Research Article Open Access
Carbon and Nutrient Release Patterns during Leaf litter Decomposition in Boter-Becho Forest, Southwestern Ethiopia
Talemos Seta1*, Sebsebe Demissew1, Zerihun Woldu1 and Mulugeta Lemenih2
1Department of Plant Biology and Biodiversity management, Addis Ababa University, Ethiopia
2Regional Head in Forestry and NRM, FARM-Africa, Ethiopia
- *Corresponding Author:
- Talemos Seta
Department of Plant Biology and
Biodiversity Management
Addis Ababa University, Ethiopia
Tel: +256772354281
E-mail: talemos.seta@yahoo.com
Received Date: November 30, 2016 Accepted Date: December 26, 2016 Published Date: December 30, 2016
Citation: Seta T, Demissew S, Woldu Z, Lemenih M (2016) Carbon and Nutrient Release Patterns during Leaf litter Decomposition in Boter- Becho Forest, Southwestern Ethiopia. J Ecosys Ecograph 6: 222. doi:10.4172/2157-7625.1000222
Copyright: © 2016 Seta T et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Carbon and nutrient release patterns from decomposing leaves in the forest involve initial leaching, a net immobilization by microbes and a net release phase where the nutrient mass decreases. The aim of this study was to assess the effect of disturbance and season (dry and wet) in the carbon (C) and nutrient release patterns from decomposing leaves. The decomposition of a mixed leaf litter in slightly disturbed (SD) and highly disturbed (HD) sites of Boter-Becho forest was investigated during one year. Litterbags of a mixed leaf litter (20 g each) were buried at a depth of 15 cm in the soils. Residual of mixed leaf litter was monthly retrieved, oven-dried (80°C for 24 h) and weighed. Initial leaf litter and decomposing leaf litter were analyzed for C, total nitrogen (N), available phosphorus (P) and potassium (K). Differences in C and nutrients remaining between seasons and site were evaluated by one way ANOVA at P<0.05. Simple linear regression analysis was performed to predict the nutrient release pattern using initial litter chemistry. The results showed that there was no significant difference between the initial litter chemistry and nutrient release except for K (P=0.021). Moreover, the site had no significant influence on the C and nutrient release patterns except for K (P=0.013). A significant difference was observed (P<0.001) in C and nutrient release pattern between wet and dry season in both sites being greater the release in the wet season. This could be because of the higher temperature and rainfall in wet season which increase microbial activity and thus decomposition rate and nutrient release. Therefore, the rate of decomposition, C and nutrient release pattern in the Boter-Becho forest mainly depend on the climatic factors but not on the initial litter chemistry or site disturbance.
Keywords
Carbon; Decomposition; Dry season; Litter bags; Nutrients; Wet season
Introduction
In forest ecosystems, faster decomposition rates have been associated with lower C/N ratios [1] and high N and P concentration [2,3]Litter containing high N concentration is often decomposed at a rapid rate due to the growth of soil microbes is limited by the low N availability in soils [4]. Carbon (C) and nutrient dynamics during the decomposition of plant residues are related to the relative availability of C and N in litter to the microbial population. If C: N is less than 20, N will be released by enhancing the decomposition of materials while C: N is higher than 20, there will be N immobilization until decomposition lowers the C: N ratio [5,6]. Moreover, other indices that incorporate C chemistry and nutrient content, such as lignin: N or C: P ratios are often negatively correlated with early decay rates in various studies [7,8]. Leaf toughness (resistance to physical damage) is also an important factor determining the velocity of leaf litter decomposition, being slower the decomposition of tougher leaves [9].
Rapid mass loss was strongly correlated with the soluble labile C content during the early decomposition phase [10,11]. This is due to the fact that the amount of C, and the chemical composition of the molecules containing C (e.g., starch, cellulose, lignin), are also strong determinants of decomposition rate [10]. For example, starch is easier to break down and provides a greater energy yield than complex lignin molecules. Therefore, litter containing a high amount of starch is broken down faster than litter containing high amounts of lignin. However, no single parameter explains the C-mineralization during litter decomposition process in the soil being a combination of environmental and biological factors involved [4,10].
Nutrient release pattern from decomposing leaves in tropical forests involves three major phases [1,10]: (1) an initial phase where leaching and nutrient release dominate; (2) a net immobilization (i.e., net accumulation) phase where nutrients increase due to the presence of microbes; and (3) a net release phase where the nutrient mass decreases. However, not all of these stages always appear in practical experiments. For instance, the immobilization phase could be absent, particularly in litter with high N concentrations [4]. Moreover, N and P dynamics can also be characterized by an early immobilization followed by net release [12].
[13-15]P availability in many tropical sites is very low compared to N which is attributed to low P supply through weathering of parent material and tight absorption of orthophosphate in the wide-spread oxisols and ultisols . P concentration of leaf litter represents important factor controlling leaf litter decomposability in the soil of the tropical forests [12].
Potassium (K), calcium (Ca) and magnesium (Mg) are essential macronutrients for energy metabolism, photosynthesis and membrane transport of plants [17]. These nutrients are available to forest trees from the litterfall [18], rarely limit microbial processes and they are rapidly lost from the decomposing litter [19]. Many authors have described the dynamics of K, Ca, and Mg during litter decomposition being K leached out quickly from decomposing litters, Ca decreased as carbon loss during litter decomposition, and Mg often showed an intermediate release pattern [20-24]. K is not a structural material and it exists mainly in solution in plant cells thus the mobile K is leached out quickly from decomposing litters. The late phase of K dynamic, on the other hand, is characterized by seasonal changes regardless of the initial litter quality [20]. For instance, Blair [24] reported that K concentrations decreased rapidly during litter decomposition of three tree species (Cornus florida , Acer rubrum and Quercus prinus ) then continued declining throughout the two years study being 91%the net loss of K.
Ethiopia possess up to12 types of ecosystems [25] due to a big range of climate (rainfall, temperature, humidity, exposure to wind) resulted from its topography and latitudinal position. The Ethiopian highlands contribute to more than 50% of the African land area [26]. In Ethiopia, the montane moist forest ecosystem comprises high forests which are mainly found in southwestern part of the country. A very few studies related to carbon and nutrient dynamics have been conducted in forest ecosystems of Ethiopia. For instance, Gindaba et al. [27] determined the nutrient composition and short term release (12 weeks study period) of nutrients from decomposing leaves of the two tree species Croton macrostachyus and Milletia ferruginea around Wondo Genet, Southeast Ethiopia. Nigatu and Michelsen (1994) studied the litter production and decomposition of the exotic species such as Cupressus lusitanica , Eucalyptus globulus , Pinus patula , and one indigenous tree Juniperus procera . These studies have considered litter decomposition dynamics and nutrient release only for a few selected tree species, being scarce the experimental studies considering several native tree species in Ethiopian montane forests. The objective of the present study was (1) examining the effect of seasonal variation on nutrient dynamics (2) characterizing the carbon and nutrient release pattern in SD and HD sites of moist evergreen montane forests in southwestern Ethiopia.
Materials and Methods
Study area
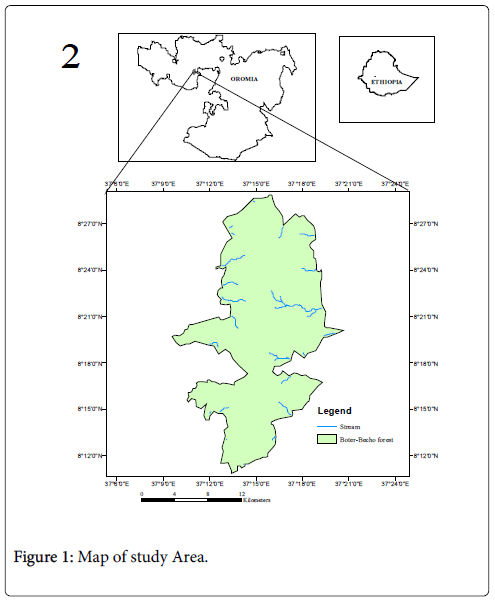
Boter-Becho forest is one of the national forest priority areas located in Jimma Zone of Oromia National Regional State, between Tiro-Afeta and Limu-Kosa districts of Jimma zone, at about 223 km southwest of Addis Ababa, the capital city of Ethiopia (Figure1). The location coordinates taken at the centre of the forest is 08021’56.4’’N and 037016’25.4’’E. The area lies along a volcanic mountain ridge, running almost north to south, and rising to a series of small peaks, the highest of which is 3200 m above sea level. The Eastern part of the ridge is sharply steep, but more gradual in western side. The hills are divided by numerous valleys. The forest is dominated by Acacia spp. in the lower altitude and high montane forest in slopes and valleys (around 2900 m). Most of the valleys along the forest ridge contain only seasonal water course remaining dry in mainly from December to March. The soil type is Nitosol, which is the characteristic soil type in Ethiopian highlands. The Soil beneath forest is reddish-brown and well-drained, and typically of the forest soil derived from Ethiopian plateau. The rock dominating the southern part of the ridge are volcanic and the southeast principally comprises Olivine and basaltic, and Kaolinite, halloysite and iron oxides dominate their clay mineralogy. The parent rocks in the area also include ignimbrite and agglomerate [28]. Boter-Becho together with Tiro forest covers a total area of about 37,787 ha. However, due to the restructuring of districts in Jimma zone, Tiro forest was administratively separated from Boter- Becho forest in 2010. Since then, the total area of each forest has not been determined yet despite the approximate boundary being used (Figure 1).
The forest is classified under moist evergreen montane forest [25]. The forest vegetation in Boter-Becho is mainly characterized by a mixture of the tree species such as Olinia rochetiana , Polyscias fulva , Pouteria adolfi-friedricii , Schefflera volkensii , Syzygium guineense Subsp. afromontanum, Allophylus abyssinicus, Croton macrostachyus, Juniperus procera, Hagenia abyssinica and Erica arborea. Also appeared large-sized trees such as Podocarpus falcatus and broadleaved tree such as Pouteria adolfi-friederici. There are some patches of Arundinaria alpina in wet sheltered valleys.
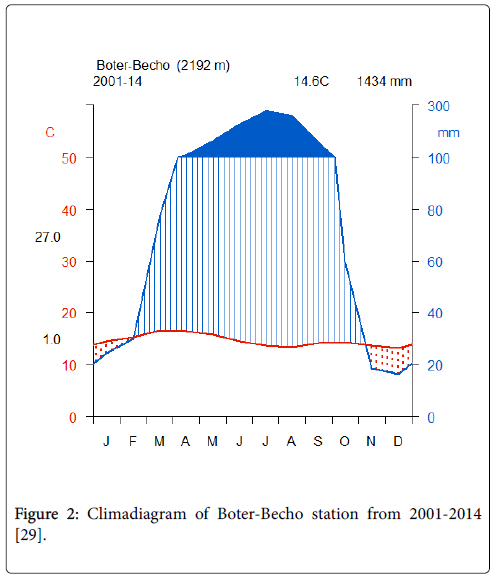
The climate in Boter-Becho is warm and temperate with an annual rainfall of 1434 mm and mean annual temperature of 14.6°C. Boter- Becho possess unimodal rainfall pattern with along rainy season from March to September and a dry season from October to February. The mean monthly rainfall is 183.6 mm and 29.6 mm in wet and dry season respectively. The mean annual temperature is 14.9 and 14.2°C in wet and dry season respectively (Boter-Becho climate station; [29] (Figure 2).
Litter bag experiment
The study was conducted at two sites of the Boter-Becho forest (Ranged from 2278-2626 m altitude) with different grade of disturbance; slightly disturbed (SD) and highly disturbed (HD). The SD site had not suffered of logging, browsing or grazing and only disturbed by human trampling. The HD site had signs of human trampling, browsing and grazing by domestic animals, and illegal cutting of large trees likely for honey bee harvesting, and fuel-wood collection (author's personal observation and information from the forest guards). The forest canopy of HD site was more open than the SD site due to intensity of disturbance. The tree species dominating SD site included Allophylus abyssinicus , Croton macrostachyus , Chionanthus mildbraedii, Ficus sur , Macaranga capensis Milletia ferruginea Subsp. darassana, Olea capensis subsp. macrocarpa , Olinia rochetiana , Podocarpus falcatus , Polyscias fulva , Pouteria adolfifriedricii , Schefflera volkensii,Syzygium guineense Subsp. afromontanum . The plant species dominating the HD site included Albizia gummifera, Apodytes dimidiata, Brucea antidysentrica, Calpurnia aurea , Celtis africana , Chionanthus mildbraedii, Croton macrostachyus, Ehretia cymosa, Milletia ferruginea Subsp. darassana , Oxyanthus speciosus , Podocarpus falcatus , Pouteria adolfi-friedricii and Teclea nobilis .
Three plots of 30 × 30 m size were randomly selected in each SD and HD site for litter bag experiment. A composite sample of freshly-fallen leaves was collected from the forest floor in each plot, air-dried for one week in the laboratory. The leaves collected were not in contact with the soil and not physically damaged. Litter decomposition was studied using the litter bag method. Thus, 20 (± 0.01) g of each composite sample were used to fill litter bags (25 × 25 cm and 2 mm mesh size which were then tied and closed according to Salinas et al. [30]. The electronic balance (DIAL-O-GRAM, OHAUS, USA) with a capacity of 2610 g and precision of (± 0.01) were used for the measurement of leaf litter. At the centre of each plot, 24 litter bags were buried in the 15 cm of top soil. The soil pH ranges from 4.0 to 6.7 and 4 to 6.2 in SD and HD sites, respectively. The litter bags were made of nylon clothes which contained the leaf litter collected in the plot. Thus, a total of 144 leaf litterbags (24 * 3 plots * 2 sites) were used for the litter decomposition experiment. Two litter bags from each plot were randomly retrieved 12 times at monthly intervals from 04 March 2014 to 03 February 2015. One year decomposition period was divided into wet (seven months) and dry (five months) seasons. Even if the experiment was not separate, the dry season data were calculated from October to the February to determine carbon and nutrient release pattern. The content of each bag was oven-dried at 80°C for 24 hours then weighed taking care not to lose any material and removing exogenous materials, such as visible animals and fine roots.
To correct for leaf litter water content sub-samples from the air-dry leaf litter collected in the forest plots were weighed before and after they were oven-dried at 80°C to constant weight. A conversion factor was calculated from each subsample as:
CF=Oven-dried weight (g)/Air-dried weight (g)
To get the oven-dried weight (hereafter dry weight) for each litter bag sample, the air-dried weight was multiplied by the corresponding CF:
Oven-dried weight (g)=Air-dried weight (g) * CF
A subsample of each air-dried leaf litter initially collected in each plot and litter collected at each sampling time were ground to pass through 1 mm sieve size of a Wiley mill and analyzed for C, N, P, and K. The organic C concentration was determined using Walkley Black Method [31], total N concentration was analyzed by sulphuric acid digestion followed by distillation and titration [31]. P concentration was determined using sulphuric acid digestion followed by colorimetric determination [31]. K concentration was analyzing using flame photometry after the sample has been digested by sulphuric acid [32]. Soil pH ( 1:2.5 (v/v) soil: water suspension) and soil moisture using soil moisture meter (4 in 1 Digital PH Sunlight Soil Moisture Meter, KC300) were determined in each sampling time.
Statistical analysis
The C and nutrient content of the decomposing leaf litter was calculated as
where, N is the percentage of the element remaining in the litter, Ci=is the concentration of the element in litter at the time of sampling; Co=is the initial concentration of element in the litter; Mi is the mass of dry matter at the time of sampling, M0 is the initial dry mass of the leaf litter and i is the time of sampling, from 1 to 12 months [33]. The percentage of nutrient released from the litter mass was calculated as 100-N. The differences in C, N, P, and K concentrations remaining in decomposing leaf litter between dry (five months) and wet (seven months) season, and also between SD and HD sites were evaluated by one way ANOVA (P< 0.05). As the dependent variables were correlated with each other, simple linear regression analysis was used to predict the relationship between initial litter chemistry and percentage C and nutrients (NPK) remaining. The rank order of the C and nutrient release pattern was determined from the percentage of the net release of each element at the end of the experiment period. To account for statistically significant differences, F and P values are presented in parentheses with between- and within-group degrees of freedom presented as a subscript of F. All the statistical analysis was done using SPSS version 20 [34] and R3.1.1 [35].
Results
Carbon and nitrogen release pattern
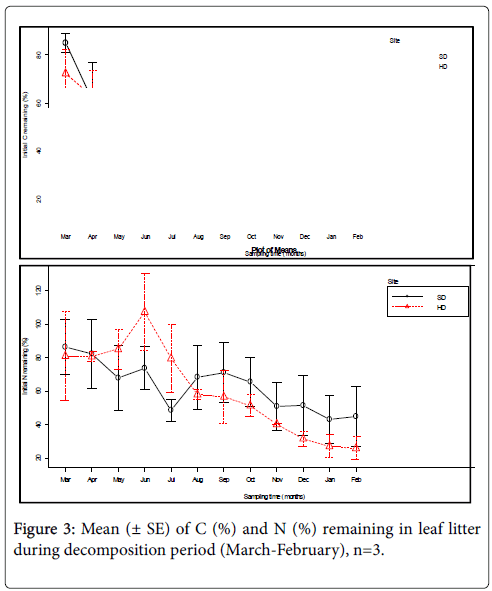
There was a rapid rate of carbon loss from the decomposing leaf litter in the first five months (0-150 days) for SD sites and in the first four months (0-120 days) for HD sites after the onset of the litter decomposition. The carbon remaining (%) in SD sites started to increase from July to August and then eventually decreases up to November of the study year. Finally the decrease in the C remaining (%) continues until the end of the experiment in SD sites. Similarly, the C remaining (%) during litter decomposition in HD sites remains more or less the same from June to August and then started progressively to decrease up to October. There was a little increase observed in November and then a rapid decrease up to the end of the experiment in HD sites (Figure 3). An average of about 56.26% of the initial carbon was remained by releasing 43.74% into the soil in SD site for the first five months. Similarly, in HD sites, 56.84% of the initial carbon was remained by releasing 43.16% into the soil in the first five months. The release of carbon into the soil continues progressively in both sites of the forest as was depicted in the Figure below. Finally, at the end of 365 days of decomposition period, 44.26% and 40.30% of the initial carbon concentration was remained by releasing 55.74% and 59.7% into the soil system in SD and HD sites, respectively.
When both sites of the forest compared, there was not statistically significant difference in the carbon concentration (F1, 70=0.628, P=0.431) remaining (%) during decomposition. Similar to the carbon concentration, the differences were not significant for all other nutrients remaining (%) between SD and HD sites of the forest (Table 1). As a matter of fact, the carbon release from the organic matter of leaf litter increases as decomposition period increases in both sites of the forest. It was observed that the release pattern of carbon from decomposing leaf litter in both sites depend on seasonal variation of a year which depend on the climatic variables (Figure 3). As evidence, the higher carbon release was observed in wet season of a decomposing period in both sites of the forest. The mean difference was strongly significant between dry and wet season at P<0.001. This might be because of the influence of decomposition by the decomposers activity in the soil system as they get suitable soil moisture, temperature and rainfall.
| C and Nutrients remaining (%) | SD (Mean ± SE) |
HD (Mean ± SE) |
F1,70 | P-value |
|---|---|---|---|---|
| Initial C (%) remaining | 44.26 ± 3.80 | 40.30 ± 3,24 | 0.628 | 0.431 |
| Initial N (%) remaining | 62.75 ± 4.60 | 60.24 ± 5.40 | 0.126 | 0.723 |
| Initial P (%) remaining | 66.50 ± 7.23 | 73.55 ± 7.04 | 0.031 | 0.487 |
| Initial K (%) remaining | 42.86 ± 5.23 | 26.9 ± 3.43 | 6.487 | 0.013* |
*The difference is significant at 0.05 level (2-tailed), n=72
Table 1: One way ANOVA test for initial carbon and nutrient (%) remaining between SD and HD sites of the decay period.
An increase in N remaining (%) was observed in the first four sampling periods (0-120 days) in HD sites of the forest but a slight decrease in the first three sampling periods (0-90 days) in SD sites. A rapid decrease in N remaining (%) was noted from June to August and a slight decrease continues from September to the end of the experiment in HD sites. The monthly rapid decreasing pattern was observed in May (90 days), July (150 days) and November (210 days) in nitrogen remaining (%) in decomposing leaf litter in SD sites. A slight increase in June (120 days), August (180 days) and September (210 days) was observed for N remaining (%) during decomposition in SD sites. Nevertheless, a slight decrease of N (%) continues up to the end of the decomposition period in SD sites as can be observed from December to February in Figure 3.
The result showed an inconsistent decrease and increase in N remaining (%) during litter decomposition. The one way ANOVA test showed significant difference (P<0.001) in N (%) remaining in decomposing leaf litter between wet and dry seasons but not significant between the two sites of the forest (Tables 1 and 2). At the end of 365 days of decomposition period, about 62.75% and 60.25% of the initial N (%) remained in decomposing leaf litter by releasing 37.25% and 39.75% into the soil system in SD and HD sites of the forest respectively. Here, the N dynamics indicate the larger immobilization than mineralization in both sites of the forest showing that N is not limiting element in the forest ecosystem (Table 2). Even though the difference between the two sites was not significant, the relatively higher value of N was released in the HD than SD site. This may be due the presence nitrogen fixing dominant tree species of family Leguminaceae such as Albizia gummifera, Calpurnia aurea and Milletia ferruginea Subsp. darassana and also due to the more disturbance effect in HD site.
| C and Nutrients remaining (%) | Wet (Mean ± SE) |
Dry (Mean ± SE) |
F1,70 | P-value |
|---|---|---|---|---|
| C (%) remaining | 28.53 ± 2.63 | 52.09 ± 3.05 | 30.76 | <0.001** |
| N (%) remaining | 43.08 ± 3.81 | 74.65 ± 4.35 | 26.92 | <0.001** |
| P (%) remaining | 44.05 ± 4.51 | 88.58 ± 6.68 | 25.68 | <0.001** |
| K(%) remaining | 24.46 ± 3.74 | 42.32 ± 4.60 | 8.07 | 0.006* |
Note:**The difference is significant at 0.01 level.
*The difference is significant at 0.05 level.
Table 2: Initial carbon and nutrient concentration analyzed from the litter mixtures in both sites.
Phosphorus (P) and potassium (K) release pattern
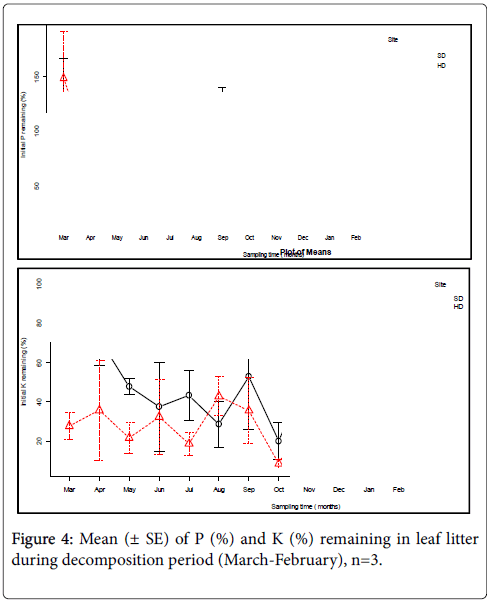
The P remaining (%) in the decomposing leaf litter showed a large temporal variability in the course of decomposition experiment. The concentration of P decreased during the first three months (0-90 days) over the initial one and then simultaneously increased in the fourth sampling time (120 days) for both sites of the forest. The release of P in the first three months was comparatively faster in SD sites than HD sites showing the rapid P mineralization. In contrast, P immobilization was simultaneously observed in the fourth sampling time at both sites.
At SD sites, the P immobilization (increased P concentration over the initial one) was observed from July to September sampling time and then P mineralization (the rapid decrease of the initial P concentration remaining or fast release of P into the soil system from the litter) was observed from September up to the end of the experiment (Figure 4). At HD sites, a slight increase in P remaining (%) was observed in the June, August, October, and November, sampling time and a fast decrease was observed in July and September, and from November up to the end the decomposition experiment (Table 3).
| Site | Plot | C (%) | N (%) | K (%) | P (%) | C:N |
|---|---|---|---|---|---|---|
| SD | SD1 | 26.13 | 2.61 | 0.0045 | 0.0045 | 10.01 |
| SD2 | 24.78 | 3.63 | 0.0039 | 0.0049 | 6.83 | |
| SD3 | 26.91 | 3.66 | 0.0048 | 0.0051 | 7.35 | |
| HD | HD1 | 27.89 | 4.26 | 0.0133 | 0.0044 | 6.54 |
| HD2 | 24.38 | 4.68 | 0.0147 | 0.0036 | 5.21 | |
| HD3 | 27.3 | 2.71 | 0.0088 | 0.0046 | 10.07 |
Note: SD1, SD2,SD3 are plots in slightly disturbed sites and HD1,HD2,HD3 are plots in highly disturbed sites
Table 3: Initial carbon and nutrient concentration analyzed from the litter mixtures in both sites.
The initial P (%) remaining in the decomposing leaf litter was not significantly different (P=0.487) between the two sites but significant (P<0.001) between wet and dry seasons of the decomposition period (Tables 1 and 2). Despite the inconsistent release pattern, the P remaining (%) at the end of 365 days was 66.50% and 73.50% by releasing 33.50% and 26.44% in SD and HD sites respectively. This pattern indicated the slow rate P- mineralization throughout the course of leaf litter decomposition experiment.
A rapid decrease of K remaining (%) in the first four months (0-120 days) of decomposition periods was observed indicating fast K mineralization/K- release into the soil system in SD sites. An increase in K remaining (%) was noted in July, September, and November sampling times during decomposition at the same site. In contrast, a drastic decrease in K remaining (%) was noted in August and October, and similarly a very slow decrease was observed in the last three months from December to February sampling time in SD sites. In case of HD sites, there was an increase in the K remaining (%) in the first two sampling time (0-60 days) observed. On top of this, there was also an increase in K remaining (%) during decomposition in the June, August and November sampling time indicating still the K- increase in the HD sites. At the same site, a decrease in K (%) over the initial one was observed in the May, July and October sampling period indicating K- release to the soil system and apparently a slow decrease continues after November till the end of the decomposition experiment (Figure 4).
When both sites compared in terms of the K remaining (%) during the litter decomposition, the mean difference was statistically significant (P=0.013). Similarly, the mean difference of K (%) remaining was significant between wet and dry season (P=0.006). At the end of the decomposition period, the K remaining (%) was found to be 42.85% and 26.91% by releasing 57.15% and 73.09% of the initial concentration into the soil system in SD and HD sites of the forest, respectively.
One way ANOVA test showed the non-significant difference in C and nutrients (%) remaining in decomposing leaf litter of the two sites (Table 1). The linear regression analysis also showed that there was no correlation between initial leaf litter chemistry and C, N, P concentration (P>0.05) released during the decomposition. A significant positive correlation (P=0.021) was observed between initial litter K and K released at the end of the decomposition experiment.
Discussion
Nutrient release pattern at different stages of decomposition
The present study showed significant differences between dry and wet seasons in concentrations of C, N, P and K in decomposing leaf litter in both SD and HD sites of the forest despite the litter types and not separated experiment (Tables 1 and 2). This means that the higher release was observed in wet season than dry season. This was due to the fact that the mean annual temperature of the area in the wet season is greater than in the dry season which facilitates the rapid ecosystem processes in the forest system. However, the differences between the two forest sites were not significant (P>0.05) except for initial K (%) remaining. The lack of difference between the two sites may be due to similar edaphic and climatic conditions (temperature and rainfall), the proximity of the two sites and the overlap of some forest species. Therefore, the local disturbance and the plant species composition did not seem to have a considerable impact on the C, N and P dynamics in the Boter-Becho forest, contrasting with other studies which found an influence of the species composition on the pattern of nutrient release [36]. In the case of K, the local disturbance showed an impact on K dynamics (P=0.013) as K is the most leachable cation and nonstructural element compared to others. The K release was higher in HD than SD sites probably due to the higher disturbance intensity in the HD sites which may enhance further leaching. The seasonal variation have showed a significant effect on the leaf litter decomposition in both sites of the forest as concluded in the study conducted by Hasanuzzaman and Hossain [37] in which the nutrient concentration differ significantly between dry and wet season of the decomposition experiment done on tree species on tropics.
Even if there was a decrease in P (%) remaining in the whole experimental period, an intermittent decrease (net mineralization) and increase (net accumulation or immobilization) was observed in the months of decomposition period (Figure 4). In general, an increase in P (%) remaining at some point of decomposition in both sites of the same forest can be supported by other similar studies [37,38]. According to Xuluc-Tolosa et al. [38], P (%) increased initially and then declined towards the end of the experiment. In contrast, a rapid rate of P release was observed at early decomposition period in the present study. This is attributed to a significant portion of P in leaves are inorganic forms and leaching might explain a major part of the P release from the leaf residues [13,14] otherwise there was no significant difference observed between initial litter P content and respective P mineralization (t=0.247, P=0.82). Similarly, Kwabiah et al. [15] reported that 54-82% of total P in leaves from six agroforestry tree species was in water-soluble forms, of which a significant proportion could be lost by leaching. Consequently, it might be expected that variability in P supply is an important control of litter decomposition in the tropical forests [12]. However, this is not the case for the Boter- Becho forest as evidenced from the P-net accumulation/increase observed at some point of decomposition particularly in between May and September. This may be attributed to a net accumulation in residual leaf litter showing that the P is not a limiting element particularly, in the study forest.
According to Heal et al. [5], N is one of the factors limiting rate of litter decomposition as it determines the growth and turnover of microbial biomass, mineralizing the organic carbon. Berg and Laskowski [4] identified an accumulation phase followed by a release phase for the litter N during decomposition among other models. In the case of HD sites, it follows a similar pattern by characterizing early immobilization in the first four months (0-120 days) and the release or mineralization eventually continues. However, N dynamics in the SD site follows another model where a leaching phase (phase I) is followed by an accumulation (phase II) and release phase (phase III) as described in Berg and Laskowski [4]. Accordingly, early N release was observed in the first three months (0-90 days) following an immobilization and then a release in the SD sites compared to relatively low N release in HD sites. This fluctuation of N remaining (%) in decomposing leaf litter during the experiment period may be due to the difference species composition though not considered in species-wise and availability of N (%) in the soil system of the forest. The researcher may also relate these results in such a way that Npoor leaf litter tends to accumulate N and Nrich leaf litter tends to release N even though it is difficult to explain this trend directly from the present study.
A peak increase of N (%) remaining in decomposing leaf litter was measured fourth sampling time (June, 120 days) for both sites of the forest. The reasons for this increase in wet season could be the result of uptake of an increased pool of available rhizosphere N and nitrogen fixation, particularly for the leguminous species which have contributed to an increase N level during the wet season. It is true that both sites have leguminous species (Milletia ferruginea Subsp. darassana ) identified and their leaves were included in the forest leaf mixture considered in litterbag experiment. Similar conclusion was made by many other authors like (Nigatu and Michelsen [14], Tolsma et al. [39]; Teklay [13]). Nevertheless, from the present study, the soil pH has shown a significant correlation (r=0.369, P=0.027, n=36) with the initial N (%) in decomposing litter in SD sites but not in HD sites (r=0.19, P=0.264 ). In the SD sites, the soil pH may have an impact on the N-mineralization. Moreover, the level of soil pH is not significantly different with the remaining analyzed mineral nutrients. The soil PH measured in SD sites range from 4.41 to 6.6 whereas in HD sites it ranges from 4.01 to 6.14. N-mineralization is restricted at low pH levels being the optimal pH for soil biomass growth has been established near neutrality. Nevertheless, in soils with pH values between 4 and 5, a significant N mineralization has been noted indicating that the microorganisms can be adapted to acid conditions [40].
A slight descent continues up to the end of the experiment by releasing this nutrient to the forest soil. High N loss observed in July (150 days) of decomposition in SD sites (Figure 3) may be attributed to stimulation of microbial activity and decomposition due to wetter conditions in the forest unlike the influence by the initial litter chemistry. Moreover, Heal et al. [5] mentioned that net release or net immobilization of total nitrogen can be estimated from the organic material’s C: N ratio or N concentration. Thus, if the C: N ratio is <20 or the N concentration >2.5%, N will be released by enhancing rapid decomposition of materials. If C: N ratio is much higher than 20, N is likely to be immobilized until decomposition and respiration lower the C: N ratio. All samples collected from forest leaf litter in the present study have shown initial C: N ratio of less than 20. Thus, the N release in the two sites of the forest has shown a tendency to slightly increase through time in the decomposition period in both sites of the forest. Leaching also can be explained for N release as much as 25% of it in the leaves may be removed by leaching [41] particularly at the early phase of decomposition.
A rapid decrease in the K (%) from the decomposing leaf litter was observed in the first four months (0-120 days) in SD sites of the forest and in the March, May, July and October sampling times in the HD sites. The decrease in K (%) was much higher in wet season than the dry season and was significantly different and similarly, the mean difference in K (%) remaining between the two sites was significant (P=0.013 ). The faster decrease of K (%) from the decomposing leaf litter in the present study was mainly because K is a non-structural element, highly mobile and most leachable cation during decomposition which was supported by various similar works by various authors (Berg and Laskowski [4]; Guo and Sims [42]; Tisdale et al. [43]; Hasanuzzaman and Hossain [37]). Moreover, K is not incorporated in to organic structures, and hence is less affected by leaf chemistry and soil faunal activity [44,45] but from the present study it was determined that the initial litter chemistry strongly correlated with the K release in both sites (R=0.879, p=0.021 ) and it seems that initial litter content of K determines the release of K. Therefore, K is the limiting element which may highly determine the plant growth and development in the Boter-Becho forest. Moreover, with regard to mineralization, Taylor noted that 85% of K and 50% of P loss in the leaves by leaching in tropical conditions.
Generally, the decrease of initial carbon and nutrients concentration in early stage observed from the present study may be due to the loss of the soluble forms of nutrients at the initial stages of decomposition which was also noted in a study by Mahmood et al. [46]. On the other hand, a slower decrease of initial carbon and nutrients (%) towards the later stages of leaf litter decomposition may be due to microbial oxidation of refractory components, physical and biological fragmentation. Similar observations were noted by various authors (Mahmood et al. [47]; Hasanuzzaman and Hossain [37]). At some points of decomposition in wet season, increased initial nutrients (N, P, and K) concentration in decomposing leaf litter was observed (Figures 3 and 4). This phenomenon is attributed to immobilization in the residual leaf litter (microbial or non-microbial) acting the decomposing leaf litter as a surface for fungi or heterotrophic organisms [4,47-49].
Conclusion
The pattern of carbon and nutrient dynamics clearly followed the pattern of mass loss in Boter-Becho forest (manuscript in preparation). The two sites of the forest did not show significant difference in carbon and major nutrients except K since both sites are in the same forest with similar edaphic, climatic variables and some forest species overlap among others. This showed that the local disturbance did not seem to have a considerable effect on the C, N and P dynamics in the forest. However, it seems that K is the limiting element in the forest soil of Boter-Becho for plants as a rapid release was noted throughout the decomposition period. In contrast to other studies, the initial litter quality did not show significant relationships with the C and nutrient release in the decomposition period except for K in Boter-Becho forest. A significant variation of C and nutrient release observed between wet (March to September) and dry season (October to February) may be due to the later stage of decomposition and the variation in climate (rainfall and temperature). A rapid decrease of mass remaining of C and nutrients in the wet season of both sites is due to the temperature in wet season is higher and warmer than that of dry season even if the experiment was not separate. The higher temperature in this season (March to September) with optimum rainfall enhances the rate of decomposition and hence the C and nutrient release to the soil and to the atmosphere. Due to the larger warming in the June to August season in Africa as predicted by Hulme et al. [50], there will be between 2 and 6°C warmer in 100 years. Thus, factors that increase the rate of decomposition and hence carbon and nutrient release could serve to increase the amounts of carbon-based gases in the atmosphere. It can also be concluded that a global warming might have an increasing influence on the rate of wet season decomposition in Boter-Becho forest which will in turn increase carbon based gases to the atmosphere. Though one year decomposition data are the first step study in nutrient release pattern in the forest, it is not enough to clearly extrapolate to long term nutrient release patterns. Therefore, further study should be sought on different forests of the country and different parts of the same forest by including microbial role in litter decomposition and the general carbon and nutrient release patterns.
Acknowledgements
The authors would like to thank the local people of Boter-Becho village in supporting data collection. Moreover, Hawassa University, college of Agriculture was duly acknowledged for analyzing the litter chemical samples. Our appreciation also goes to the anonymous reviewers of this manuscript.
References
- Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Blackwell Scientific Publications, Oxford.
- Bosatta E, Staaf H (1982) The control of Nitrogen turn-over in Forest litter. Oikos 39: 143-151.
- Wang Q, Wang S, Huang Y (2008) Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. For Ecol Manage 255: 1210-1218.
- Berg B, Laskowski R (2006) Litter Decomposition: A Guide to Carbon and Nutrient Turnover. Adv Ecol Res 38: 448.
- Heal OW, Anderson JM, Swift MJ (1997) Plant litter quality and decomposition: An historical overview. In: Cadisch G, Giller KE editors. Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, UK.
- Hill PW, Jones DL, Marshal C, Farrar JF (2006) Temporal and spatial dynamics of soil solution C and N concentrations during Lolium perrene establishment and the effect of elevated CO2 and N additions. Soil Biol Biochem 38: 1290-1297.
- Thomas K, Jijeesh CM, Seethalakshmi KK (2014) Litter production, decomposition and nutrient mineralization dynamics of Ochlandra setigera: A rare bamboo species of Nilgiri Biosphere Reserve, Indian J For Res 25: 579-584.
- Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD (2006) Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 9: 46-62.
- Sundarapandian SM, Swamy PS (1999) Litter production and leaf-litter decomposition of selected tree species in tropical forests at Kodayar in the Western Ghats, India. For Ecol Manage 123: 231-244.
- Berg B, McClaugherty C (2008) Plant Litter: Decomposition, Humus Formation, Carbon Sequestration. 2nd ed. Berlin: Springer.
- Harmon ME, Sexton J, Caldwell BA, Carpenter SE (1994) Fungal sporocarp mediated losses of Ca, Fe, K, Mg, Mn, N, P, and Zn from conifer logs in the early stages of decomposition. Can J Forest Res 24: 1883-1893.
- Vitousek P, Sanford RLJ (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17: 137-167.
- Teklay T (2007) Decomposition and nutrient release from pruning residues of two indigenous agroforestry species during the wet and dry seasons. Nutr Cycl Agroecosys 77: 115-126.
- Nigatu L, Michelsen A (1994) Litterfall and nutrient release by decomposition in three plantations compared with a natural forest in the Ethiopian highland. For Ecol Manage 65: 149 -164.
- Kwabiah AB, Stoskopf NC, Voroney RP, Palm CA (2001) Nitrogen and Phosphorus Release from Decomposing Leaves under Sub-Humid Tropical Conditions. Biotropica 33: 229-240.
- Vitousek PM, Turner DR, Parton WJ, Sanford RL (1994) Litter decomposition on the Mauna Loa environmental matrix, Hawaii: Patterns, mechanisms and Models. Ecology 75: 418-429.
- Slovic S (1997) Tree physiology. In: Huttl RF, Schaaf W (editors), Magnesium deficiency in forest ecosystems. Kluwer Academic, Dordrecht. pp: 101-214.
- Likens GE, Bormann FH (1995) Biogeochemistry of a forested ecosystem. Springer-Verlag, New York. p. 162.
- Anderson JM, Ineson P, Huish SA (1983) Nitrogen and cation mobilization by soil fauna feeding on leaf litter and soil organic matter from deciduous woodlands. Soil Biol Biochem 15: 463-467.
- Osono T, Takeda H (2004) Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J For Res 9: 23-31.
- Adams MB, Angradi TR (1996) Decomposition and nutrient dynamics of hardwood leaf litter in the Fernow whole-watershed acidification experiment. Forest Ecol Manag 83: 61-69.
- Hasegawam M, Takeda H (1996) Carbon and nutrient dynamics in decomposing pine needle litter in relation to fungal and faunal abundances. Pedobiologia 40: 171-184.
- Berg B, Cortina J (1995) Nutrient dynamics in some decomposing leaf and needle litters in a Pinussylvestris forest. Scand J Forest Res 10: 1-11.
- Blair JM (1988) Nitrogen, sulphur and phosphorus dynamics in decomposing deciduous leaf litter in the southern Appalachians. Soil Biol Biochem 17: 827-830.
- Friis I, Demissew S, Breugel PV (2010) Atlas of the Potential Vegetation of Ethiopia. The Royal Danish Academy of Sciences and Letters, Copenhagen, Denmark. p. 306.
- Yalden DW (1983) The extent of high-ground in Ethiopia compared to the rest of Africa. SINET: Ethiop J Sci 6: 35-39.
- Gindaba J, Olsson M, Itanna F (2004) Nutrient composition and short-term release from Croton macrostachyus Del. and Millettia ferruginea (Hochst.) Baker leaves. Biol Fertil Soils 40: 393-397.
- FAO (1998) The soil and terrain database for northeastern Africa. Land and Water Digital Media Series 2. FAO, Rome.
- NMSA (2015) National Metereological Service Agency, Ethiopia.
- Salinas N, Malhi Y, Meir P, Silman M, Roman Cuesta, et al. (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189: 967-977.
- Anderson JM, Ingram JSI (1993) Tropical Soil Biology and fertility. A hand book of method. 2nd ed. Wallington, UK.
- Allen SE, Grimshaw HM, Parkinson JA, Quarmby C, Roerts JD (1974). Chemical Analysis of Ecological Materials. Blackwell Scientific Publications, Oxford, London. p. 566.
- Bockheim JG, Jepson EA, Helsey DM (1991) Nutrient dynamics in decomposing leaf litter of four tree species in northern Wisconsin. Can J Forest Res 21: 267-286.
- IBM Corp (2011) IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
- R Development Core Team (2013). A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria.
- Torreta NK, Takeda H (1999) Carbon and Nitrogen dynamics of decomposing leaf litter in a tropical hill evergreen forest. Eur J Soil Biol 35: 57-63.
- Hasanuzzaman M, Hossain M (2014) Leaf Litter Decomposition and Nutrient Dynamics Associated with Common Horticultural Cropland Agroforest Tree Species of Bangladesh. Int J Forest Res 2014: 10.
- Xuluc-Tolosa FJ, Vester HFM, Ramirez-Marcial N, Castellanos-Albores J, Lawrence D (2003) Leaf litter decomposition of tree species in three successional phases of tropical dry secondary forest in Campeche, Mexico. For Ecol Manage 174: 401-412.
- Tolsma DJ, Ernest WHO, Verweij RA, Vooijs R (1987) Seasonal Variation of Nutrient concentration in a semi-arid savana ecosystem in Botswana. J Ecol 75: 755-770.
- Shah Z, Adams WA, Haven CDV (1990) Composition and activity of the microbial population in an acidic upland soil and effects of liming. Soil Biol Biochem 22: 257-263.
- Vitousek P (1984) Litterfall, nutrient cycling and nutrient limitation in tropical forests. Ecology 65: 285-298.
- Guo L, Sims REH (2002) Eucalypt litter decomposition and nutrient release under a short rotation forest regime and effluent irrigation treatments in New Zealand: II. Internal effects. Soil Biol Biochem 34: 913-922.
- Tisdale SL, Nelson WL, Beaton JD, Havlin JL (1993) Soil Fertility and Fertilizers. 5th edn, Prentice Hall, New Delhi, India.
- Tian G, Kang BT, Brussaard L (1992) Effects of chemical composition on N, Ca, and Mg release during incubation of leaves from selected agroforestry and fallow species. Biogeochemistry 15: 1-17.
- Ribeiro C, Madeira M, Araujo MC (2002) Decomposition and nutrient release from leaf litter of Eucalyptus globules grown under different water and nutrient regimes. For Ecol Manage 171: 31-41.
- Mahmood H, Saberi O, Misri K, Japar Sidik B (2007) Nutrients dynamics associated with leaf litter degradation of Bruguieriaparviflora (Whight and Arnold) at Kuala Selangor Mangrove Forest, Malaysia. Indian J For 30: 325-330.
- Mahmood H, Siddique MRH, Abdullah SMR, Saha S, Ghosh DC, et al. (2014) Nutrient dynamics associated with leaching and microbial decomposition of four abundant mangrove species leaf litter of the Sundarbans, Bangladesh. Wetlands 34: 439-448.
- Hossain M, Siddique MRH, Rahman MS, Hossain Z, Hasan, MM (2011) Nutrient dynamics associated with leaf litter decomposition of three agroforestry tree species (Azadirachtaindica, Dalbergia sissoo and Melia azedarach) of Bangladesh. J Forest Res 22: 577-582.
- Lin YM, Liu JW, Xiang P, Lin P, Ding ZH, et al. (2007) Tannins and nitrogen dynamics in mangrove leaves at different age and decay stages (Jiulong River Estuary, China). Hydrobiologia 583: 285-295.
- Hulme M, Doherty R, Ngara T, New M, Lister D (2001) Africa climate change: 1900-2100. Climate Res 17: 145-168.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 5908
- [From(publication date):
December-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 4738
- PDF downloads : 1170