Research Article Open Access
Cardiovascular Drug Medication Adherence Assessed by Dried Blood Spot Analysis
Sangeeta Tanna and Graham Lawson*Leicester School of Pharmacy, Faculty of Health and Life Sciences, De Montfort University, UK
- *Corresponding Author:
- Graham Lawson
Leicester School of Pharmacy, Faculty of Health and Life Sciences
De Montfort University, Leicester, LE1 9BH, UK
Tel: 44-116-257-7129
E-mail: glawson@dmu.ac.uk
Received date: December 05, 2013; Accepted date: January 27, 2014; Published date: January 29, 2014
Citation: Tanna S, Lawson G (2014) Cardiovascular Drug Medication Adherence Assessed by Dried Blood Spot Analysis. J Anal Bioanal Tech S12: 006. doi: 10.4172/2155-9872.S12-006
Copyright: © 2014 Tanna S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The use of dried blood spot (DBS) collection cards was investigated for the detection of therapeutic drugs used in cardiovascular therapy for assessing medication adherence. A liquid chromatography-high resolution mass spectrometry (LC-HRMS) method was developed for the determination of, bisoprolol, ramipril, and simvastatin. Whole blood spiked with target analytes was used to produce 30 μl blood spots on specimen collection cards for calibration purposes. An 8 mm disc was cut from the dried blood spot and extracted using methanol: water (70:30, v/v). The High Resolution MS detection, at the precise mass of the target drugs, was carried out in electrospray positive ion mode. The LC-HRMS method successfully identified control volunteers who were known to be either adherent or non-adherent. There were no false positives from volunteers taking other cardiovascular drugs or from volunteers receiving no medication. Potentially therefore the system would identify non–adherent patients based on the absence of the target analyte in the DBS sample provided.
Keywords
Keywords: Dried blood spot (DBS); LC-HRMS; Bisoprolol; Ramipril; Simvastatin; Compliance
Abbreviations
PK: Pharmacokinetics; DBS: Dried Blood Spot; LCHRMS: Liquid Chromatography-High Resolution Mass Spectrometry
Introduction
Cardiovascular disease covers a range of disorders of the heart and blood vessels, namely hypertension (high blood pressure), angina, heart attack, stroke and heart failure. It is one of the biggest killers worldwide and in the UK in 2009 accounted for one in three of all deaths [1]. There are currently around 2.7 million people in the UK living with the disease and the current medical care of such patients uses a combination of cardiovascular drugs to treat high blood pressure and statins to lower cholesterol [1]. Research by Lewin [2] showed that as many as 50% of patients ignore or do not adhere to their prescribed cardiac medication after the first year of commencing treatment. More recent research by Michael [3] indicated that 34% of patients stopped taking at least one prescription item whilst 12% stopped taking all their drugs within a month of leaving hospital. This type of medicine wastage is estimated to cost the NHS £396 million annually [4] whilst in the USA poor medication adherence is tied to an estimated $290 billion [5] annually in increased medical costs. Not only are there costs due to the wastage of drugs but non-adherence to medication can result in high rates of complications, hospital re-admissions or even death [6]. All of these have additional cost burdens which must be carried by the healthcare provider. Medication related hospital admissions cost about $100 billion per year in the USA [5].
The simple blood spot test being developed provides an elegant check of adherence to the prescribed medication by confirming the presence of the drug/s in the blood sample. Very sophisticated analysis is needed in this test since the volume of blood provided is typically 30 micro-litres versus the 3.0-5.0 ml required for a conventional liquid blood sample. From a pharmacokinetic (PK) standpoint the level of drug to be detected will depend on the prescribed dose, the bio-availability, the patient’s metabolic rate and the time the sample was collected after the initial dose. Literature PK data, for the target drugs, has therefore been collated in order to help define the detection parameters required of the analytical instrumentation. It should also be noted that certain foods e.g. grapefruit juice produce significant variations in the expected blood levels of certain drugs [7] used in the treatment of CV problems.
Methodology
We have already developed and reported an analytical procedure to identify the presence of target CV drugs in volunteer’s blood presented in the form of dried blood spots on sample cards [8]. In this work volunteers, with known prescription adherence were used to test the capability of the system to distinguish between cases of adherence and non-adherence rather than to test patient adherence to prescription. Once the capability of the system was confirmed the approach would be extended to assess patient adherence to prescribed medication versus more formal methods such as pill count. This study has received ethical approval from the De Montfort University Research Ethics Committee.
Target drugs
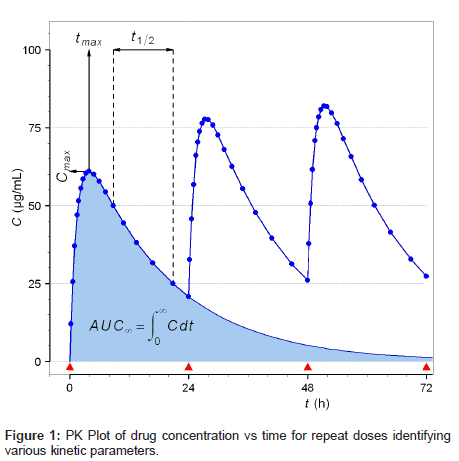
The selection of specific cardiovascular medication was informed by discussion with practicing clinicians and a survey of drugs taken by potential volunteer colleagues. Table 1 identifies the frequently prescribed CV drugs in the UK and details the essential PK information which will define the detection parameters required for this investigation. The aim of this work is to investigate if the presence of specified target drugs can be confirmed in blood samples from individual patients and therefore the possible ranges in the responses from different volunteer/drug combinations is more important than a mean value. Specifically therefore the worst case scenario, i.e. the lowest likely drug concentration in the DBS sample, must be considered. This will occur for a combination of low dose, rapid elimination (short thalf) with a low cmax developed in the shortest time (tmax). In this respect simvastatin should pose the major challenge. The other major factor affecting the level of drug to be detected is the time delay between the patient taking the medication and the subsequent provision of the blood spot sample.
| Drug | Dose range/mg | Cmax /ng/ml | Tmax/h | Thalf/h |
|---|---|---|---|---|
| Bisoprolol [9] | 2.5/5/10* | 37-87 | 1.5-4 | 5-16 |
| Ramipril [10] | 2.5/5*/10 | 11-31 | na | 4-6 |
| Amlodipine [11-13] | 5*/10 | 5-7 | 5-8 | 35-50 |
| Valsartan [14] | 40/80/160* | 879-3874 | 2-8 | 3.5-14 |
| Doxasozin [15] | 1/2/4/8*/16 | 67(17.6) | 2.7-5 | 20.5 (6.1) |
| Simvastatin [7] | 10/20/40* | 5-40 | 2-3 | 1.3-2.7 |
| Atenolol [9] | 25/50*/100 | 159-377 | 1.5-6.0 | 4-11 |
*Indicates dose taken for the data cited
Table 1: PK Data for frequently prescribed CV drugs.
The effects of these parameters are demonstrated in Figure 1 which shows the concentration of the drug in blood as a function of time after dosing. As can be seen the data in Figure 1 represents repeat dosing, as expected in the patient situation, whereas the majority of literature studies [16-19] focus on drug levels in blood after a single dose. This situation is shown by the shaded area to the left hand side of Figure 1 and indicates that for short values of t1/2 the level of drug available for detection rapidly decreases. More importantly for this investigation, Figure 1 shows the development of the steady-state concentration of the drug (after 36 hours) which should be maintained for any patient following a fixed dose drug regimen. Under adherence conditions the steady state level is the minimum level of drug available for detection. For non-adherence the level of the drug in blood is usually nondetectable after 5 half-lives or approximately 10 days abstinence for drugs with a long t1/2 such as amlodipine. An indication of patient nonadherence will be derived from a combination of no signal above the instrument noise level and the passage of at least 5 half-lives abstinence.
Volunteers
In the initial phase of the research volunteers were selected from informed colleagues, already prescribed one or more of the selected drugs, to provide control blood samples at specified times after taking known medication. Several volunteers known to take none of the target drugs provided baseline reference DBS samples.
The eight volunteers, 3 male and 5 female had ages between 30 and 66 provided 105 well documented DBS samples where the delay time between dose and blood spot collection was recorded. It should be noted that replicate samples were collected and several of the volunteers were prescribed more than one of the target drugs. Nine DBS samples were also collected from two volunteers (1 male and 1 female) who were known not to be prescribed any of the target drugs.
Sample collection
Capillary blood spot samples were collected in replicate on an Ahlstrom 226 sample card. A 2 mm lancet was used and the blood was collected as a single drop into each of the designated areas on the card and not as smears. The blood was then allowed to dry for circa 2 hours under ambient conditions and the card was then sealed into a small plastic bag for transport and further storage.
Sample analysis
The blood spot samples were analysed using a validated method based on solvent extraction followed by liquid chromatography– high resolution mass spectrometry (LC-HRMS) [8]. The analysis and validation of data for Ramipril, Bisoprolol and Simvastatin obtained from an 8 mm disc (approximately 20 micro-litres of blood) punched from the centre of the DBS sample has been detailed (Lawson et al.) elsewhere [8] and will not be repeated here.
Prior to all analyses authentic samples of the drugs were run in order to determine both the correct precise mass, used to uniquely identify that compound, and also the retention time under the LC conditions chosen (Table 2). These trials also led to the confirmation of the presence of both ramipril and ramiprilat [17] and that the sodium adduct of simvastatin was the most abundant ion under the analytical conditions used.
| Drug | Precise mass/(m/z) | Retention time/min |
|---|---|---|
| Bisoprolol | 326.2326 | 1.41 |
| Ramipril/Ramiprilat | 417.2384 / 389.2071 | 1.51 |
| Simvastatin/Na adduct | 418.2719 / 441.2611 | 2.30 |
Table 2: Analytical data for selected drugs.
Results
The purpose of the research was to develop a simple method to demonstrate either adherence or non-adherence to prescribed CV medication. Patients, discharged from hospital with prescribed longterm medication, will be expected to take the medicine as directed under normal living conditions. Therefore in this series of experiments there is not the usual control of conditions such as fasting, delivery of medication and other regimens during the trial period. Volunteers were asked not to change their habits during the periods they were collecting samples for this investigation. In several instances repeat samples were provided from similar time points on sequential days and these were compared for evidence of any significant differences indicative of the effects of diet on drug concentrations. Simvastatin is one good example in this area of study where the consumption of grapefruit juice can lead to a 10X elevation of the drug concentration in blood.
Data for controlled medication volunteers
The dried blood spot samples collected provided 388 drug concentration/time data points from volunteers who accurately recorded their medication procedures for each of the selected drugs they were prescribed. In addition to this group were the baseline (no prescribed medication) volunteers where the analysis of the DBS samples confirmed no detectable signal above the LOQ, as predicted, for the particular drug at the appropriate precise mass/ retention time combination [8].
The data was collated into four six hour periods after taking the medication. Assuming a notional average morning dose time of 7.00 am this could correspond to samples being collected during the morning, the afternoon, overnight and finally very early in the morning. This latter batch of samples should provide the greatest challenge for the methodology since it corresponds to the longest delay post dose. The results from the baseline samples confirmed there was no interference with the determination of the target drugs. The detection capability is recorded in Table 3 where a dash indicates no sample collected for this time delay. In work reported by Lawson et al. [8] previously we had been unable to detect low levels of simvastatin corresponding to the 18- 24 hour delay post dosing. An instrumental problem leading to poor performance under certain elution conditions (e.g. the retention time for simvastatin) has been identified and corrected.
| Drug | Dose/mg | 0-6 | 6-12 | 12-18 | 18-24 Delay/h |
|---|---|---|---|---|---|
| Bisoprolol | 5 | 32/32 | 24/24 | 16/16 | 8/8 |
| Ramipril | 5 | - | 2/2 | - | - |
| Simvastatin | 20 | 5/5 | 5/5 | 5/5 | - |
| No drug | na | 37/37 | 31/31 | 21/21 | 8/8 |
Table 3: Correct identification of presence/absence of target drugs versus sample collection time delay.
The results (Table 3) demonstrate that the system produced the anticipated signal outcomes for both groups of volunteers. Furthermore there were no false positive indications resulting from a second CV drug being prescribed. These results highlight the potential of this approach to indicate patient non adherence.
It is interesting to note that following the detection of an unexpectedly high level of simvastatin in a sample, the volunteer explained that to ensure all the drugs were taken they were combined and taken at lunch time rather than some in the morning and some in the evening. In this instance the presence of one CV drug, not monitored in this exercise, led to an increase in the bioavailability of the statin. The data from the system led to the identification of an incidence of incorrect adherence to the prescription.
Conclusion
The blood spot test has been shown to be able to provide adherence/ non-adherence information reliably for all the test subjects’ trialled. Highlighting the issue of incorrect adherence to prescriptions may provide a means to help alleviate the problem. The information from this test has the potential to aid clinical decisions and to improve the patient’s cardiovascular care. This test could also help the NHS save money in terms of unused pills and un-necessary hospital readmissions.
Acknowledgements
The authors thank the Gunn and Carter Fellowship for financial support.
The authors also thank Professor Kamlesh Khunti from the University of Leicester for advice on clinical aspects of the study.
References
- Anon British Heart Foundation statistics database (2010) Economic costs. Coronary Heart Disease 2010 Economics.
- Lewin J (2011) NONADHERENCE: A problem for patients, a problem for our health system.
- Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, et al. (2006) Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 166: 1842-1847.
- Allender S, Scarborough P, Peto V, Rayner M, Leal J, et al. (2008) European Cardiovascular Disease Statistics. European Heart Network, Brussels.
- Clark M (2011) 2010 Benchmarks in medication adherence: Programs, Challenges and Results.
- Barat I, Andreasen F, Damsgaard EM (2001) Drug therapy in the elderly: what doctors believe and patients actually do. Br J Clin Pharmacol 51: 615-622.
- Lilja JJ, Neuvonen M, Neuvonen PJ (2004) Effects of regular consumption of grapefruit juice on the pharmacokinetics of simvastatin. Br J Clin Pharmacol 58: 56-60.
- Lawson G, Cocks E, Tanna S (2013) Bisoprolol, ramipril and simvastatin determination in dried blood spot samples using LC-HRMS for assessing medication adherence. J Pharm Biomed Anal 81-82: 99-107.
- Lewis R, Maclean D, Ioannides C, Johnston A, McDevitt DG (1988) A comparison of bisoprolol and atenolol in the treatment of mild to moderate hypertension. Br J Clin Pharmacol 26: 53-59.
- Hosie J, Meredith P (1991) The pharmacokinetics of ramipril in a group of ten elderly patients with essential hypertension. J Cardiovasc Pharmacol 18 Suppl 2: S125-127.
- Shah S, Asnani A, Kawade D, Dangre S, Arora S, et al. (2012) Simultaneous Quantitative Analysis of Olmesartan Medoxomil and Amlodipine Besylate in Plasma by High-performance Liquid Chromatography Technique. J Young Pharm 4: 88-94.
- Vincent J, Harris SI, Foulds G, Dogolo LC, Willavize S, et al. (2000) Lack of effect of grapefruit juice on the pharmacokinetics and pharmacodynamics of amlodipine. Br J Clin Pharmacol 50: 455-463.
- Meredith PA, Elliott HL (1992) Clinical pharmacokinetics of amlodipine. Clin Pharmacokinet 22: 22-31.
- Zaid AN, Cortesi R, Qaddomi A, Khammash S (2011) Formulation and bioequivalence of two valsartan tablets after a single oral administration. Sci Pharm 79: 123-135.
- Chung M, Vashi V, Puente J, Sweeney M, Meredith P (1999) Clinical pharmacokinetics of doxazosin in a controlled-release gastrointestinal therapeutic system (GITS) formulation. Br J Clin Pharmacol 48: 678-687.
- Gonzalez O, Iriarte G, Rico E, Ferreirós N, Maguregui MI, et al. (2010) LC-MS/MS method for the determination of several drugs used in combined cardiovascular therapy in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 878: 2685-2692.
- Tan A, Jin W, Deng F, Hussain S, Musuku A, et al. (2009) Bioanalytical method development and validation using incurred samples--simultaneous quantitation of ramipril and ramiprilat in human EDTA plasma by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 877: 3673-3680.
- Tseng C-M, Huang C-C, Ho M-C, Chen Y-A, Shieh Y-H, et al. (2007) Bioequivalence assessment of two simvastatin tablets in healthy Taiwanese volunteers. J Food and Drug Analysis 15: 15-19.
- Peste G, Bibire N, Apostu M, Vlase A, Oniscu C (2009) A new liquid chromatography-tandem mass spectrometry method for determination of bisoprolol in human plasma samples. J Biomedicine and Biotechnology 1-8.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16559
- [From(publication date):
specialissue-2014 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 11886
- PDF downloads : 4673