Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Short Communication Open Access
Cardiac Sympathetic Nerve Plasticity and Heart Failure
| Hideaki Kanazawa and Keiichi Fukuda* | |
| Department of Cardiology, Keio University School of Medicine, Japan | |
| Corresponding Author : | Keiichi Fukuda Department of Cardiology Keio University School of Medicine 35 Shinanomachi, Shinjuku-ku Tokyo 160-8582, Japan E-mail: kfukuda@a2.keio.jp |
| Received November 30, 2015; Accepted January 19, 2016; Published January 21, 2016 | |
| Citation: Kanazawa H, Fukuda K (2016) Cardiac Sympathetic Nerve Plasticity and Heart Failure. J Pain Relief 5:223. doi:10.4172/2187-0846.1000223 | |
| Copyright: © 2016 Kanazawa H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Pain & Relief
| Introduction |
| To establish the treatment strategy for heart failure (HF), progress in the pathophysiological elucidation of HF is important. Recent studies revealed the existence of a cross-talk, which occurs through various humoral factors, between cardiomyocytes and cardiac sympathetic nerves (CSNs). Axon growth, denervation, and functional alteration of sympathetic nerves have been noted in HF cases. By using molecular biological approaches, a new adaptation mechanism involving the autonomic nervous system (ANS) has been developed for HF. In this review, we focus on the concept of cardiac autonomic nerve plasticity in HF. |
| Anatomy and Function of the CSNs and their Alterations in Diseased Heart |
| The heart is abundantly innervated, and its performance is tightly controlled by both sympathetic and parasympathetic efferent nerves. The CSNs extending from the sympathetic neuronal body in stellate ganglia uses norepinephrine (NE) as a neurotransmitter and increases the heart rate (chronotropic response) and conduction velocity (dromotropic response), as well as myocardial contraction (inotropic response) and relaxation (lusitrophic response). Sympathetic innervation density, which is the highest in the subepicardium, is stringently regulated in the heart [1]. |
| Cardiac innervation density is altered in diseased hearts, which can lead to unbalanced neural activation and lethal arrhythmias. The pathology of HF involves various abnormalities in the sympathetic nerve terminals. During the transition to overt HF, the sympathetic neural tone is upregulated. On the other hand, there is a paradoxical reduction in NE synthesis concomitant with the downregulation of tyrosine hydroxylase (TH), the rate-limiting enzyme in innervated neurons; NE reuptake into the sympathetic nerve terminals; and decrease in the NE levels in the myocardium [2-4]. This discrepancy between the anatomical or functional integrity and the catecholaminergic properties of the cardiac sympathetic nervous system in HF is long standing. However, the molecular mechanisms underlying the reduction in the catecholaminergic property of the CSN system in HF remain poorly understood. |
| Factors Regulating the CSN Density |
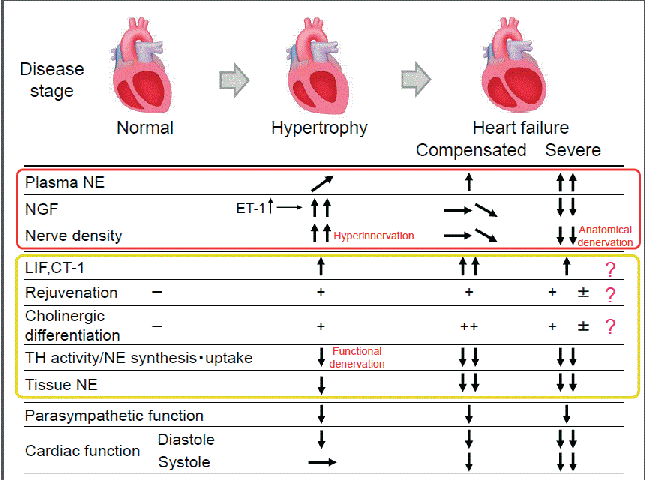
| The nerve growth factor (NGF), which is known as a sympathetic nerve regulation factor, is a member of the neurotrophin family [5]. NGF is critical for the differentiation, survival, and synaptic activity of the peripheral sympathetic nerves. The expression of NGF in the target organ is believed to correspond to the sympathetic nerve density [6]. Moreover, NGF is reported to be upregulated in cardiac hypertrophy, leading to sympathetic hyperinnervation [3] NGF upregulation is specifically promoted by endothelin-1 (ET-1), a known cardiac hypertrophic factor. Thus, the ET-1/NGF pathway is critical for the anatomical modulation of the CSNs [7]. In contrast, long-term exposure to high plasma NE concentration caused a reduction in myocardial NGF, and sympathetic nerve fiber loss in severe decompensated HF, resulting in the so-called anatomical denervation due to NGF depletion [8]. A previous report showed that this attenuation of NGF was caused by mechanical stretching and α-1 adrenergic stimulation through the calcineurin-nuclear factor of the activated T-cell signaling pathway [9]. |
| Rejuvenation of CSNs in HF |
| The genes encoding the fetal phenotype for the β-myosin heavy chain and α-skeletal muscle actin are reported to be upregulated in hypertrophic cardiomyocytes. This rejuvenation phenomenon of cardiomyocytes occurs because of isoform changes that might play a crucial role in the biological defense mechanism. Interestingly, the hyperinnervated sympathetic nerves induced by the upregulated NGF in the hypertrophic heart also express immature neuronal markers such as PSA-NCAM (polysialylated isoforms of neural cell adhesion molecule) and GAP 43 (growth-associated protein 43) [3]. HF leads to the upregulation of various growth factors and cytokines in the heart. LIF (leukemia inhibitory factor) and CT-1 (cardiotrophin-1) are members of the IL-6 (interleukin-6) family, which can induce these fetal genes in the diseased heart [3]. However, a paradoxical deterioration of NE synthesis occurs concomitantly with the downregulation of TH and the reuptake of NE into the sympathetic nerve terminals. Taken together, these results suggest that CSN dysfunction is accompanied by neuronal rejuvenation and the so called“functional denervation due to rejuvenation” mechanism. |
| As CSNs are mediated by changes in cardiac-derived humoral factors, cardiac sympathetic properties are also altered in chronic HF. Less dense TH-positive neurons were observed in an experimental animal model and in autopsy specimens from patients with HF [10]. Furthermore, many neurons in the sympathetic ganglion and left ventricle express parasympathetic markers such as choline transporter and choline acetyltransferase. This was thought to represent the cholinergic transdifferentiation of cardiac adrenergic neurons into cholinergic neurons, which were induced by cardiac-derived IL-6-related cytokines such as the LIF and CT-1 [10]. Thus, we confirmed that sympathetic neuron-specific gene targeting of glycoprotein (gp) 130, an IL-6 cytokine family receptor, revealed that sympathetic nerves do not undergo cholinergic transdifferentiation in the left ventricle. More interestingly, control mice had significantly improved survival rates and ventricular functions than sympathetic nerve-specific gp130-deficient mice, suggesting that this phenomenon might be an adaptive response that protects the heart from excessive sympathetic discharge. Taken together, these results indicate that the IL-6 family of cytokines secreted from the failing myocardium act as negative modulators of sympathetic function by means of rejuvenation and cholinergic differentiation through a gp130 signaling pathway.10) |
| Plasticity of the CSNs |
| Both cardiac sympathetic and parasympathetic nerves are known to develop from a neural crest cell with same genetic origin. The ability of cardiac sympathetic neurons to change their function in HF suggests that sympathetic neurons exhibit diverse plasticity that enables them to adapt to changes in the environment. The functional alterations in CSNs that occur in HF are believed to be associated with pluripotent potentials (dedifferentiation and transdifferentiation) that are based on their plasticity. From a cardioprotective perspective, this phenomenon is a notable concept for the elucidation of sympathetic abnormalities in HF. However, the rationale for the functional and anatomical changes in CSNs that occur in HF is difficult to explain. The complicated pathogenesis of HF includes many factors, such as disease phase and etiology, which contribute to its spatial and temporal complexity (Figure 1). |
| HF and the Parasympathetic Nerves |
| The parasympathetic nerve function reportedly starts declining in the early stages of HF [11]. As a result of the reduced parasympathetic nerve function, the heart rate increases, which works as a compensatory mechanism for HF. However, this also reduces the threshold for the development of fatal arrhythmia. As a result, the reduced parasympathetic nerve function promotes the progression of HF, and worsens the prognosis. The muscarinic 2 (M2) receptor is well recognized among the muscarinic receptors distributed in the myocardium. Recently, however, it has been reported that the M3 receptor is also present in the myocardium and that its expression increases during HF [12]. Additionally, the stimulation of the M3 receptor is reported to protect the myocardium by signaling the promotion of antioxidative and antiapoptotic activities as well as the defense mechanisms against ischemia [13]. It also improves the cardiac function, prevents remodeling, and improves the prognosis after HF or after myocardial infarction by activating the vagus nerve [14]. Acetylcholine has been shown to exert antiarrhythmic effects by inducing expression of the hypoxia-inducible factor HIF-1α, suppressing apoptosis, and reducing cytotoxicity [15], as well as by improving the function of connexin 43 [16]. On the basis of the results of previous studies, direct and indirect stimulations of the parasympathetic nerve functions during HF, which increase cardioprotective effects, are initiated through various mechanisms. Furthermore, the changes in the expression of the muscarinic receptors during HF, as well as the previously mentioned functional changes in the cholinergic nerves of the CSNs, are possibly a generalized purposive adaptation. |
| Conclusions |
| The upregulation of the CSN activity observed in HF is a primitive but important compensatory mechanism. However, this adaptive reaction causes further myocardium damage, decreases cardiac function, and promotes fatal arrhythmia, all of which contribute to poor prognosis [17,18]. Therefore, several compensatory mechanisms should exist to maintain homeostasis electrophysiologically and cardioprotectively. We expect that further elucidation of the molecular mechanism of CSN plasticity should eventually result in a new treatment strategy for HF. |
| References |
References
- Kawano H, Okada R, Yano K (2003) Histological study on the distribution of autonomic nerves in the human heart.Heart Vessels 18: 32-39.
- Chidsey CA, Braunwald E, Morrow AG, Mason DT (1963) Myocardial norepinephrine concentration in man. effects of reserpine and of congestive heart failure.N Engl J Med 269: 653-658.
- Kimura K,Ieda M, Kanazawa H, Yagi T, Tsunoda M, et al. (2007) Cardiac sympathetic rejuvenation: a link between nerve function and cardiac hypertrophy.Circ Res 100: 1755-1764.
- Himura Y, Felten SY, Kashiki M, Lewandowski TJ, Delehanty JM, et al. (1993) Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation 88: 1299-1309.
- Snider WD (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us.Cell 77: 627-638.
- Heumann R, Korsching S, Scott J, Thoenen H (1984) Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues.EMBO J 3: 3183-3189.
- Ieda M, Fukuda K, Hisaka Y, Kimura K, Kawaguchi H, et al. (2004) Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression.J Clin Invest 113: 876-884.
- Kimura K, Kanazawa H, Ieda M, Kawaguchi-Manabe H, Miyake Y, et al. (2010) Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. AutonNeurosci 156: 27-35.
- Rana OR,Saygili E, Meyer C, Gemein C, Krüttgen A, et al. (2009) Regulation of nerve growth factor in the heart: the role of the calcineurin-NFAT pathway.J Mol Cell Cardiol 46: 568-578.
- Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H (2010) Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest 120: 408-421.
- Ishise H,Asanoi H, Ishizaka S, Joho S, Kameyama T, et al. (1998) Time course of sympathovagal imbalance and left ventricular dysfunction in conscious dogs with heart failure.J ApplPhysiol (1985) 84: 1234-1241.
- Wang Z, Shi H, Wang H (2004) Functional M3 muscarinic acetylcholine receptors in mammalian hearts.Br J Pharmacol 142: 395-408.
- Yang B, Lin H, Xu C, Liu Y, Wang H, et al. (2005) Choline produces cytoprotective effects against ischemic myocardial injuries: evidence for the role of cardiac m3 subtype muscarinic acetylcholine receptors. Cell PhysiolBiochem 16: 163-174.
- Olshansky B,Sabbah HN, Hauptman PJ, Colucci WS (2008) Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy.Circulation 118: 863-871.
- Kakinuma Y, Ando M, Kuwabara M, Katare RG, Okudela K, et al. (2005) Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1alpha. FEBS Lett 579: 2111-2118.
- Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, et al. (2005) Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation 112: 164-170.
- Bristow MR,Minobe W, Rasmussen R, Larrabee P, Skerl L, et al. (1992) Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms.J Clin Invest 89: 803-815.
- Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, et al. (2000) Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101: 1960-1969.
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 10008
- [From(publication date):
January-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9174
- PDF downloads : 834
