Research Article Open Access
Cannabis Smoke Causes Up-Regulation of Akt and Bax Protein in Subfertile Patients Sperm Cells
Sreyashi Mitra1, Rinku Saha2, Sayantan Bhattacharyya1, Kushal K Kar3, Alex C Verghese4, Manabendra Dutta Choudhury2, Parag Nandi5, Shubhadeep Roychoudhury2 and Nabendu Murmu1*1Department of Signal Transduction and Biogenic Amines, Chittaranjan National Cancer Institute, Kolkata, India
2Department of Life science and Bioinformatics, Assam University, Silchar, India
3Mediland fertility clinic, Mediland hospital and Research centre Itkhola, Silchar, Assam, India
4Scientific & Laboratory Director, ASTRA Fertility Group,4303, Village Centre Court, Mississauaga, ON L4Z 1S2, Canada
5Cradle Fertility Centre, 26 Banamali Ghosal lane, Kolkata-700034, India
- Corresponding Author:
- Nabendu Murmu
PhD, Department of Signals Transduction and Biogenic Amines
Chittaranjan National Cancer Institute, 37-S.P. Mukherjee Road
Kolkata, India, Pin-700026
Tel No: 91-9831340813
E-mail: nabendu.murmu@cnci.org.in
Received date: September 30, 2015; Accepted date: October 30, 2015; Published date: November 07, 2015
Citation: Mitra S, Saha R, Bhattacharyya S, Kar KK, Verghese A, et al. (2015) Cannabis Smoke Causes Up-Regulation of Akt and Bax Protein in Subfertile Patient’s Sperm Cells. J Addict Res Ther 6:247. doi:10.4172/2155-6105.1000247
Copyright: © 2015 Mitra S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: Emerging worldwide evidences in support of adverse effects of cannabis smoke indicate its significant role in declining male fertility. The aim of the present study was to compare the percentage of damaged sperm cells and the expression profiles of cell survival protein p-Akt and pro-apoptotic protein Bax in non-smoker, tobacco smoke addicted and cannabis smoke addicted subfertile subjects. Method: Semen samples were collected from 80 male subjects of reproductive age group in Southern Assam of North-East India. 46 (57.5%), 25 (31.25 %) and 9 (11.325%) of these subjects were found to be cigarette smokers, cannabis smokers and non-smokers respectively. ROS levels in semen samples were measured by chemiluminescence assay. Sperm DNA integrity were assessed by acridine orange test, toluidine blue staining and TUNEL assay. Expression profiles of p-Akt and Bax were observed by flow cytometry and western blot analysis.
Results: Among three groups, the cannabis smoke addicted subjects showed the highest level of seminal ROS production along with the highest percentage of sperm DNA damage, chromatin abnormalities and apoptotic cells. High expression of Bax and low expression of p-Akt was observed in non-smoker and tobacco smoke addicted subjects. Conversely, cannabis smoke addicted group showed the highest expression of both p-Akt and Bax proteins.
Conclusion: The present study indicates cannabis smoke addiction to be more detrimental for male reproductive health compared to the tobacco smoke. The over-expression of both Akt and Bax proteins among cannabis smokers suggest that the up-regulation of pro-survival protein Akt, during sperm meiotic division could have triggered the oxidative apoptosis of sperm cells via the up-regulation of pro-apoptotic protein Bax.
Keywords
Infertility; Cannabis addiction; ROS; DNA damage; Apoptosis; Akt; Bax
Introduction
Since last few decades, a sharp decline in male fecundity has been observed all over the world. In addition to congenital abnormalities, environmental and occupational exposures, changed lifestyle factors were also found to impact male reproductive health [1-3]. Several studies reported direct association of excessive tobacco and alcohol consumption with the declining male fertility [4,5]. Emerging studies have also correlated addiction to cannabis smoke with poor semen quality of men [6,7].Contents of cannabis smoke reduce antioxidant defence mechanism and increase oxidative stress in seminal plasma [8,9].
It has been estimated that around 150 million people across the globe were addicted to cannabis in the beginning of this millennia [10].The hallucinogenic effects caused by this recreational drug, entices people of different age groups and socio-economic classes in different countries all over the world [11,12]. ‘Cannabis’ is a generic term used for marijuana, hashish and hash oil and it is derived from the Cannabis sativa plant [13]. Δ9-Tetrahydrocannabinol (THC) is the unique compound of cannabis with major psychoactive effects and is said to act upon a specific cannabinoid receptor (CB1) in the brain [14,15]. In the 1990s, it was observed that cannabinoid compounds are naturally synthesized in human body from fatty acid derivatives termed as endogenous cannabinoids or endocannabinoids [16,17]. Endocannabinoids modulate several pathophysiologic processes like neuropathic pain, movement disorders such as Parkinson disease, Huntington disease and many other conditions like atherosclerosis, obesity as well as reproductive health [18]. However, the association between cannabis smoking and cancer is highly disputed as different case-control studies had inferred different results [19,20]. Both cannabinoid [21] and nicotine [22] receptors are coupled to the protein kinase B (Akt) signalling pathway. Akt is a serine threonine kinase which induces anti-apoptotic signal and inhibits apoptosis. However, the role of Akt coupled with cannabinoid receptor, varies from one disease to another. In Alzheimer’s disease, activation of cannabionoid receptor [23] and subsequent activation of Akt pathway can prevent brain cell death caused by the production of beta-amyloid protein [24]. Conversely, the Akt signalling cascade inhibits apoptosis and promotes tumour progression in cancer. Downstream signalling cascade of Akt has Bax protein. Bax is a member of the Bcl-2 (pro-apoptotic) family of proteins with accelerates apoptosis induced by a variety of stimuli [25,26].It promotes mitochondrial cytochrome cleakage by dimerization and insertion into mitochondrial membrane which eventually leads to the nuclear fragmentation of the cell [27].
Several plausible theories explain the probable mechanism of abnormal sperm formation [28,29]. Recent advances in the field of genetics and molecular biology provided a great impetus to explore sperm chromosomal abnormalities at molecular level [30]. These studies suggested that mutation during sperm meiotic division may trigger DNA fragmentation and subsequent apoptosis of the cells in humans and experimental animals [31-33]. On the other hand, several literatures revealed that DNA damage in sperm cells is associated with elevated levels of reactive oxygen species (ROS) production causing oxidative stress [34-36]. At lower level, ROS play an important role in sperm maturation and functions such as capacitation and acrosome reaction [37]. However, increased ROS production, beyond the antioxidant capacity limit in seminal plasma often resulted in cell and DNA damage. The polyunsaturated fatty acid (PUFA) content makes the sperm cells susceptible to the peroxidation in the presence of seminal ROS, resulting in the up-regulation of apoptotic pathways [38- 42].
The aim of the present study was to compare the seminal oxidative stress, percentage of sperm DNA damage and expression profiles of Akt and pro-apoptotic protein Bax in sperm cells of three groups of non-smoker, tobacco smoke addicted and cannabis smoke addicted subfertile subjects in Southern Assam of North-East India.
Materials and methods collection of semen samples
Total (n=80) semen samples were collected for the study with 96% power from the subjects of reproductive age group (25-40 years) living in Southern Assam of North-East India. Each subject was asked to sign an informed consent form approved by Institutional Ethical Committee (IEC), Assam University, Silchar and were asked to fill in a questionnaire. The subjects were instructed 2-3 days of sexual abstinence prior to semen ejaculation by masturbation into a sterile, wide mouthed, labeled container. Semen samples were collected as per guidelines of latest edition of World Health organization (WHO, 2010) manual. Patients with specific congenital abnormalities like-hypospadias, cryptorchisdism and other systemic diseases that may impair reproductive capacity such as hepatic, renal, endocrine, autoimmune diseases along with HIV infected patients were excluded from the study. 46 (57.5%), 25 (31.25 %) and 9 (11.325%) subjects were found to be cigarette smokers, cannabis smokers and non- smokers respectively. Semen analysis categorized these samples into two specific categories- Oligoasthenozoospermia and Teratozoospermia. Patients identified as Azoospermic (no sperm cells in seminal ejaculate) were also excluded from this study.
Measurement of reactive oxygen species
ROS levels in seminal ejaculates were measured by chemiluminescence assay using luminol (5-amino-2, 3- dihydro-1, 4-phthalazinedione; Sigma, St Louis, MO) as the probe. 10 μL of 5 mmol/L luminol prepared in dimethyl sulfoxide (Sigma Chemical) were added to 400 μL of the washed sperm suspension. Negative controls were prepared by replacing the sperm suspension with 400 μL phosphate buffered saline. Positive control included 400 μL of PBS and 50 μL of hydrogen peroxide (30%; 8.8 M) in triplicates. Chemiluminescence was measured for 15 minutes using a luminometer (Promega Glomax 20/20 Luminometer).The results were expressed as relative light units (RLU)/ sec/106 sperm [43].
Tests for sperm DNA integrity acridine orange test (AOT):
Acridine orange test (AOT) is a simple microscopic procedure based on acidic conditions to denaturant DNA followed by staining with acridine orange. The AOT measures the metachromatic shift of AO fluorescence from green (native DNA) to red (denatured DNA). Sperm smears were fixed in Carnoy’s solution (60% ethanol, 30% chloroform and 10% glacial acetic acid) and were subsequently stained with acridine orange solution (0.02% acridine orange in citratephosphate buffer, pH 2.5) according to the procedure of Tejada et al. [44]. After 5 minutes of staining, each smear was washed with distilled water, covered with a cover slip and sealed with a synthetic resin to prevent the smear from drying. Smears were examined within 1 day using a Leica fluorescence microscope (Leica DM 4000 B) with the following filter combination: 450 nm to 490 nm excitation and 520 nm barrier filters. All spermatozoa with fully compacted nuclei were examined. Nuclei of 300 spermatozoa were scored on the basis of their fluorescence (green/red). In recent studies percentage of DNA fragmentation has been represented as DFI (DNA fragmentation Index).
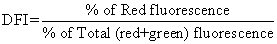
Toluidin blue staining for sperm chromatin abnormalities
Toluidine blue (TB) staining had been reported to be a sensitive test for incomplete DNA structure and packaging [45,46]. A thin smear was prepared and the air-dried and fixed in freshly prepared 96% ethanolacetone (1:1) at 4°C for 1 hour. After that the samples were hydrolyzed in 0.1 N HCL at 4°C for 5 minutes. Thereafter, the slides were rinsed 3 times in distilled water for 2 minutes and finally stained with 0.05% TB (in 50% McIlvaine’s citrate phosphate buffer, pH 3.5, Merck) for 5 minutes at room temperature. The slides were rinsed briefly in distilled water. Under light microscopic evaluation, a total of 300 spermatozoa were counted in different areas of each slide using oil immersion with × 200 magnifications. Sperm cell heads with good chromatin integrity stained light blue and those of diminished integrity were deep blue in colour.
TUNEL assay of sperm cells
Apoptosis in sperm cells was determined by the TUNEL technique using TACS® 2 TdT DAB kit (Trevigen, Catalogue No.4810-30-K). The terminal deoxynucleotidyl transferase-mediated (TdT) deoxyuridine triphosphate (dUTP) nick end labeling assay (TUNEL) is a direct quantification of sperm DNA fragmentation. dUTP is incorporated at single stranded and double stranded DNA fragments in a reaction catalyzed by the enzyme TdT. The DNA breaks based on the incorporated dUTP are then labelled and counter stained with 1% Methyl Green [47]. Sperm cells are then classified as TUNEL positive (deep brown), negative (green) and expressed as a percentage of the total sperm in the population. A total of 300 spermatozoa were counted in different areas of each slide using oil immersion under bright field microscope with × 400 magnifications.
Flow cytometric analysis
The sperm cell pellets were suspended in 900 μL in PBS and centrifuged at 4000 rpm for 5 minutes at room temperature. The pellet was resuspended in 900 μL PBS and this step was repeated twice. The final pellet was resuspended in 100 μL PBS and the number of cells were counted using haemocytometer. 5 x 106 sperm cells were dissolved again in 100 μL PBS and the suspension incubated with 0.5% TritonX- 100 for 20 minutes at room temperature followed by PBS wash. The cells were next incubated with Alexa Fluor 488 tagged anti p-Akt and Bax antibodies for 1hour at room temperature. After two consecutive PBS washes, the cells were fixed using 100μL paraformaldehyde. Prior to acquiring, 200 μL sheath fluid was added. Expression of proteins were obtained using BD FACS Calibur machine.
Protein extraction and western blot analysis
To prepare a whole sperm cell extract aliquots of 0.5 ml samples were centrifuged at 7500 g for 5 minutes at room temperature and the supernatant was discarded. The resulting sperm pellet was resuspended in 500 μL extraction medium (2% SDS, 28% sucrose, 12.4 mM N,N,N9,N9-tetramethylethylenediamine and 185 mM Tris–HCl, pH 6.8) and immediately incubated for 5 minutes at 100°C. After centrifugation the concentration of proteins in the supernatant was measured using BSA kit (Thermo Scientific) as per the manufacturer’s protocol. Finally, extracts were stored at -20°C until used for western blot analysis [48]. The proteins were subjected to SDS-PAGE and electro-transferred to nitrocellulose membranes. The membrane was blocked with 5% non-fat dry milk in Tris-buffer saline (20 mM Tris HCL and 137 mM NaCl, pH-7.5) for 1 hour at room temperature. Immunogenicity was detected by incubation of the membrane overnight with appropriate primary antibodies p-Akt and Bax (1:200) and specific proteins were detected by the enhanced chemiluminescence system (Biovision ECL Western Blot substrate). The Signal intensity of the band was detected by densitometer (Bio Rad, GS 800).β-actin expressions were tested for confirming equal distributions of proteins.
Statistical analysis
Statistical analyses were performed using Graph Pad Prism 5.00 gold software package. t-test was used to compare the means. Median values were calculated as 25th and 75th percentile. Spearman rank correlation coefficient (r) was calculated to find the correlation between variables. P ≤ 0.05 was taken to be statistically significant.
Results
The results of the present study showed no significant deviation (p>0.05) in the mean age (in years) and average BMI (body mass index) (Kg/m2) of non-smoker, tobacco smoke addicted and cannabis smoke addicted subjects [Mean age, non-smokers-29.28 ± 1.97, tobacco smokers-29.63 ± 2.95, cannabis smokers - 29.08 ± 2.43; BMI, non-smokers-24.14 ± 1.28, tobacco smokers-24.08 ± 1.44, cannabis smokers-24.22 ± 1.41] which could be the contributing factors of their fertility status.
ROS production in semen samples
The oxidative stress was measured by assessing the production of ROS level in seminal ejaculates of three groups of subjects. The highest and the lowest ROS production was observed in subjects addicted to cannabis smoke [161.5 (154; 163.25) (RLU/sec/106 sperm)] and in non-smoker group respectively [123.25 (132; 136.8) (RLU/sec/106 sperm)] whereas, the tobacco smoke addicted subjects showed the intermediate level of ROS production [144.5 (142; 153.5) (RLU/sec/106 sperm)] (Table 1).
| Variables | Non-smoker | Tobacco smoker | Cannabis smoker | p value |
|---|---|---|---|---|
| (n=9) | (n=46) | (n=25) | ||
| ROS production [(RLU)/sec/106sperm] | 123.25(132;136.8)** | 144.5(142;153.5)** | 161.5(155;163.6)** | |
| 36.33±3.97 (47.53) | 41.82±8.14a (53.32) | 51.77±4.61a,b (62.17) | p<0.001 | |
| % of Sperm DNA damage | ||||
| (DFI) | 36.81±4.46 | 45.06±2.80a | 52.83±4.67a,b | p<0.001 |
| % of Sperm chromatin abnormalities | ||||
| 37.93±3.84 | 42.92±1.98a | 52.44±2.87a,b | p<0.001 | |
| % of Apoptotic Sperm Cells |
** Value s are represented as median (25th; 75th percentile)
Percentages are calculated as Mean ± SD
DFI- DNA Fragmentation Index
ap<0.01 statistically significant compared to non-smoker group.
bp<0.01 statistically significant compared to tobacco addicted group
Table 1: Relative ROS production and percentage of Sperm cell DNA integrity among three groups of patients.
Acridine orange fluorescence study of sperm nuclei
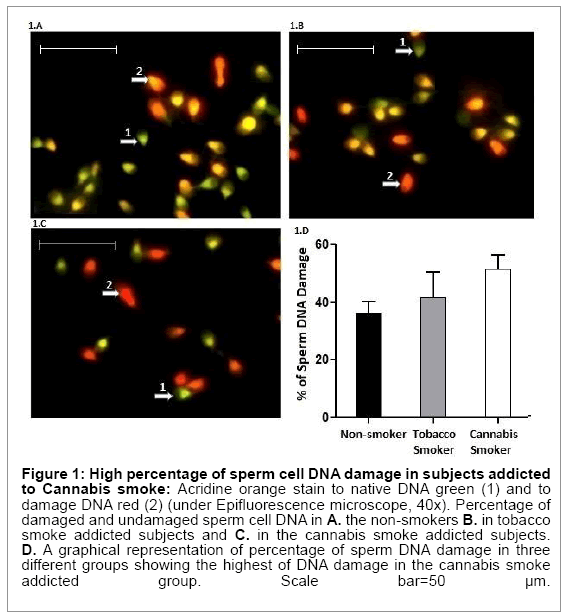
Table 1 summarizes significant differences (p<0.001) in the percentages of sperm cell DNA damage (red fluorescence; single stranded/denatured DNA) among three groups of subjects [cannabis smokers- 51.77 ± 4.61, non-smokers-36.33 ± 3.97, tobacco smokers-41.82 ± 8.14 respectively] (Figure 1A-1D).
Figure 1: High percentage of sperm cell DNA damage in subjects addicted to Cannabis smoke: Acridine orange stain to native DNA green (1) and to damage DNA red (2) (under Epifluorescence microscope, 40x). Percentage of damaged and undamaged sperm cell DNA in A. the non-smokers B. in tobacco smoke addicted subjects and C. in the cannabis smoke addicted subjects. D. A graphical representation of percentage of sperm DNA damage in three different groups showing the highest of DNA damage in the cannabis smoke addicted group. Scale bar=50 μm.
Assessment of sperm chromatin abnormalities among different subjects
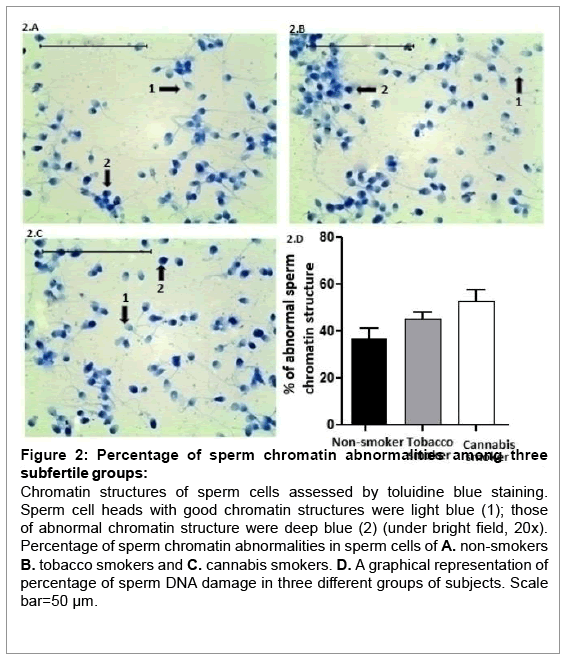
The percentage of abnormal sperm chromatin structure and its condensation was compared between the three groups by Toluidine Blue staining (Table 1). The results showed highest percentage (52.83 ± 4.67) of sperm chromatin abnormalities among cannabis smokers. Tobacco smokers (45.06 ± 2.80) and non- smokers (36.81 ± 4.46) also showed moderately high percentage of sperm chromatin abnormalities (Figure 2A-2D).
Figure 2: Percentage of sperm chromatin abnormalities among three subfertile groups:
Chromatin structures of sperm cells assessed by toluidine blue staining. Sperm cell heads with good chromatin structures were light blue (1); those of abnormal chromatin structure were deep blue (2) (under bright field, 20x). Percentage of sperm chromatin abnormalities in sperm cells of A. non-smokers B. tobacco smokers and C. cannabis smokers. D. A graphical representation of percentage of sperm DNA damage in three different groups of subjects. Scale bar=50 μm.
Variation in the percentage of apoptotic sperm cells among three groups
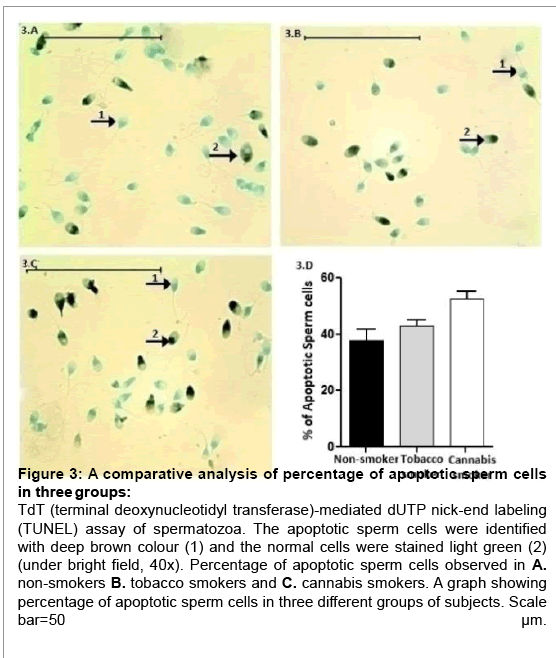
The TUNEL assay results were again consistent with the DNA fragmentation results. The overall incidence of sperm cell apoptosis was significantly different (p<0.001) among the three groups of subjects (Table 1). The highest percentage (52.44 ± 2.87) of apoptotic sperm cells were observed again in subjects addicted to cannabis smoke. Tobacco smokers (42.92 ± 1.98) and non-smokers (37.93 ± 3.84) also showed moderately high percentage of apoptotic sperm cells (Figure 3A-3D).
Figure 3: A comparative analysis of percentage of apoptotic sperm cells in three groups:
TdT (terminal deoxynucleotidyl transferase)-mediated dUTP nick-end labeling (TUNEL) assay of spermatozoa. The apoptotic sperm cells were identified with deep brown colour (1) and the normal cells were stained light green (2) (under bright field, 40x). Percentage of apoptotic sperm cells observed in A. non-smokers B. tobacco smokers and C. cannabis smokers. A graph showing percentage of apoptotic sperm cells in three different groups of subjects. Scale bar=50
Correlation between increasing seminal ROS production and percentage of DNA damage, chromatin abnormalities and apoptosis in sperm cells
The Spearman correlation analysis showed significant (p<0.0001) positive correlations between increasing seminal ROS production and percentage of sperm DNA damage, chromatin abnormalities and the percentage of apoptotic sperm cells in cannabis smoke addicted subjects. However, no significant association between these parameters was observed among non-smokers and tobacco smokers (Table 2).
| Groups | % of Sperm DNA damage | % of Sperm chromatin abnormalities | % of Apoptotic Sperm Cells |
|---|---|---|---|
| r p | r p | r p | |
| Non-smoker | 0.025 NS | 0.050 NS | -0.666 NS |
| Tobacco smoker | 0.021 NS | 0.084 NS | -0.1098 NS |
| Cannabis smoker | 0.9980 <0.0001 | 0.9955 <0.0001 | 0.9928 <0.0001 |
NS-Not Significant; r-spearman’s rank correlation coefficient
Table 2: Comparison of sperm DNA integrity among three groups of patients.
Differential expression of P-Akt and Bax protein in sperm cells of three groups
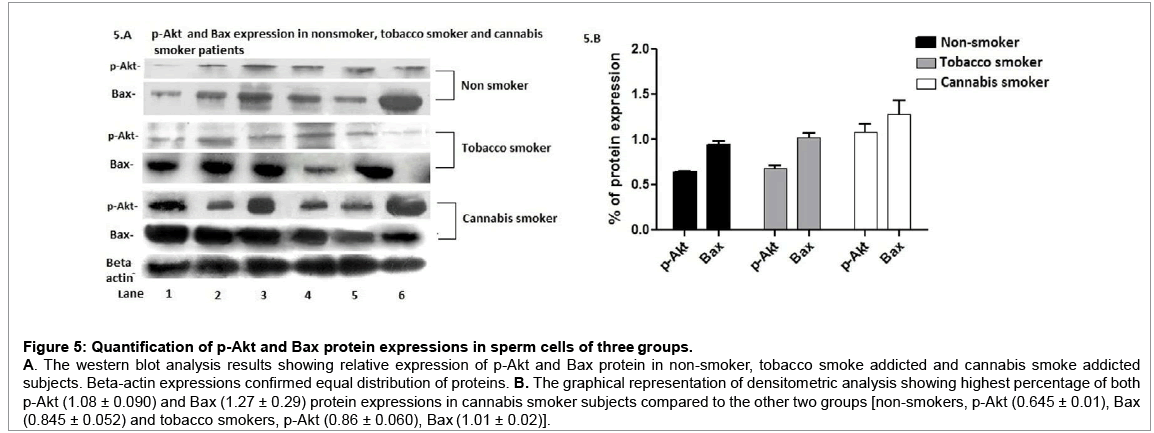
The flow cytometric analysis showed high positive expression of Bax protein in all three groups of subjects, whereas high expression of p-Akt was observed among cannabis smokers only (Figure 4A-4D). This observation was further confirmed by the western blot analysis results (Figure 3). The quantitative densitometric analysis showed highest percentage (1.27 ± 0.15) of Bax protein expression in cannabis smoke addicted subjects compared to the non-smokers and tobacco smokers (0.845 ± 0.052 and 1.01 ± 0.02 respectively). The highest percentage (1.08 ± 0.090) of p-Akt was also observed in cannabis patients’ sperm cells, whereas the other two groups [non-smokers (0.645 ± 0.01), tobacco smokers (0.86 ± 0.060)] showed comparatively low percentage of p-Akt expressions (Figure 5A and 5B).
Figure 4: Analysis of p-Akt and Bax protein expressions in Non-smoker, Tobacco smokers and Cannabis smokers:
Forward-angle light scatter (FSC) versus side-angle light scatter (SSC) dot plots obtained by flow cytometry is represented here. The gates used to select the events for subsequent Alexa fluor 488 analyses and were determined depending on the event position in the dot plot, related with its FSC versus SSC. A. Non-smoker subjects showing 25.5% Bax positive sperm cells and only 3.5% p-Akt positive cells. B. Subjects addicted to tobacco smoke showing 32.1% Bax positive sperm cells and only 4.2% p-Akt positive cells C. 44.6% Bax positive and 30.5% p-Akt positive sperm cells were observed in cannabis smokers. D. A graph showing relative expressions of Bax and p-Akt proteins in three groups of subjects by flow cytometry.
Figure 5: Quantification of p-Akt and Bax protein expressions in sperm cells of three groups.
A. The western blot analysis results showing relative expression of p-Akt and Bax protein in non-smoker, tobacco smoke addicted and cannabis smoke addicted subjects. Beta-actin expressions confirmed equal distribution of proteins. B. The graphical representation of densitometric analysis showing highest percentage of both p-Akt (1.08 ± 0.090) and Bax (1.27 ± 0.29) protein expressions in cannabis smoker subjects compared to the other two groups [non-smokers, p-Akt (0.645 ± 0.01), Bax (0.845 ± 0.052) and tobacco smokers, p-Akt (0.86 ± 0.060), Bax (1.01 ± 0.02)].
Discussion
Worldwide evaluation of different infertility cases across the world suggests that the largest percentage of patients experience idiopathic infertility [49]. A proper understanding of the underlying mechanism causing male factor infertility requires insightful analysis of molecular and cellular events involved in human spermatogenesis [50]. Spermatogenesis is a complex process which produces mature sperm cells from undifferentiated germ cells via mitosis and meiosis. Previous work by Zhuang et al. [33] showed that chromosomal aberration during spermatogenesis causes apoptosis of haploid sperm cells and decline in the fertilizing potential [33]. An important review work by Diemer and his co-worker discussed about different genetic disorders during spermatogenesis [51]. A study by Jamieson et al. [52] showed that chromosomal aberration has profound effect on spermatogenesis and in some cases few mature spermatozoa are produced which eventually undergo apoptosis. On the other hand, increasing evidences have strongly correlated declining semen parameters with addiction to tobacco and cannabis smoke [4,6,7]. Some of the significant studies by Rossato et al. and Kolodny et al. have linked rampant cannabis consumption with declining fertility rate in men [53,54]. In particular, it has been revealed by the works of Schuel H et al. and Berdyshev EV et al. [55,56]that cannabinoids influence human sperm functions, leading to a reduction of their fertilizing ability in both invertebrates and vertebrates. In the present study we observed the highest percentages of seminal ROS production, damaged sperm cell DNA, chromatin abnormalities and apoptotic sperm cells in cannabis addicted individuals compared to the non-smokers and tobacco smokers. The percentage of apoptotic sperm cells was also high in the tobacco smokers which could be one of the predominant causes of their declining fertility status. The comparative analysis of our study suggested that cannabis consumption could be more deleterious for the fertility status of an addicted individual compared to those addicted to tobacco smoke. Previous works by Yamaguchi and his co-worker [57] as well as Gardai et al. [58] showed that over-expression of Akt suppresses the localization of Bax to mitochondria and subsequent apoptosis of the cell. Interestingly, in this study we observed high expression of both Akt and pro-apoptotic protein Bax in sperm cells of cannabis smokers but comparatively lower expression of Akt in the other two groups of subjects. Although the precise molecular mechanism behind this phenomenon is yet to be explored, one plausible hypothesis would be up-regulation of Akt during sperm meiotic division. Mutation during sperm meiotic division might trigger the up-regulation of Akt protein or the activation of Akt may occur naturally. A work by Andersen et al. showed that activation of Akt and the subsequent phosphoinositide 3-kinase signalling pathway promotes cell growth and differentiation in oocytes [59]. Furthermore, another work by Veronique Nogueira et al. suggested that Akt induces oxidative senescence and sensitizes cells to undergo apoptosis [60]. It can be hypothesized that the upregulation of pro-survival protein Akt during sperm meiotic division could not induce the survival of haploid sperm cells as they do not divide. Alternatively, Akt triggered the oxidative apoptosis of the sperm cells via pro-apoptotic Bax protein. This could be the possible reason behind the synchronous expression of Akt and Bax in the sperm cells of cannabis smokers. In conclusion, the present study clearly shows that cannabis smoke is far more detrimental for the male reproductive health. Additionally, it causes up-regulation of Akt protein which in turn triggers the oxidative apoptosis of sperm cells via Bax protein. Although in-depth molecular studies need to be executed for a better understanding of these phenomena.
Acknowledgements
This entire work was funded by department of Signal transduction and Biogenic Amines (STBA), Chittaranjan National Cancer Institute (CNCI), Department of Life science and Bioinformatics, Assam University, Silchar, Assam, India. Dr. Jaydeep Biswas, Director, Chittaranjan National Cancer Institute (CNCI), Kolkata. Department of Biotechnology (DBT), New Delhi, India. We are thankful for their kind cooperation and support.
References
- Singh R, Hamada AJ, Bukavina L, Agarwal A (2012) Physical deformities relevant to male infertility. Nat Rev Urol 9: 156-174.
- Joffe M (2003) Infertility and environmental pollutants. Br Med Bull 68: 47-70.
- De Rosa M, Zarrilli S, Paesano L, Carbone U, Boggia B, et al. (2003) Traffic pollutants affect fertility in men. Hum Reprod 18: 1055-1061.
- Kovac JR, Khanna A, Lipshultz LI (2015) The effects of cigarette smoking on male fertility. Postgrad Med 127: 338-341.
- Muthusami KR, Chinnaswamy P (2005) Effect of chronic alcoholism on male fertility hormones and semen quality. FertilSteril 84: 919-924.
- Eisenberg ML (2015) Invited Commentary: The Association between Marijuana Use and Male Reproductive Health. Am J Epidemiol 182: 482-484.
- duPlessis SS, Agarwal A, Syriac A (2015) Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet.
- Yousefniapasha Y, Jorsaraei G, Gholinezhadchari M, Mahjoub S, Hajiahmadi M, et al. (2015) Nitric oxide levels and total antioxidant capacity in the seminal plasma of infertile smoking men. Cell J 17: 129-136.
- Sarafian TA, Magallanes JA, Shau H, Tashkin D, Roth MD (1999) Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. Am J Respir Cell MolBiol 20: 1286-1293.
- Iversen L (2000) The Science of Marijuana, Oxford University Press.
- Clough AR, Lee KS, Cairney S, Maruff P, O'Reilly B, et al. (2006) Changes in cannabis use and its consequences over 3 years in a remote indigenous population in northern Australia. Addiction 101: 696-705.
- Hall W, Degenhardt L (2007) Prevalence and correlates of cannabis use in developed and developing countries. CurrOpin Psychiatry 20: 393-397.
- Hall W, Degenhardt L (2009) Adverse health effects of non-medical cannabis use. Lancet 374: 1383-1391.
- Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, et al. (2013) The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One 8: e76635.
- Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61-65.
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, et al. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946-1949.
- Felder CC, Veluz JS, Williams HL, Briley EM, Matsuda LA (1992) Cannabinoid agonists stimulate both receptor- and non-receptormediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. MolPharmacol 42:838–845.
- Pacher P, Bátkai S, Kunos G (2006) Theendocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58: 389-462.
- Sewell RA, Cohn AJ, Chawarski MC (2008) Doubts about the role of cannabis in causing lung cancer. EurRespir J 32: 815-816.
- Penson DF (2015) Re: Association between Cannabis Use and the Risk of Bladder Cancer: Results from the California Men's Health Study. J Urol 194: 667.
- Gómez del Pulgar T, Velasco G, Guzmán M (2000) The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J 347: 369-373.
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, et al. (2003) Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111: 81-90.
- Panikashvili D1 Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, et al. (2001) An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413: 527-531.
- Leker RR, Shohami E, Abramsky O, Ovadia H (1999) Dexanabinol; a novel neuroprotective drug in experimental focal cerebral ischemia. J NeurolSci 162: 114-119.
- Zhang N, Chen X (2015) Potential role of O-GlcNAcylation and involvement of PI3K/Akt1 pathway in the expression of oncogenic phenotypes of gastric cancer cells in vitro. BiotechnolApplBiochem .
- Pawlowski J, Kraft AS (2000) Bax-induced apoptotic cell death. ProcNatlAcadSci U S A 97: 529-531.
- Chiu SM, Xue LY, Usuda J, Azizuddin K, Oleinick NL (2003) Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Br J Cancer 89: 1590-1597.
- He J, Xia M, Tsang WH, Chow KL, Xia J (2015) ICA1L forms BAR-domain complexes with PICK1 and is crucial for acrosome formation in spermiogenesis. J Cell Sci 128: 3822-3836.
- Ma B, Qi H, Li J, Xu H, Chi B et al. (2015) Triptolide disrupts fatty acids and peroxisome proliferator- activated receptor (PPAR) levels in male mice testes followed by testicular injury: A GC-MS based metabolomics study.Toxicology2:84-95
- Kohn TP, Clavijo R, Ramasamy R, Hakky T, Candrashekar A, et al. (2015) Reproductive outcomes in men with karyotype abnormalities: Case report and review of the literature. Can UrolAssoc J 9: E667-670.
- Jiang H, Wang L, Cui Y, Xu Z, Guo T, et al. (2014) Meiotic chromosome behavior in a human male t(8;15) carrier. J Genet Genomics 41: 177-185.
- Vendrell X, Ferrer M, García-Mengual E, Muñoz P, Triviño JC, et al. (2014) Correlation between aneuploidy, apoptotic markers and DNA fragmentation in spermatozoa from normozoospermic patients. ReprodBioMed Online 28: 492–502.
- Zhuang X, Huang J, Jin X, Yu Y, Li J, et al. (2014) Chromosome aberrations and spermatogenic disorders in mice with Robertsonian translocation (11; 13). Int J ClinExpPathol 7: 7735-7743.
- Kumar V, Abbas AK, Aster JC (2012) Robbins Basic Pathology.(9th edn), Saunders.
- Gunes S, Al-Sadaan M, Agarwal A (2015) Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online 31: 309-319.
- Lopes S, Jurisicova A, Sun JG, Casper RF (1998) Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 13: 896-900.
- Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. FertilSteril 79: 829-843.
- Fraczek M, Szkutnik D, Sanocka D, Kurpisz M (2001) [Peroxidation components of sperm lipid membranes in male infertility]. Ginekol Pol 72: 73-79.
- Dandekar SP, Nadkarni GD, Kulkarni VS, Punekar S (2002) Lipid peroxidation and antioxidant enzymes in male infertility. J Postgrad Med 48: 186-189.
- Agarwal A, Tvrda E, Sharma R (2014) Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. ReprodBiolEndocrinol 12: 45.
- Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, et al. (1998) Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. BiolReprod 59: 1037-1046.
- Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN (2014) Oxidative stress and male reproductive health. Asian J Androl 16: 31-38.
- Kashou AH, Sharma R, Agarwal A (2013) Assessment of oxidative stress in sperm and semen. Methods MolBiol 927: 351-361.
- Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S (1984) A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. FertilSteril 42: 87-91.
- Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J, Zalkalns J (2001) Comparative study of cytochemical tests for sperm chromatin integrity. J Androl 22: 45-53.
- Erenpreiss J, Jepson K, Giwercman A, Tsarev I, Erenpreisa J, et al. (2004) Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod 19: 2277-2282.
- Gorczyca W, Gong J, Darzynkiewicz Z (1993) Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyltransferase and nick translation assays. Cancer Res 53: 1945-1951.
- Grasa P, Colas C, Gallego M, Monteagudo L, Blanco TM et al. (2009) Changes in content and localization of proteins phosphorylated at tyrosine, serine and threonine residues during ram sperm capacitation and acrosome reaction. Reproduction 137: 655–667.
- Fisch H, Lipshultz LI (1992) Diagnosing male factors of infertility. Arch Pathol Lab Med 116: 398-405.
- Yang KT, Tang CJ, Tang TK (2015) Possible Role of Aurora-C in Meiosis. Front Oncol 5: 178.
- Diemer T, Desjardins C (1999) Developmental and genetic disorders in spermatogenesis. Hum Reprod Update 5: 120-140.
- Jamieson CR, van der Burgt I, Brady AF, van Reen M, Elsawi MM, et al. (1994) Mapping a gene for Noonan syndrome to the long arm of chromosome 12. Nat Genet 8: 357-360.
- Rossato M, Popa FI, Ferigo M, Clari G, Foresta C (2004) Human Sperm Express Cannabinoid Receptor Cb1, the Activation of Which Inhibits Motility, Acrosome Reaction, and Mitochondrial Function. J ClinEndocrinolMetab 90: 984 –991.
- Kolodny RC, Masters WH, Kolodner RM, Toro G (1974) Depression of plasma testosterone levels after chronic intensive marihuana use. N Engl J Med 290: 872-874.
- Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S (1994) Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. ProcNatlAcadSci USA 91:7678 –7682.
- Berdyshev EV (1999) Inhibition of sea urchin fertilization by fatty acid ethanolamides and cannabinoids. CompBiochemPhysiol C PharmacolToxicolEndocrinol 122: 327-330.
- Yamaguchi H, Wang HG (2001) The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20: 7779-7786.
- Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, et al. (2004) Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J BiolChem 279: 21085-21095.
- Andersen CB, Roth RA, Conti M (1998) Protein kinase B/Akt induces resumption of meiosis in Xenopus oocytes. J BiolChem 273: 18705-18708.
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, et al. (2008) Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14: 458-470.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 12016
- [From(publication date):
December-2015 - Dec 26, 2024] - Breakdown by view type
- HTML page views : 11227
- PDF downloads : 789