
- Qualls Z, Brown D, Ramlochansingh C, Hurley LL, Tizabi Y (2014) Protective effects of curcumin against rotenone and salsolinol-induced toxicity: Implications for Parkinson’s disease. Neurotox Res 25: 81-89.
- Mravec B (2006) Salsolinol, a derivate of dopamine, is a possible modulator of catecholaminergic transmission: A review of recent developments. Physiol Res 55: 353-364.
- Nagasawa Y, Ueoka R, Yamanokuchi R, Horiuchi N, Ikeda T, et al. (2011) Isolation of salsolinol, a tetrahydroisoquinoline alkaloid, from the marine sponge Xestospongia cf. vansoesti as a proteasome inhibitor. Chem Pharm Bull (Tokyo) 59: 287-290.
- MusshoffF, Lachenmeier DW, Schmidt P, Dettmeyer R, Madea B (2005) Systematic regional study of dopamine, norsalsolinol and (R/S)-salsolinol levels in human brain areas of alcoholics. Alcohol Clin Exp Res 29: 46-52.
- Kuklinski NJ, Berglund EC, Engelbreksson J, Ewing AG (2010) Determination of salsolinol, norsalsolinol and twenty-one biogenic amines using micellar electrokinetic capillary chromatography-electrochemical detection. Electrophoresis 31: 1886-1893.
- Maruyama W, Takahashi T, Minami M, Takahashi A, Dostert P, et al. (1993) Cytotoxicity of dopamine-derived 6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolines. Adv Neurol 60: 224-230.
- Maruyama Y, Teraoka H, Iwata H, Kazusaka A, Fujita S (2001) Inhibitory effects of endogenous dopaminergic neurotoxin, norsalsolinol on dopamine secretion in PC12 rat pheochromocytoma cells. Neurochem Int 38: 567-572.
- Kobayashi H, Fukuhara K, Tada-Oikawa S, Yada Y, Hiraku Y, et al. (2009) The mechanisms of oxidative DNA damage and apoptosis induced by norsalsolinol, an endogenous tetrahydroisoquinoline derivative associated with Parkinson’s disease. J Neurochem 108: 397-407.
- Scholz J, Toska K, Luborzewski A, Maass A, Schünemann V, et al. (2008) Endogenous tetrahydroisoquinolines associated with Parkinson's disease mimic the feedback inhibition of tyrosine hydroxylase by catecholamines. FEBS J 275: 2109-2121.
- Naoi M, Matsuura S, Takahashi T, Nagatsu T (1989) A N-methyltransferase in human brain catalyses N-methylation of 1,2,3,4-tetrahydroisoquinoline into N-methyl-1,2,3,4-tetrahydroisoquinoline, a precursor of a dopaminergic neurotoxin, N-methylisoquinolinium ion. Biochem Biophys Res Commun 161: 1213-1219.
- Zhang Y, Wang L, Mu X, Duan J, Zhu Y, et al. (2011) Assessment of salsolinol N-methyltransferase activity in rat peripheral lymphocytes by liquid chromatography-electrospray time-of-flight mass spectrometry. Anal Bioanal Chem 399: 3541-3545.
- Maruyama W, Sobue G, Matsubara K, Hashizume Y, Dostert P, et al. (1997) A dopaminergic neurotoxin, 1(R), 2(N)-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, N-methyl(R)salsolinol, and its oxidation product, 1,2(N)-dimethyl-6,7-dihydroxyisoquinolinium ion, accumulate in the nigro-striatal system of the human brain. Neurosci Lett 223: 61-64.
- Takahashi T, Deng Y, Maruyama W, Dostert P, Kawai M, et al. (1994) Uptake of a neurotoxin-candidate, (R)-1,2-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline into human dopaminergic neuroblastoma SH-SY5Y cells by dopamine transport system. J Neural Transm Gen Sect 98: 107-118.
- Maruyama W, Narabayashi H, Dostert P, Naoi M (1996) Stereospecific occurrence of a parkinsonism-inducing catechol isoquinoline, N-methyl(R)salsolinol, in the human intraventricular fluid. J Neural Transm 103: 1069-1076.
- Akao Y, Maruyama W, Shimizu S, Yi H, Nakagawa Y, et al. (2002) Mitochondrial permeability transition mediates apoptosis induced by N-methyl(R)salsolinol, an endogenous neurotoxin and is inhibited by Bcl-2 and rasagiline, N-propargyl-1(R)-aminoindan. J Neurochem 82: 913-923.
- Jantas D, Pytel M, Mozrzymas JW, Leskiewicz M, Regulska M, et al. (2008) The attenuating effect of memantine on staurosporine-, salsolinol- and doxorubicin-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurochem Int 52: 864-877.
- Maruyama W, Naoi M, Kasamatsu T, Hashizume Y, Takahashi T, et al. (1997) An endogenous dopaminergic neurotoxin, N-methyl-(R)-salsolinol, induces DNA damage in human dopaminergic neuroblastoma SH-SY5Y cells. J Neurochem 69: 322-329.
- Arshad A, Chen X, Cong Z, Qing H, Deng Y (2014) TRPC1 protects dopaminergic SH-SY5Y cells from MPP+, salsolinol and N-methyl-(R)-salsolinol-induced cytotoxicity. Acta Biochim Biophys Sin (Shanghai) 46: 22-30.
- Mao J, Ma H, Xu Y, Su Y, Zhu H, et al. (2013) Increased levels of monoamine-derived potential neurotoxins in fetal rat brain exposed to ethanol. Neurochem Res 38: 356-363.
- Wang R, Qing H, Liu XQ, Zheng XL, Deng YL (2008) Iron contributes to the formation of catechol isoquinolines and oxidative toxicity induced by overdose dopamine in dopaminergic SH-SY5Y cells. Neurosci Bull 24: 125-132.
- Deng Y, Luan Y, Qing H, Xie H, Lu J, et al. (2008) The formation of catechol isoquinolines in PC12 cells exposed to manganese. Neurosci Lett 444: 122-126.
- Deng Y (2001) ADTIQ was found in the PD patients' brains. 121th Annual meeting of the American neurological association in Chicago.
- Xie BJ, Xiong Y, Ullah K, Peng L, Lin FK, et al. (2013) Determination of endogenous neurotoxin 1-Acetyl-6,7-Dihydroxyl-1,2,3,4-Tetrahydroisoquinoline in rat substantia nigra by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Lett 46: 2828-2834.
- de Arriba SG, Stuchbury G, Yarin J, Burnell J, Loske C, et al. (2007) Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells - protection by carbonyl scavengers. Neurobiol Aging 28: 1044-1050.
- Lu J, Randell E, Han Y, Adeli K, Krahn J, et al. (2011) Increased plasma methylglyoxal level, inflammation and vascular endothelial dysfunction in diabetic nephropathy. Clin Biochem 44: 307-311.
- Xie B, Lin F, Peng L, Ullah K, Wu H, et al. (2014) Methylglyoxal increases dopamine level and leads to oxidative stress in SH-SY5Y cells. Acta Biochimica Et Biophysica Sinica 46: 950-956.
- Song DW, Xin N, Xie BJ, Li YJ, Meng LY, et al. (2014) Formation of a salsolinol-like compound, the neurotoxin, 1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, in a cellular model of hyperglycemia and a rat model of diabetes. Int J Mol Med 33: 736-742.
- Xie B, Lin F, Ullah K, Peng L, Ding W, et al. (2015) A newly discovered neurotoxin ADTIQ associated with hyperglycemia and Parkinson's disease. Biochem Biophys Res Commun 459: 361-366.
- Kohta R, Kotake Y, Hosoya T, Hiramatsu T, Otsubo Y, et al. (2010) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline binds with tubulin beta, a substrate of parkin and reduces its polyubiquitination. J Neurochem 114: 1291-1301.
- Wąsik A, Romańska I, Michaluk J, Kajta M, Antkiewicz-Michaluk L (2014) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline, an endogenous neurotoxic compound, disturbs the behavioral and biochemical effects of L-DOPA: In vivo and ex vivo studies in the rat. Neurotox Res 26: 240-254.
- Wasik A, Kajta M, Lenda T, Antkiewicz-Michaluk L (2014) Concentration-dependent opposite effects of 1-benzyl-1,2,3,4-tetrahydroisoquinoline on markers of apoptosis: In vitro and ex vivo studies. Neurotox Res 25: 90-99.
- Kotake Y, Sekiya Y, Okuda K, Ohta S (2007) Cytotoxicity of 17 tetrahydroisoquinoline derivatives in SH-SY5Y human neuroblastoma cells is related to mitochondrial NADH-ubiquinone oxidoreductase inhibition. Neurotoxicology 28: 27-32.
- Kotake Y, Sekiya Y, Okuda K, Ohta S (2014) Detection of a novel neurotoxic metabolite of Parkinson's disease-related neurotoxin, 1-benzyl-1,2,3,4-tetrahydroisoquinoline. J Toxicol Sci 39: 749-754.
- Contu VR, Kotake Y, Toyama T, Okuda K, Miyara M, et al. (2014) Endogenous neurotoxic dopamine derivative covalently binds to Parkinson's disease-associated ubiquitin C-terminal hydrolase L1 and alters its structure and function. J Neurochem 130: 826-838.
- Angot E, Steiner JA, Lema Tomé CM, Ekström P, Mattsson B, et al. (2012) Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS ONE 7: e39465.
- Sahay S, Ghosh D, Dwivedi S, Anoop A, Mohite GM, et al. (2015) Familial Parkinson disease-associated mutations alter the site-specific microenvironment and dynamics of alpha-synuclein. J Biol Chem 290: 7804-7822.
- van de Berg WD, Hepp DH, Dijkstra AA, Rozemuller JA, Berendse HW, et al. (2012) Patterns of alpha-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonism Relat Disord 18 Suppl 1: S28-30.
- Lu J, Sun F, Ma H, Qing H, Deng Y (2015) Comparison between alpha-synuclein wild-type and A53T mutation in a progressive Parkinson’s disease model. Biochem Biophys Res Commun 464: 988-993.
- Bendor JT, Logan TP, Edwards RH (2013) The function α-Synuclein. Neuron 79: 1044-1066.
- Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M (2010) Seeded aggregation and toxicity of {alpha}-synuclein and tau: Cellular models of neurodegenerative diseases. J Biol Chem 285: 34885-34898.
- Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, et al. (2014) Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75: 351-362.
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, et al. (2012) A progressive mouse model of Parkinson's disease: The Thy1-aSyn ("Line 61") mice. Neurotherapeutics 9: 297-314.
- Dokleja L, Hannula MJ, Myöhänen TT (2014) Inhibition of prolyl oligopeptidase increases the survival of alpha-synuclein overexpressing cells after rotenone exposure by reducing alpha-synuclein oligomers. Neurosci Lett 583: 37-42.
- Xu B, Wu SW, Lu CW, Deng Y, Liu W, et al. (2013) Oxidative stress involvement in manganese-induced alpha-synuclein oligomerization in organotypic brain slice cultures. Toxicology 305: 71-78.
- Pandey N, Schmidt RE, Galvin JE (2006) The alpha-synuclein mutation E46K promotes aggregation in cultured cells. Exp Neurol 197: 515-520.
- Coskuner O, Wise-Scira O (2013) Structures and free energy landscapes of the A53T mutant-type alpha-synuclein protein and impact of A53T mutation on the structures of the wild-type alpha-synuclein protein with dynamics. ACS Chem Neurosci 4: 1101-1113.
- Zhang XF, Thompson M, Xu YH (2016) Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab Invest 96: 496-507.
- Liu Y, Sun JD, Song LK, Li J, Chu SF, et al. (2015) Environment-contact administration of rotenone: A new rodent model of Parkinson's disease. Behav Brain Res 294: 149-161.
- Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, et al. (2011) Parkinson's disease risk from ambient exposure to pesticides. Eur J Epidemiol 26: 547-555.
- Ishido M (2007) Melatonin inhibits maneb-induced aggregation of alpha-synuclein in rat pheochromocytoma cells. J Pineal Res 42: 125-130.
- McDowall JS, Brown DR (2016) Alpha-synuclein: Relating metals to structure, function and inhibition. Metallomics 8: 385-397.
- Bai J, Zhang Z, Liu M, Li C (2016) α-synuclein-lanthanide metal ions interaction: Binding sites, conformation and fibrillation. BMC Biophys 9: 1.
- Bates CA, Fu S, Ysselstein D, Rochet JC, Zheng W (2015) Expression and transport of alpha-synuclein at the blood-cerebrospinal fluid barrier and effects of manganese exposure. ADMET DMPK 3: 15-33.
- Binolfi A, Rodriguez EE, Valensin D, D'Amelio N, Ippoliti E, et al. (2010) Bioinorganic chemistry of Parkinson's disease: Structural determinants for the copper-mediated amyloid formation of alpha-synuclein. Inorg Chem 49: 10668-10679.
- Lucas HR, Lee JC (2011) Copper(II) enhances membrane-bound alpha-synuclein helix formation. Metallomics 3: 280-283.
- Zhang J, Cai T, Zhao F, Yao T, Chen Y, et, al. (2012) The role of alpha-synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int J Biol Sci 8: 935-944.
- Su LY, Li H, Lv L, Feng YM, Li GD, et al. (2015) Melatonin attenuates MPTP-induced neurotoxicity via preventing CDK5-mediated autophagy and SNCA/alpha-synuclein aggregation. Autophagy 11: 1745-1759.
- Zaheer F, Slevin JT (2011) Trichloroethylene and Parkinson disease. Neurol Clin 29: 657-665.
- Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, et al. (2010) Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J Neurochem 112: 773-783.
- Bourdenx M, Daniel J, Genin E, Soria FN, Blanchard-Desce M, et al. (2016) Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy 12: 472-483.
- Leyva-Gómez G, Cortés H, Magaña JJ, Leyva-García N, Quintanar-Guerrero D, et al. (2015) Nanoparticle technology for treatment of Parkinson’s disease: The role of surface phenomena in reaching the brain. Drug Discov Today 20: 824-837.
- Coccini T, Manzo L, Bellotti V, De Simone U (2014) Assessment of cellular responses after short- and long-term exposure to silver nanoparticles in human neuroblastoma (SH-SY5Y) and astrocytoma (D384) cells. Scientific World Journal 2014: 259765.
- Feng X, Chen A, Zhang Y, Wang J, Shao L, et al. (2015) Application of dental nanomaterials: Potential toxicity to the central nervous system. Int J Nanomedicine 10: 3547-3565.
- Wu J, Wang C, Sun J, Xue Y (2011) Neurotoxicity of silica nanoparticles: Brain localization and dopaminergic neurons damage pathways. ACS Nano 5: 4476-4489.
- Alvarez YD, Fauerbach JA, Pellegrotti JV, Jovin TM, Jares-Erijman EA, et al. (2013) Influence of gold nanoparticles on the kinetics of α-synuclein aggregation. Nano Lett 13: 6156-6163.
- Joshi N, Basak S, Kundu S, De G, Mukhopadhyay A, et al. (2015) Attenuation of the early events of alpha-synuclein aggregation: A fluorescence correlation spectroscopy and laser scanning microscopy study in the presence of surface-coated Fe3O4 nanoparticles. Langmuir 31: 1469-1478.
- Wu J, Xie H (2016) Effects of titanium dioxide nanoparticles on alpha-synuclein aggregation and the ubiquitin-proteasome system in dopaminergic neurons. Artif Cells Nanomed Biotechnol 44: 690-694.
- Jowaed A, Schmitt I, Kaut O, Wüllner U (2010) Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients' brains. J Neurosci 30: 6355-6359.
- Tan YY, Wu L, Zhao ZB, Wang Y, Xiao Q, et al. (2014) Methylation of alpha-synuclein and leucine-rich repeat kinase 2 in leukocyte DNA of Parkinson's disease patients. Parkinsonism Relat Disord 20: 308-313.
- Desplats P, Spencer B, Coffee E, Patel P, Michael S, et al. (2011) Alpha-synuclein sequesters Dnmt1 from the nucleus: A novel mechanism for epigenetic alterations in lewy body diseases. J Biol Chem 286: 9031-9037.
- Harrison IF, Dexter DT (2013) Epigenetic targeting of histone deacetylase: Therapeutic potential in Parkinson's disease? Pharmacol Ther 140: 34-52.
- Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, et al. (2011) Phenylbutyrate up-regulates the DJ-1 protein and protects neurons in cell culture and in animal models of Parkinson disease. J Biol Chem 286: 14941-14951.
- Monti B, Gatta V, Piretti F, Raffaelli SS, Virgili M, et al. (2010) Valproic acid is neuroprotective in the rotenone rat model of Parkinson’s disease: Involvement of alpha-synuclein. Neurotox Res 17: 130-141.
- Du Y, Wang F, Zou J, Le W, Dong Q, et al. (2014) Histone deacetylase 6 regulates cytotoxic alpha-synuclein accumulation through induction of the heat shock response. Neurobiol Aging 35: 2316-2328.
- Du G, Liu X, Chen X, Song M, Yan Y, et al. (2010) Drosophila histone deacetylase 6 protects dopaminergic neurons against {alpha}-synuclein toxicity by promoting inclusion formation. Mol Biol Cell 21: 2128-2137.
- Su M, Shi JJ, Yang YP, Li J, Zhang YL, et al. (2011) HDAC6 regulates aggresome-autophagy degradation pathway of alpha-synuclein in response to MPP+-induced stress. J Neurochem 117: 112-120.
- Palm T, Bahnassawy L, Schwamborn J (2012) MiRNAs and neural stem cells: A team to treat Parkinson's disease? RNA Biol 9: 720-730.
- Nelson PT, Wang WX, Rajeev BW (2008) MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol 18: 130-138.
- Hunsberger JG, Fessler EB, Chibane FL, Leng Y, Maric D, et al. (2013) Mood stabilizer-regulated miRNAs in neuropsychiatric and neurodegenerative diseases: Identifying associations and functions. Am J Transl Res 5: 450-464.
- Zhang Z, Cheng Y (2014) miR-16-1 promotes the aberrant α-synuclein accumulation in parkinson disease via targeting heat shock protein 70. Scientific World Journal 2014: 938348.
- Kabaria S, Choi DC, Chaudhuri AD, Mouradian MM, Junn E (2015) Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson's disease. FEBS Lett 589: 319-325.
- Kong Y, Liang X, Liu L, Zhang D, Wan C, et al. (2015) High throughput sequencing identifies microRNAs mediating alpha-Synuclein toxicity by targeting neuroactive-ligand receptor interaction pathway in early stage of drosophila Parkinson's disease model. PLoS ONE 10:e0137432.
- Wang ZH, Zhang JL, Duan YL, Zhang QS, Li GF, et al. (2015) MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting alpha-synuclein expression in MPTP-induced Parkinson's disease mouse. Biomed Pharmacother 74: 252-256.
- Niu M, Xu R, Wang J, Hou B, Xie A (2016) MiR-133b ameliorates axon degeneration induced by MPP(+) via targeting RhoA. Neuroscience 325: 39-49.
- Su C, Yang X, Lou J (2016) Geniposide reduces alpha-synuclein by blocking microRNA-21/lysosome-associated membrane protein 2A interaction in Parkinson disease models. Brain Res 1644: 98-106.
- Thome AD, Harms AS, Volpicelli-Daley LA, Standaert D (2016) GmicroRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J Neurosci 36: 2383-2390.
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283: 9089-9100.
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65: 1272-1284.
- Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ (2014) Soluble, prefibrillar alpha-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem 289: 21490-21507.
- Plotegher N, Gratton E, Bubacco L (2014) Number and Brightness analysis of alpha-synuclein oligomerization and the associated mitochondrial morphology alterations in live cells. Biochim Biophys Acta 1840: 2014-2024.
- Requejo-Aguilar R, Bolaños JP (2016) Mitochondrial control of cell bioenergetics in Parkinson's disease. Free Radic Biol Med 100: 123-137.
- Kurnik M, Gil K, Gajda M, Thor P, Bugajski A (2015) Neuropathic alterations of the myenteric plexus neurons following subacute intraperitoneal administration of salsolinol. Folia Histochem Cytobiol 53: 49-61.
- Kheradpezhouh M, Shavali S, Ebadi M (2003) Salsolinol causing Parkinsonism activates endoplasmic reticulum-stress signaling pathways in human dopaminergic SK-N-SH cells. Neurosignals 12: 315-324.
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, et al. (2011) Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci 31: 14508-14520.
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, et al. (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in non-transgenic mice. Science 338: 949-953.
- Parkinson J (2002) An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 14: 223-236.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 6408
- [From(publication date):
February-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 5472
- PDF downloads : 936