Research Article Open Access
Carbon Isotopic Data Validate the New Model of Carbon Turnover
Ivlev AA*Russian State Agrarian University of K.A. Timiryazev, Moscow, Russia
- *Corresponding Author:
- Ivlev AA
Russian State Agrarian University of KA
Tel: +7 (499) 976-0480
Fax: +7 (499) 976-2910
E-mail: aa.ivlev@list.ru
Received date: October 24, 2016; Accepted date: December 19, 2016; Published date: December 26, 2016
Citation: Ivlev AA (2016) Carbon Isotopic Data Validate the New Model of Carbon Turnover. J Marine Sci Res Dev 6:218. doi: 10.4172/2155-9910.1000218
Copyright: © 2016 Ivlev AA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
A new global redox carbon cycle model is suggested. It claims that lithospheric plates’ movement exerts an impact on photosynthesis development. The impact is realized via periodic injections of CO2 coming from zones of plates’ collisions. Carbon dioxide is derived from oxidation of sedimentary organic carbon in thermochemical sulfate reduction proceeding in subduction zones. Carbon turnover is considered as a conversion of the element from the oxidized state (CO2 + HCO3- + CO3-) into the reduced state produced in photosynthesis and in the following transformation. The isotopic data confirm the validity of the model. They explain the observed correlation of carbon isotope composition of sedimentary organic matter with geologic age. It was found that the difference between carbon isotope composition of organic matter and that of coeval carbonates is an analog of the carbon 13C isotope discrimination in photosynthesis used for modern plants. The periodicity of isotopic characteristics correlates with periodicity of climatic changes, mass extinctions, with the irregularity of stratigraphic distribution of rocks rich in organic matter and other periodic events in biosphere.
Keywords
Global carbon cycle; Isotope fractionation; CO2 assimilation and photorespiration; Sedimentary organic carbon; Carbonates; Orogenic and Geosynclynal periods; Ecological compensation point; Lithospheric plates; Climate cycles; Mass extinctions
Introduction
As early as 1926 famous Russian geochemist Vernadsky [1] put forward an idea that Earth crust processes interact with Biosphere processes. The present work develops and elaborates this idea. The new interpretation claims: the moving lithospheric plates exert an impact on photosynthesis development via CO2 injections into “atmospherehydrosphere” system, produced in zone of plates’ collisions.
To prove this assertion, a new approach to carbon turnover is needed. That is why; a redox carbon cycle model has been worked out. It is characterized by three main features that distinguish it from other models [2-4].
The Special Features of the Redox Carbon Cycle Model
The first feature is linked with the assertion that CO2, which fills “atmosphere-hydrosphere” system and determines photosynthesis development, is produced in zone of lithospheric plates’ collisions. Carbon dioxide is derived from the oxidation of the sedimentary organic matter in the thermochemical sulfate reduction.
The second feature claims that carbon turnover depends on lithospheric plates’ movement, which is irregular. It consists of shortterm period of intense movement and quick rates of plates. It provides a number of plates’ collisions favorable for thermochemical sulfate reduction and producing considerable amounts of CO2. In long-term period of quiet plates’ movement when the plates’ rate is low, weathering and photosynthesis become dominant and CO2 concentration in the “atmosphere-hydrosphere” system drops.
The third feature, characterizing the model, is the assertion that carbon turnover is not a simple transition of the element between geospheres and biosphere, but is a conversion of carbon from the oxidized state, presented by CO2, bicarbonate and carbonate ions, into the reduced state, presented by different biogenic forms, produced in photosynthesis, and by the products of their following transformations. The reverse transition is realized via respiration of living organisms, via microbial and chemical oxidation accompanying transformations of the “living” matter after the burial. Among the oxidative processes the final oxidation of organic matter in the subduction zone is dominant.
Thus, the global redox carbon cycle formally can be presented as a closed loop consisting of the two branches-oxidative and reductive. It has two remarkable points, one of which is photosynthesis, where the oxidized carbon species turn into the reduced state; another point provides the reverse transition in a number of oxidation processes.
Two Known Geological Concepts are the Basis for the Model
The proposed model is based on two geologic concepts-plate tectonics [5,6] and orogenic cycles [7]. The first proves that plates, covering the entire Earth surface, are in permanent movement. The movement reminds that of escalator. In some places of the Earth, in the zone of the mid-atlantic ridge, where the crust is most subtle, magma erupts onto the surface and, coming into the contact with ocean water, hardens to form a new plate. It pushes other plates, causing them to move. In other places of the Earth, along the continent margins and island arcs, the plates, moving towards each other, collide. One of them, bending and moving down under the other, is absorbed by magma. Area, where the collisions occur, is called the subduction zone.
According to the second concept, the Earth crust fluctuates permanently, but with different intensity. Short-term periods of high intensity, termed orogenic, correspond to periods of great volcanism and strong volcanic and magmatic eruptions. In long-term quiet period, termed geosynclynal, the activity of geologic processes slows down; weathering and photosynthesis become dominant.
The Essence of the Redox Carbon Cycle Model
I have combined both concepts and supposed that short-term orogenic periods correspond to the time of intense movement of the lithospheric plates, when frequent collisions, provide massive entry of CO2 into “atmosphere-hydrosphere” system. Long-term geosynclynal periods correspond to the time of dominant role of photosynthesis, what leads to the considerable depletion of CO2 in the system. The active tectonic activity slows down.
Best of all to understand the carbon cycle functioning is to study the variations of the main components of photosynthesis reaction (1)
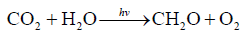 (1)
(1)
within the orogenic cycle. The tentative variations of the concentrations of the components are presented on Figure 1. In the orogenic period CO2, coming from the subduction zone, fills the “atmosphere-hydrosphere” system and reaches its maximal concentration. Note, that because of the equilibrium in the system [8]
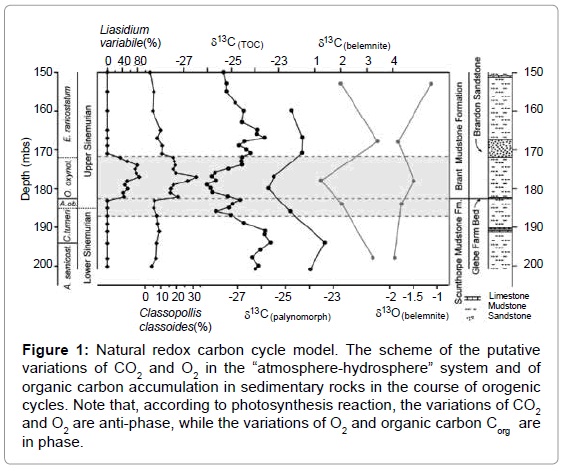
Figure 1: Natural redox carbon cycle model. The scheme of the putative variations of CO2 and O2 in the “atmosphere-hydrosphere” system and of organic carbon accumulation in sedimentary rocks in the course of orogenic cycles. Note that, according to photosynthesis reaction, the variations of CO2 and O2 are anti-phase, while the variations of O2 and organic carbon Corg are in phase.
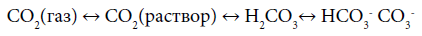
other inorganic species are described by the same curve as CO2.
In the subsequent geosynclynal period due to photosynthesis the CO2 concentration falls down reaching minimal meaning by the end of the period. The corresponding variations are described by exponential curve what is typical to the chemical reactions.
The changes of O2 and organic matter are presented by the antiphase curves relative to CO2 curve, since they are the products of the reaction (1) whereas CO2 is the substrate. It should be also underlined that we have replaced the total “living matter” in the reaction (1) by the sedimentary organic matter, since the residence time of the sedimentary organic matter within the carbon cycle, is greater than the life time of the “living” matter.
Isotopic Variations of Organic Matter in the Course of Orogenic Cycles
To illustrate the special role of carbon isotope data in substantiation of the redox carbon cycle model, additional information on carbon isotope fractionation in photosynthesis is needed.
As known, photosynthesis consists of two reciprocal processes: CO2 assimilation and photorespiration. From recent works it was found that each of these processes is accompanied by carbon isotope fractionation with the effect of opposite sign. The CO2 assimilation results in 12C enrichment of biomass, whereas photorespiration leads to the accumulation in biomass of 13C. Thus carbon isotope composition of the total biomass is determined by both isotope effects. The above processes are in reciprocal relations, i.e., the strengthening one of them is accompanied by the weakening of the other. Contribution of each process into isotope composition of biomass depends on the CO2/ O2 concentration ratio in the environment, since intensification of photorespiration leads to the enrichment of biomass in 13C, whereas intensification of CO2 assimilation leads to 12C enrichment. The last is especially meaningful bearing in mind that with photosynthesis emergence average O2 concentration in the atmosphere steadily grew up. The O2 concentration growth occurred also within geosynclynal period of orogenic cycle.
Taking the above into consideration, one should conclude that in orogenic period the “living matter”, and hence the buried organic matter has maximal enrichment in 12C, whereas in geosynclynal period of the cycle organic matter is gradually enriched in 13C reaching maximal 13C enrichment by the end of the cycle.
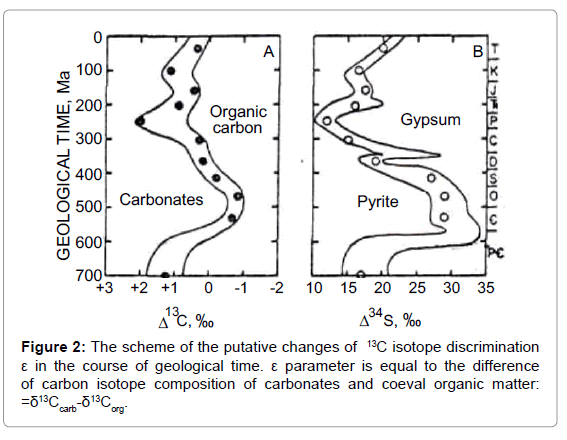
One more important notion is the following. Considering the actualism principle [8], the carbonates can be regarded as analog of the carbon dioxide in the past atmosphere, whereas coeval sedimentary organic matter can be considered as analog of the “living” matter. Following this logic, the difference between carbon isotope composition of carbonates and that of coeval organic matter maybe regarded as analog of 13C isotope discrimination in the past photosynthesis. It was designated as ε parameter [9].
Taking into account that isotope ratio of carbonates doesn’t depend on O2 concentration; it is convenient to use the ε parameter variations to analyze the impact of CO2/O2 ratio within the cycle. Within the cycle ε parameter varies from the maximal value in orogenic period, when CO2/O2 ratio is maximal, to the minimal value by the end of geosynclynal period when CO2/O2 ratio reaches minimal meaning (Figure 2).
Another important relation may be deduced from the assumed depletion of inorganic pool of CO2 in geosynclynal period. The depletion of the inorganic pool is accompanied with an isotope effect of photosynthesis and hence it should give rise to the so-called Raleigh effect. It means that in the course of geosynclynal period the residual CO2 in the “atmosphere-hydrosphere” system and the newly produced “living” mater are consistently enriched in 13C. The “heaviest” organic carbon is produced by the end of geosynclynal period.
Note that in geosynclynal period there is O2 growth in the atmosphere what stimulates photorespiration and results in additional accumulation of 13C in organic matter. At the same time a decrease of CO2 concentration in the system results in the temperature drop on the Earth surface. It achieves the minimal meaning by the end of the geosynclynal period and causes glaciations. Thus high oxygen concentration, low temperatures (glaciations) and 13C enrichment of organic matter are three traits, indicating the end of the cycle.
The Arguments In Favor of the Redox Carbon Cycle Model; The Dependence of Carbon Isotope Composition of Sedimentary Organic Carbon on O2 Concentration
Hayes et al. [9] who examined carbon isotope ratio of sedimentary organic matter in Precambrian and Phanerozoic along the geologic scale found the sequential 13C enrichment of organic matter, what corresponded to the growth of O2 in the atmosphere.
The examination of sedimentary organic matter at three successive periods has discovered that during the Neoproterozoic: from 800 to 750 Ma δ13C values of organic carbon were greater than 32%; from 685 to 625 Ma δ13C were between 32% and 28%, and in Phanerozoic up to the Early Oligocene interval the values were less than 28%, reaching up to 22%.
Average O2 concentration in this interval grew up from some percent in late Proterozoic [10] to approximately 20-25% in Late Paleocene [11,12].
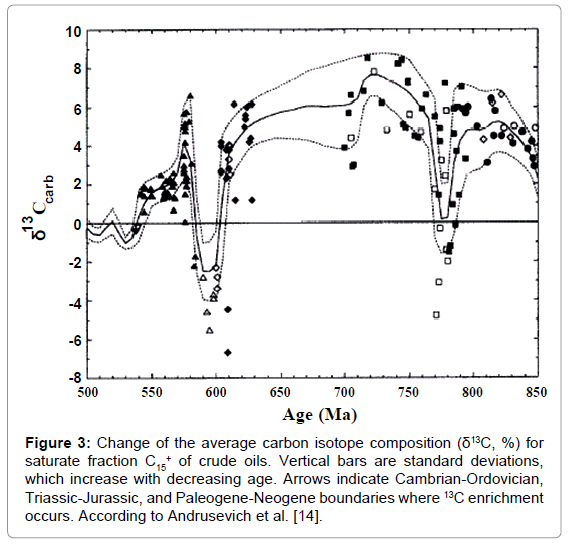
The oils show the same tendency (Figure 3) [13,14]. There are three successive steps of 13C enrichment corresponding to the growth of the average concentration of O2 in geologic time. This phenomenon is explained by the fact, that oils are the derivatives of organic matter and inherit its carbon isotope composition from them. It also relates to all oil fractions.
Figure 3: Change of the average carbon isotope composition (δ13C, %) for saturate fraction C15+ of crude oils. Vertical bars are standard deviations, which increase with decreasing age. Arrows indicate Cambrian-Ordovician, Triassic-Jurassic, and Paleogene-Neogene boundaries where 13C enrichment occurs. According to Andrusevich et al. [14].
It was also disclosed [9] that organic matter related to the “ice house” period was always enriched in 13C as compared with that related to the interglacial periods.
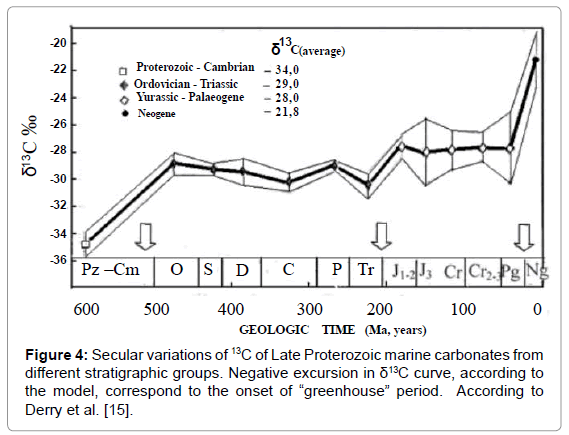
Isotopic Data on Precambrian Carbonates
Figure 4 presents carbon isotope data on Precambrian carbonates. Data were taken from work of Derry et al. [15], who gathered all the available data at that time. They disclosed great isotopic variations which could be explained in the frame of the suggested model. Two narrow peaks, corresponding to the abrupt negative excursions, according to the model, correspond to two short-term orogenic periods of two different orogenic cycles. Negative sign of the excursions within shortterm intervals correspond to “greenhouse effect” typical to orogenic periods. Additional support for the explanation is a proximity of each peak to two glacial episodes: the Sturtian (ca. 780 Ma) and Varangian (ca. 600 Ma) correspondingly. In accordance with the model, abrupt cooling always proceeded by orogenic period.
Carbon and Sulfur Cycles Coupling Source of CO2
Here we illustrate how isotopic data and non-isotopic arguments prove one of the most important model’s assertions: CO2, produced in oxidation of sedimentary organic matter by means of thermochemical sulfate reduction, occurs in subduction zones in the orogenic period. It was also shown that sulfate reduction is the point of coupling of carbon and sulfur natural cycles.
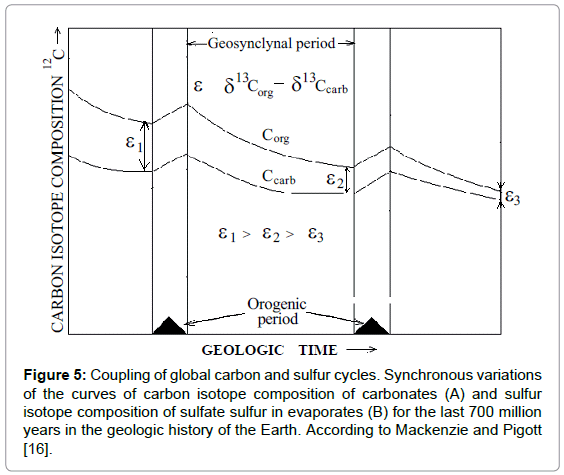
Temporal curves on Figure 5 demonstrate synchronous isotopic variations of carbon and sulfur in marine carbonates and gypsum (sulfates) in geologic time [16]. Such synchronism prompts itself that both cycles are somehow bound. Each curve has two differently directed humps. Next to them there are inscriptions, made by the authors, to indicate minerals of sedimentary rocks that were mostly spread at the corresponding periods. Carbonates and pyrites correspond to the humps in the lower parts of the secular curves. Organic matter and gypsum correspond to the humps in the upper part of the curves respectively.
Figure 5: Coupling of global carbon and sulfur cycles. Synchronous variations of the curves of carbon isotope composition of carbonates (A) and sulfur isotope composition of sulfate sulfur in evaporates (B) for the last 700 million years in the geologic history of the Earth. According to Mackenzie and Pigott [16].
If we compare the above substances with the substrates and the products of sulfate reduction, it is easy to see that the substances, corresponding to the lower humps on the curves, coincide with the reaction products, whereas the substances, corresponding to the upper humps on the curves coincide with the reaction substrates. It explains the opposite directions of the humps.
Analysis of the isotopic changes of carbon and sulfur proves that the coincidence is not accidental.
Prior to the analysis of the dynamics of carbon and sulfur isotopic variations presented by the curves on Figure 5, firstly, we should note that thermochemical sulfate reduction is followed by sulfur isotope fractionation [17,18]. Secondly, due to periodic character of the reaction, the substrate pool of sulfates is periodically depleted. The depletion is followed by the Raleigh effect. The more the reaction proceeds and the more the substrate pool is depleted, the greater the residual substrate (gypsum) is enriched with a “heavy” sulfur isotope 34S. As it follows from the analysis of the lower part of the curves, the enrichment of gypsum with 34S is accompanied with the enrichment of carbonates with a “light” carbon isotope 12C. The enrichment of sulfates in 34S evidences in favor of high extent of sulfate conversion. At the same time high extent of sulfate conversion proves that another reaction product CO2 should be also produced in a considerable amount. The produced CO2 inherits its “light” carbon isotope composition from organic matter. Hence, when “light” CO2 enters marine “carbon dioxide-carbonate” system with carbon enriched in 13C, it makes carbon in the system to be “lighter” due to the chemical isotope exchange between species.
Quite opposite picture one can deduce from the analysis of the upper parts of the curves. The 32S enrichment of gypsum evidences that the extent of sulfate conversion is low. Hence the small amounts of “light” CO2 are produced and marine carbonates become “heavier” as compared with the previous case. Thus, the coupled isotopic changes of carbon and sulfur of marine carbonates and gypsum, in addition to chemical arguments, give firm proofs that they are the results of sulfate reduction process occurring in the subduction zones.
An indirect argument in favor of sulfate reduction in subduction zone was the great abundance of sulfide oxidizing bacteria in Precambrian. It evidences for significant inflow of the reduced sulfur forms (sulfides and hydrogen sulfide) onto the Earth surface. This was favored by low oxygen concentration in the Earth’s atmosphere at that time. The sulfide oxidizing bacteria were so widely disseminated that gave grounds for Hayes et al. [9] to conclude that these bacteria were the main source of organic matter in rocks in the Proterozoic.
Some other arguments evidencing in favor the assertion that CO2 is formed in sulfate reduction in orogenic period are given in our previous works.
Ecological Compensation Point
Global photosynthesis is the key element of carbon turnover. With emergence of photosynthesis its product oxygen has begun to accumulate in the “atmosphere-hydrosphere” system. At first oxygen was a poison and destructive to the photosynthesizing organisms, but in the course of evolution they found the way to make use of oxygen having created photorespiration, which began to increase with photosynthesis development [8]. Thus, evolution worked out feedback mechanism to compensate the growing impact of oxygen. The growth of oxygen forced the organisms to evolve in direction to their compensation point. It is the state when the contribution of CO2 assimilation became equal to that of photorespiration. Below this point physical existence of organism is impossible.
The similar feedback mechanism determines the evolution of the carbon cycle to the ecological compensation point. The latter corresponds to the state when the whole amount of the reduced carbon, produced in photosynthesis, becomes equal to the carbon returning into the oxidizing forms. At this point the system becomes very sensitive to the collisions of separate plates. The orogenic cycles became shorter and finally turned into short-term oscillations, termed climatic oscillations. The identical nature of orogenic cycles and climatic oscillations is supported by the same set of traits.
Both consist of two phase: warming and cooling. Warming phase is characterized by high CO2 concentration and temperature, by low oxygen concentration. This phase is characterized by intense volcanism and magmatism, by sea level rise. In these conditions predominance of thermophylic organisms takes place.
On contrary, the cooling phase is characterized by low CO2 concentration and temperature, by increased oxygen concentration, by the reduced tectonic activity. Volcanism and magmatism are replaced by strengthening of photosynthesis and weathering processes. Sea level drops. The transition from anaerobic to aerobic environment is followed by the emergence of aerobic organisms.
It should be stressed that the transition from cooling to warming phase, accompanying with dramatic changes in the environment, results in great biotic events, such as mass extinction. It stimulates the formation of deposits rich in organic matter. The transition is always followed by the simultaneous change of isotopic shifts of different elements. It correlates with the variations of non-isotopic parameters Figure 6 [19]. It allows clearly distinguishing the onset of warming period and carrying out stratigraphic correlations.
Conclusion
• The suggested redox carbon cycle model reveals the underlying mechanisms of interaction of biosphere and Earth crust processes. It was found that lithospheric plates’ movement exerts impact on photosynthesis development via periodic injections of CO2 into “atmosphere-hydrosphere” system during plates’ collisions.
• The model corresponds well to the available experimental data. It allows explaining of many biosphere events in the geological past, such as climatic cycles, mass extinctions, the irregularity in stratigraphic distribution of deposits rich in organic matter, etc.
• Carbon isotope data on marine carbonates and coeval sedimentary organic matter play an important role in carbon turnover studies. The most effective use of isotopic parameters is to combine them with non-isotopic parameters and to find coherence.
References
- Vernadsky VI (1926) The Biosphere, Complete annotated ed. Copernicus Books, New York, p: 1986.
- Houghton RA (2005) The contemporary carbon cycle. In: Schlesinger WH (ed.) Biogeochemistry. Amsterdam, Elsevier Science p: 473-513.
- Milero FJ (2006) Chemical Oceanography, 3rd edn, CRC Press.
- Jansen HH (2004) Carbon cycling in earth systems-a soil science perspective. Agriculture, Ecos Environ 104: 399-417.
- Flint RF(1973) The Earth and its history. J Wiley and Sons, New York, NY.
- Monin AS (1977) Istoriya Zemli [History of the Earth]. Nauka, Leningrad [in Russian].
- Rutten MG (1971) The Origin of Life by Natural Causes. Elsevier, Amsterdam.
- Ivlev AA (2013) Chemical evolution vs. Biological evolution: Coupling Effect and Consequences. Signpost Research Publisher, Kerala.
- Hayes JM, Strauss H, Kaufman AJ (1999) The abundance of 13C in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem Geol 161: 103-125.
- Canfield DE, Teske A (1996) Late Proterozoic rise in atmospheric oxygen concentration inferred from phylogenetic and sulphur-isotope studies. Nature 382: 127-132.
- Berner RA, Canfield DE (1989) A new model for atmospheric oxygen over Phanerozoic time.Am J Sci 289: 333-361
- Lenton TM (2001) The role of land plants, phosphorous weathering and fire in the rise and regulation of atmospheric oxygen. Global Change Biol 7: 613-629.
- Andrusevich VE, Engel MH, Zumberge JE (2000) Effects of paleolatitude on the stable carbon isotope composition of crude oil. Geology 28: 847-850.
- Andrusevich VE, Engel MH, Zumberge JE, Brothers LA (1998) Secular, episodic changes in stable carbon isotope composition of crude oils. Chem Geol p: 59-72.
- Derry LA, Kaufman AJ, Jacobsen SB (1992) Sedimentary cycling and environmental change in the Late Proterozoic: Evidence from stable and radiogenic isotopes. Geochim et Cosmochim Acta 56: 1317-1329.
- Mackenzie FT, Pigott JD (1981) Tectonic controls of Phanerozoic sedimentary rock cycling. J Geol Soc London 138: 183-196.
- Nakai N, Jensen M(1960) Biogeochemistry of sulfur isotopes. J Earth Sci p: 30-35.
- Thode H, Monster J, Dunford H (1961) Sulfur isotope geochemistry. Geochim. Cosmochim. Acta 3: 159-174.
- Riding JR, Leng MJ, Kender S, Hesselbo SP, Feist-Burkhardt S (2013) Isotopic and palynological evidence for a new Early Jurassicenvironmental perturbation. Paleogeography, Paleoclimatology, Paleoecology p: 16-27.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 4054
- [From(publication date):
December-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 3196
- PDF downloads : 858