Review Article Open Access
Cancer of Unknown Primary Site: Not All is Lost!
Muhammad Furrukh*, Ikram Burney, Asim Qureshi and Ritu Lakhtakia
Department of Oncology, Shifa International Hospital, Pakistan
- *Corresponding Author:
- Muhammad Furrukh
Department of Oncology
Shifa International Hospital
Islamabad, Pakistan
Tel: 0092 310 3335889
E-mail: furrukh_1@yahoo.com
Received Date: November 24, 2014; Accepted Date: December 19, 2014; Published Date: December 26, 2014
Citation: Furrukh M, Burney I, Qureshi A, Lakhtakia R (2015) Cancer of Unknown Primary Site: Not All is Lost!. J Clin Diagn Res 3:115. doi:10.4172/2376-0311.1000115
Copyright: ©2015 Furrukh M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Clinical Diagnosis and Research
Abstract
A Cancer of Unknown Primary Site (CUPS) is defined as a metastatic tumor for which the site of origin remains unknown after establishing tissue diagnosis despite a standard diagnostic approach. It is still not known whether CUP is an entity with dormancy of primary as its hallmark or a distinct entity with specific genetic aberrations which define it as a primary metastatic disease. It poses a diagnostic challenge to the pathologists and often poses a therapeutic dilemma for the oncologists. The diagnostic algorithm includes; age, gender, histology, site of metastasis, distribution and natural history of disease, and expression of tissue specific markers by the malignant clones as revealed on immunostains, e.g. TTF1 for thyroid gland and adenocarcinoma lung, PSA for prostate gland, estrogen/ progesterone receptors and gross cystic disease fluid protein-15 for breast cancer, etc. Despite extensive and expensive diagnostic work up, in almost 20-45% cases the site of their origin remains unknown, however the yield rises to 70-80% after postmortem examination. While the pathology teams are working to resolve the primary site, the oncology teams are adamant on identifying subset of patients that may be treatable and potentially curable. However, CUPS remains aggressive, and generally associated with a poorer prognosis with a median survival of less than a year. The review focuses on current practices in diagnoses and treatment of an occasionally rare, and a challenging entity.
Keywords
Primary neoplasm; Unknown neoplasm; Occult primary; Unknown primary neoplasm; Neoplasm metastases; Unknown primary; Immunophenotypings; Cisplatin; Etoposide; Paclitaxel
Introduction
A cancer of unknown primary site (CUPS), previously called as metastasis of unknown origin (MUO), is defined as a metastatic tumor for which the site of origin remains unknown at the time of decision making, despite detailed history, thorough physical examination, laboratory investigations, and mandatory imaging, endoscopic evaluation, but after establishing tissue diagnosis on routinely stained sections. It is believed that 10-15% cancer cases present with metastasis and almost ~3-5% remains as CUPS [1]. Most commonly, the patient seeks medical attention because of a recent enlargement of a superficial lymph node (42%), or symptoms due to metastatic lesions in the liver (33%), lung (26%), bones (22%), mesothelial lining (9-11%), etc. The metastasis may be symptomatic or detected at imaging for screening or incidental discoveries during clinicoradiological evaluation for other illnesses. CUPS remain a mystery, being designated as a group of metastatic tumors with undetermined primary site, or for being a specific entity with distinct genetic alterations [2].
American Cancer Society (ACS) estimates 31,430 new cases of CUPS in the US in 2014 [3]. CUPS account for <5% of all cancers, and remains the 8th most frequent cancer and 4th common cause of death due to cancer in Europe. There is no gender predilection and the median age of diagnosis is ~60 years. Twenty to 45% of these presentations remain CUPS despite extensive diagnostic work up, and even after postmortem examination primary sites may only be identified in 70-80% cases [4]. In general CUPs remains aggressive, and associated with a poorer prognosis, with a median survival of 5-11 months [5] (Table 1).
| Poor prognostic features |
|---|
| Male gender |
| Adenocarcinoma metastatic to liver, lung and bones |
| Non-papillary malignant ascites (adenocarcinoma) |
| Multiple cerebral metastasis (adenocarcinoma or SCC) |
| Adenocarcinoma with lung/ pleura or bone metastasis |
| Relatively good prognostic features |
| Midline disease |
| Poor differentiation |
| Papillary adenocarcinoma peritoneal cavity |
| Adenocarcinoma axillary lymph nodes (females) |
| SCC neck or inguinal lymph nodes |
| Poorly differentiated neuroendocrine tumors |
| Men with sclerotic bone metastasis with high PSA |
| Solitary small volume resectable disease |
Table 1: Prognostic Features in CUPS
Signs and symptoms are seldom helpful. Vague symptoms may be present, such as generalized weakness, fatigue, anorexia, and weight loss, or reflective of the metastatic site: discomfort from a lump, pain, cough and hemoptysis, shortness of breath, bleeding from orifices, obstruction of a hollow viscus, low back ache, pathological fracture, etc. These symptoms do not contribute to the organ-site localization. Patients diagnosed with CUPS have early, systemic metastasis, frequently involving 3-4 organs and sometimes may be localized to unusual sites e.g. skin, scalp, heart, distant lymph nodes and kidneys [6]. The pattern of spread of metastasis may sometimes help locate the primary site, but are often erratic and not reliable in most of the cases. For metastasis above the diaphragm lung or breast could be the primary. To identify the primary site of origin with upper cervical lymphadenopathy, head and neck should be thoroughly examined e.g. larynx, pharynx, thyroid gland, etc. and when lymphadenopathy occurs along the spinal accessory chain, the nasopharynx must be looked at. Virchow’s node could be tricky as a wide variety of cancers may metastasize to this area including testis, ovary, pancreas, stomach, and superior sulcus tumor of the lung, etc. In males, a testicular primary must be searched for, when retrocrural or midline lymphadenopathy is evident on imaging.
The extensive diagnostic work up is expensive, yet meant to identify treatable and potentially curative cases. The work up also allows predictive and prognostic assessment based on the current biologic understanding of specific malignancies. The morphoimmunophenotypic evaluation of the metastasis (occasionally supplemented with molecular features) provides the backbone for further clinico-radiological search for the primary, along with additional serologic and biochemical investigations. Occasionally this may fail, where the metastatic clone may have changed its appearance and biologic characteristics which accounts for the unresolved cases.
Small volume surgically resectable disease is often curative, but a large number of cases are offered palliative systemic therapy or local irradiation directed at symptom control which attempts at improving quality of life. In some cases e.g. poorly differentiated carcinoma (germ cell tumors on immunostains), long term survival and even cure is a distinct possibility.
Materials and Methods
Data was retrieved from searches in Medscape, PubMed, Google, and from review articles published in reputable indexed medical journals (Annals of Oncology, The Oncologist, Acta Cytology, Molecular Cancer, American Journal of Clinical Pathology, Pathologist, Journal of Clinical Oncology, New England Journal of Medicine, Lancet Oncology, European Journal of Cancer, Journal of National Cancer Institute, Critical Review Oncology Hematology, Breast Cancer Research and Treatment, Clinical Translational Oncology) and through key groups e.g. National Comprehensive Cancer Network (NCCN), American Cancer Society (ACS) and European Society of Clinical Oncology (ESMO) guidelines, by writing terms like; cancer of unknown primary sites, metastases of unknown origin, immunophenotyping in diagnoses of metastases of unknown primary site, treatment of metastases of unknown primary and treatment of CUPS, neoplasm, occult primary, and unknown primary neoplasms. Key words were verified through MeSH of the ncbi website.
Diagnostic Evaluation and Essential Work-up in CUPS
Essential diagnostic work-up should evolve from the final histology and extensive tests must be avoided as these may remain fruitless in locating the primary. Key diagnostic and staging work-up is enumerated in Table 2. Baseline blood work-up and biochemistry, pertinent tumor markers are essential but not diagnostic. Extensive imaging seldom locates the primary site; however, it does define extent and bulk of metastatic disease and occasionally guides a biopsy.
| Detailed history, thorough physical examination (including PR, PV, PA, PS, ENT and Testicular exam.) |
|---|
| Baseline bloods: Full Blood Counts, LFT’s, UandE, Urine cytology, chronic hepatitis screen (HBV PCR, seek hepatology consult) |
| Fecal occult blood test (FOBT) |
| Appropriate Tumor Markers: |
| ß-HCG and AFP (midline metastases), PSA, CEA, CA 19-9 (Males) CA 125, ß-HCG, AFP, LDH, CA 15-3, CEA, CA 19-9 (Females) |
| Radiological Imaging: |
| Baseline X Ray Chest (PA/ Lateral views) - if suspicious, seek HRCT Chest |
| CT Scan Chest/ Abdomen/ Pelvis with contrast (minimal for all patients) |
| CT Scan skull base to root of neck for isolated neck node/s with suspected head and neck primary |
| PET-CT scan* |
| Radioisotope bone scan**; |
| Gallium-dototate PET-CT imaging,33 or Octreoscan and serum chromogranin in NET |
| B/L Mammograms + USG Breast***, if inconclusive, seek MRI Breast |
| T3, T4, TSH, USG thyroid gland and neck and Tc99m radio-isotope scanning**** |
| Endoscopic Evaluation (signs/ symptoms or histology directed): |
| Bronchoscopy |
| Upper/ Lower GI Endoscopy |
| Cystoscopy |
| Pan-endoscopy (DL/ IDL, Nasoscopy, Nasopharyngoscopy) |
| FNAC, Excision or Incision biopsy; |
| Careful reevaluation of previous biopsy material, review slides/ blocks and attention to previous biopsy sites. Extensive IHC panels. |
**to evaluate for any biopsy able focus, and to look for other sites of bone involvement/ bulk of metastases
***(females having poorly differentiated adenocarcinoma involving the axillary lymph nodes)
****(for suspected thyroid gland primary)
Table 2: Essential Work-up in CUPS
Establishing the Histological Diagnosis and Defining the Occult Primary Site
Histopathologic diagnosis of CUPS
The pathologic preliminary analysis is based on the following considerations, which form a guide to judicious usage of immunohistochemistry (IHC) panels of antibodies.
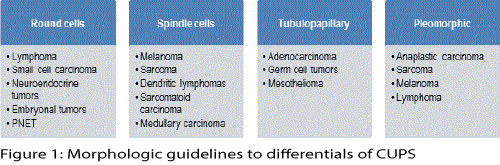
Clinical presentation: Salient findings include age and gender of patient, site of metastasis (organ/ lymph node group/ midline location) and associated symptoms and signs must be explored. Conventional knowledge and wisdom guide the initial differentials aided by; microscopic cyto-architectural features: Figure 1 is an abbreviated illustration of the value of initial differential diagnosis based on morphologic observations.
Immunophenotyping
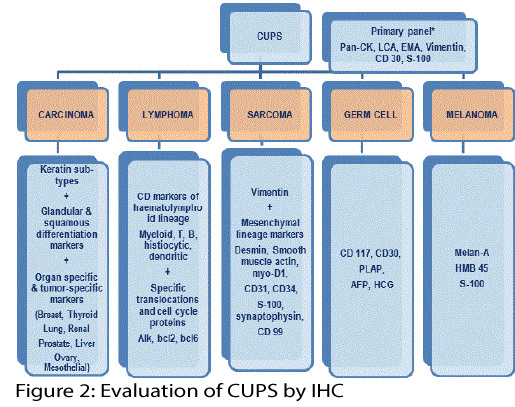
Primary panel
Figure 2 provides an overview of usage of antibody panels for metastatic differentiated tumors. An initial select primary panel could provide direction for the inclusion or exclusion of more specific secondary panel of antibodies. The typical example illustrated could confirm epithelial/mesenchymal expression, lymphoid lineage, germ cell or melanocytic differentiation. There is possibility of oversimplification and the pragmatic pathologist would tailor it based on clinico-morphologic correlation, a sound hindsight of antigenic infidelity and overlapping immunoreactivity. Examples of deviations include LCA negativity in anaplastic large cell lymphomas, CD30 expression in lymphomas and germ cell tumors, EMA positivity in epithelial sarcomas as well as few lymphomas [7,8].
Secondary panels
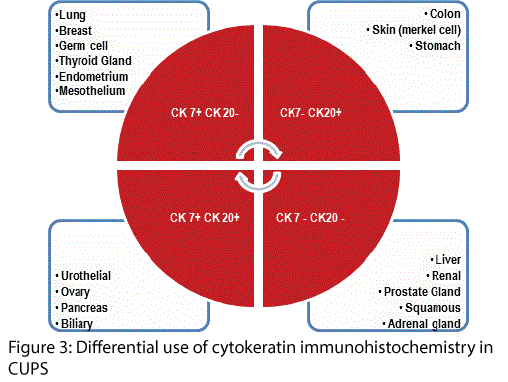
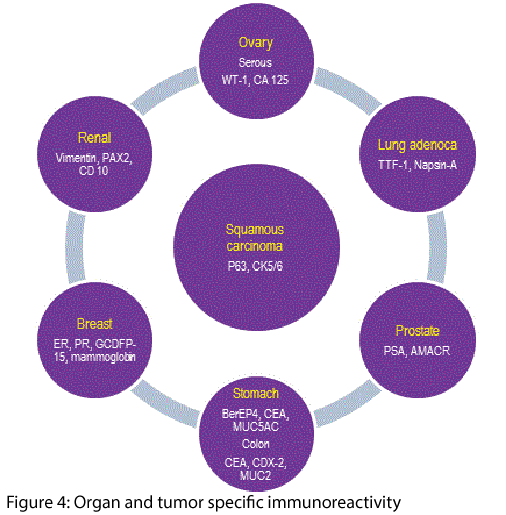
Figure 3 outlines the differential use of antibodies against cytokeratins of different molecular weights that support primary specificity in CUPS. These are invariably interpreted with select antibodies that have shown a high specificity for organs e.g. estrogen and progesterone receptors (ER/ PR) with gross cystic disease fluid protein-1 (GCDFP-1) for breast, thyroid transcription factor (TTF-1) in thyroid and lung, prostate-specific antigen (PSA) and AMACR for prostate gland, CDX2 for colo-rectum, HepPar-1 for liver, MUC subtypes for stomach and colon, melan-A for melanoma and so on - Figure 4.
The outcome of the morpho-immunophenotypic evaluation could be organ-specific (e.g. thyroid, renal, colon); or histologic-type-specific (e.g. papillary/medullary carcinoma thyroid gland, duct carcinoma breast, renal cell carcinoma); or only provide lineage differentiation (e.g. lymphoid, neuroendocrine tumor, squamous cell carcinoma).
A meta-analysis of studies addressing the utility of IHC in the diagnosis of site of origin in metastatic setting ranges between 60-70% [9]. Appropriate use of a selected panel of immunohistochemical markers (including PSA, TTF-1, GCDFP-15, CDX2, CK7 and 20, CA 125, ER, mesothelin, and lysozyme) allowed identification of the primary tumor in almost 88% of 452 adenocarcinomas from the seven most common sites (breast, colon, lung, ovary, pancreas, prostate and stomach), and to correctly identify the sites of origin in 83% of 30 metastatic sites in one study [10].
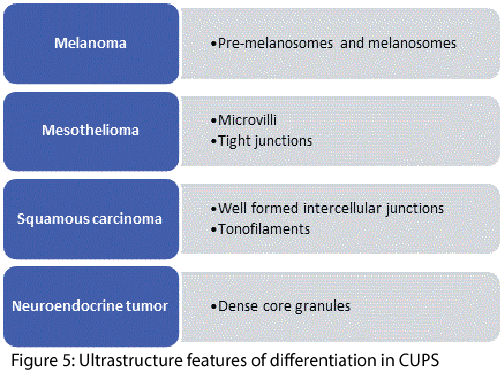
Electron Microscopy
Cancer of unknown primary site poses a difficult diagnostic dilemma; adenocarcinomas, SCC, malignant melanomas, lymphomas, neuroendocrine tumors, and sarcomas can all be very difficult to be typed if the light microscopic histology reveals poor differentiation. Electron microscopy (EM) may reveal intra-cytoplasmic lumina with long microvilli and many well-formed desmosomal junctions narrowing the diagnosis to poorly differentiated adenocarcinoma or presence of desmosomes and bundle of tonofilaments which may be associated with SCC, or dense core granules observed in neuroendocrine tumors, etc. Figure 5. Presence of site of origin was correctly identified in 59% by FNAC and in 88% with EM [11-13]. Once important histogenetic determinant in poorly differentiated tumors, its role has been grossly diminished with the ever increasing availability of newer antibodies for IHC.
Molecular Biology and Chromosomal Abnormalities
With the accumulating knowledge of genetic fingerprints of tumors there is increasing possibility of accurate diagnosis in select tumors. Microarrays and micro-RNA detection methods are being incorporated into commercially available gene signatures that shall be a shot in the arm for diagnostic success in these challenging situations. Cancer TYPEID a 92-gene RT-PCR based assay, Rosetta cancer origin test and 64 microRNA test claim 75% detection rate and 92% concordance respectively [14-16]. In a study on poorly and undifferentiated carcinomas, gene expression profiling (GEP) demonstrated accuracy of 91% compared to 71% with IHC [17]. Tissue microarray platforms which screen multigene expression in each primary tumor sample, compares it with the multigene expression of a CUPS case in order to biologically classify it. This will be followed by institution of specific therapy for the ‘biological’ primary tissue, i.e. FOLFOX4 chemotherapy with or without bevacizumab for a CUPS genetically classified as ‘colon cancer’ [18]. In addition, molecular profile assay are also useful diagnostic tools where IHC fails to predict site of origin for any histology e.g. poorly differentiated tumors without a lineage clearly defined by IHC, or malignant pleural effusion or when the biopsy specimen is too small to undergo extensive IHC testing [19].
Aneuploidy, presence of abnormalities of the short arm of chromosome 1(1p), p53 mutations, anti-apoptotic gene like bcl2 overexpression, c-kit, c-myc, and ras mutations have all been documented as precursor alterations. In addition, human epidermal growth factor receptor-2 (HER2) over-expression, co-expression of HER2 with epidermal growth factor receptor (EGFR) and cyclo-oxygenase (COX-2) have also been observed in CUPS. However, their exact role in oncogenesis remains to be defined.1 Co-overexpression of p53 and bcl2 was predictive for superior response to cisplatin based chemotherapy [20].
Tumor Markers
Serum tumor markers in patients with CUP are generally elevated in a nonspecific way. Oncologists and primary clinicians regularly order tumor markers in the initial evaluation of patients with CUP which certainly helps to narrow the differential diagnosis and may occasionally be diagnostic in individual cases. An elevated serum levels of β-HCG (human chorionic gonadotrophins) and AFP (alpha fetoprotein) in a young patient with midline metastases revealing poorly differentiated carcinoma, elevated serum CA125 in women with primary peritoneal serous adenocarcinomatosis, a high serum CA 15-3 in women with an isolated axillary node adenocarcinoma metastasis, and raised PSA in men with sclerotic bone metastases can give important clues to identify primary tumors thus guide therapies [21].
Imaging in Cancer of Unknown Primary Site
Modern imaging technologies including MRI, 18F-FDG PET and PET/CT have evolved over the past decade, enabling localization of the primary site and facilitating site-specific therapy. The PET/CT is considered to change the therapeutic plan in only 35% of patients with CUP. 18F-FDG PET or PET/CT detects the primary sites in 24-40%, compared to CT or MRI which detects the primary in 20-27%, yet the information comes from small retrospective studies. In addition, the role of PET/CT in CUP has also not been validated by prospective trials. There are several scenarios where its use may be justified e.g. SCC with cervical lymphadenopathy, where it may identify head and neck primary in nearly 50% of patients, helps guide the biopsy of the suspected primary site, and determine the extent of disease. In extracervical CUP, PET/CT may identify the solitary metastases directing local therapy e.g. surgical resection or radiation therapy, and remains a suitable choice where patients may have iodinated contrast allergy or deranged renal functions. However, in widespread metastases its utility is limited, because it cannot distinguish the primary from metastatic foci, and may result in false-positive lesions [22].
Risk Stratification and Distinct Clinico-Pathologic Groups in CUPS
A patient with CUPS may present with various clinical scenarios: (a). CUPS originating in the liver or at multiple sites, (b). CUPS diagnosed in a lymph nodes; (mediastinal-retroperitoneal, or axillary, or cervical or inguinal nodes, (c). CUPS identified from malignant ascites; (peritoneal papillary serous carcinomatosis in females, peritoneal non-papillary carcinomatosis in males or females), (d). CUPS arising as malignant effusion or pulmonary parenchymal metastasis, (e). CUPS identified in bones, (f). CUPS found in the brain, e.g. as NETs (metastatic neuroendocrine carcinomas), and (g). CUPS originating as a malignant -melanoma. It is of paramount importance to correctly identify and subsequently treat such cases [23].
Women with isolated axillary lymphadenopathy (adenocarcinoma), isolated inguinal lymphadenopathy (squamous cell carcinoma uterine cervix), or serous papillary peritoneal carcinomatosis; males with carcinoma originating in mediastinal or retroperitoneal lymph node area, and patients with high-grade carcinoma exhibiting neuroendocrine features is grouped in the ‘favourable risk CUPS’ cohort. These patients receive tailored treatment with impressive responses, which often translate into long-term survival. Approximately 70-80% of CUPS presents with high-volume metastatic deposits involving multiple sites i.e. vital viscera, soft tissue and bones, and are grouped in the ‘poor-risk CUPS’ cohort – Table 1. Poor-risk CUPS is characterized by regression or dormancy of the primary tumour, early and rapid growth of metastasis, biological aggressiveness, relative resistance to chemotherapy and is generally associated with poorer prognosis, with most fatalities occurring within a year from diagnosis. Chemotherapy regimens with low side effects profile must be used in such patients [24]. Epidemiological data indicates that CUPS behaves differently from the parent tumor in terms of response to therapy and disease course, even when biologically classified to a primary tumor group [1].
Others have stratified CUPS into two groups with different prognosis, those having a good to excellent eastern co-operative oncology group performance status (ECOG PS0-1) and a normal serum lactate dehydrogenase (LDH) levels with median life expectancy of 12 months, and another, having PS 3-4 (Table 3) with elevated LDH levels and a life expectancy less than 5 months [24,25].
| *ECOG Performance Status | |
|---|---|
| Grade | ECOG |
| 0 | Fully active, able to carry on all pre-disease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours |
| 3 | Capable of only limited self-care, confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair |
| 5 | Dead |
Table 3: Eastern Co-operative Oncology Group Performance Status (ECOG PS)[25]
Therapeutic Challenges in Treatment of CUPS
Treatment of solitary metastases or site specific metastases
Cervical lymphadenopathy: Isolated occurrence of a lymph node mass high in the neck should warrant exploration of the head and neck, and upper aero-digestive tract primary. Thorough head and neck examination should be sought; naso-pharyngoscopy, direct/indirect layrngoscopy and upper GI endoscopy. Any suspicious appearing areas must be biopsied and blind biopsies may be attempted from nasopharynx, oro-pharynx, hypopharynx, tongue base, larynx, etc; histology may prove to harbinger squamous cell carcinoma, adenocarcinoma, adeno-squamous carcinoma, or rare tumors.
Treatment includes radical neck dissection with or without adjuvant radiotherapy, local resection followed by whole neck radiotherapy, with or without weekly platinum compounds; especially for undifferentiated or poorly differentiated tumors. Combined modality treatment results in 5 year survival of 25% [1].
A low cervical, specially left sided solitary supraclavicular lymphadenopathy should guide the treating teams to explore a primary in thorax, or abdomino-pelvic viscera. For isolated lymph node(s), same principles of management apply as described for upper neck adenopathy [1,26].
Women with isolated axillary lymphadenopathy: With histologic diagnoses of adenocarcinoma as an occult primary, breast is the most likely site of origin, however, occult primary in the head and neck (thyroid gland) and upper limbs may also be looked for. ER, GCDFP-15, mammoglobulin and HER2 IHC/ FISH positivity may confirm the primary site in breasts. Imaging may help localize the primary in the breast e.g. mammography, ultrasound and MRI breast [27]. The consensus is to offer modified radical mastectomy, followed by adjuvant chest wall radiotherapy where indicated, and therapy directed according to the predictive markers. These patients are further treated with adjuvant anthracycline/ taxane combination chemotherapy, with or with-out trastuzumab, and integration of antiestrogen therapy where indicated [1,28].
Men with extragonadal midline tumors: A tumor mass in the region of mediastinum or retroperitoneum with or with-out elevated serum beta human chorionic gonadotrophins (β-hCG), alpha fetoproteins (AFP), lactate dehydrogenase and tumor staining with placental alkaline phosphatase (PLAP), OCT4 stain, or AFP should be treated as a poor risk, stage III germ cell tumor (GCT). Optimal management utilizes standard dose BEP. Response rates are nearly 60%, and in 25% a complete resolution of the disease may be seen. The overall prognosis remains poor with median OS of 1 year [29]. Residual mass(es) may have to be resected at the end of treatment based on PET-CT evaluation resulting in long term survival. Molecular genetic testing may reveal FISH for i(12p) chromosomal abnormality, confirming the diagnoses of GCT and predicting response to cisplatin based chemotherapy [1].
Inguinal lymph node metastases: Isolated inguinal node involvement should prompt examination of the uro-genital organs, ano-rectum, perineum and the lower limbs. With no other site(s) of involvement: inguinal lymphadenectomy with or without radical radiotherapy or local excision followed by adjuvant radiation therapy is considered optimal. In poorly differentiated histology, treatment with adjuvant combined modality treatment (radiotherapy with platinum drugs) is considered standard, in patients having PS 0-2. Loco-regional management offers long-term disease control in 50%– 60% of patients [30].
Men with bone metastases: Men with elevated PSA, or prostate specific acid phosphatase, PSA tumor staining, NKX3.1 IHC staining, CK7-, CK20- and having sclerotic bone metastases may undergo TRUS, and TRUS guided six quadrant prostate gland biopsy. A trial of anti-androgens is mandatory i.e. one week of pure ant-androgens, three monthly LHRH agonist and bone directed therapy using bisphosphonates should be considered. The response rates and median PFS and OS are substantially improved [1,31].
Women with peritoneal adenocarcinomatoses: In woman having elevated serum CA-125/ CEA levels, appropriate imaging, laproscopic evaluation, biopsy of ovaries and peritoneum may be carried out. IHC may reveal CK7+, ER+ and WT-1 staining. These patients are treated as stage III/ IV ovarian cancer with responses up to 80% (30%–40% complete responders) and a median survival of 36 months. Options include; primary cytoreductive surgery followed by platinum taxane combination chemotherapy with or without bevacizumab. Chemotherapy for both, the primary peritoneal carcinoma and peritoneal metastasis from occult ovarian primary incorporates identical agents [32].
Neuroendocrine Tumors (NET): Patients may present with paraneoplastic manifestations or with site-specific signs and symptoms. Patients with well differentiated type NETs (carcinoids/ islet cell tumors) frequently present with liver metastases. A thorough search of GI tract is mandatory. Upper GI and capsule endoscopy, octreotide scintigraphy and gallium-dototate PET-CT imaging are helpful in localizing primary site, as well as staging and defining bulk of the disease [33]. Serum chromogranin levels and other pertinent markers may be elevated.
The treatment of metastases from a neuroendocrine carcinoma is not modified by the identification of the primary and must take into consideration the cellular differentiation. There is no standard treatment for the forms that are well differentiated. Well-moderately differentiated sub-types usually undergo an indolent course. Observation and palliative long acting octreotide may be used to alleviate signs and symptoms in metastatic setting [34]. Poorly differentiated NETs are usually aggressive and have early appearance of metastases to other sites, and are considered chemosensitive. Palliative cisplatin-etoposide based chemotherapy is the main stay of intervention for these sub-types, with response rates range between 70-75% and durable disease free survival [1,35]. A combination involving paclitaxel, carboplatin, and etoposide is associated with response rates of 50-55% (13% CR) in one study, a two year survival rate nearly 30% and a median survival of 14.5 months, however, the combination was toxic. Surgical resection, radiofrequency ablation (RFA), trans-arterial chemo-embolization (TACE) remain therapeutic options for liver confined disease. Radiotherapy is reserved for palliation alone. Isolated lymph node metastasis may undergo surgical resection with or without adjuvant irradiation. Adjuvant combination chemotherapy is optional as these patients are at “high risk” for subsequent appearance of metastatic disease. More recently, radioisotope therapy using lutetium (Lu177) – dotatate (radio-isotope bound to monoclonal antibody targeting somatostatin receptors) has also shown promise for widespread systemic disease, with durable responses and a PFS that exceeds ~40 months [36].
CUP in unselected patients: In a phase II trial, docetaxel and carboplatin combination was associated with response rates of 32% (higher in favorable group and lower in unfavorable group) and median survival of 22.6 and 5 months respectively [37]. Another phase II trial used bevacizumab/ erlotinib combination resulting in a median survival of 7-8 months [38].
Frank Metastases or Multiple Site Involvement in CUPS
In well to moderately differentiated adenocarcinoma, the response rates to palliative chemotherapy remain in the range of 8-50%. For poorly differentiated tumors (with or without features of adenocarcinoma, or squamous cell carcinoma, or neuroendocrine features, and without elevated AFP or β-HCG) treatment should incorporate platinum based regimens, preferably platinum etoposide for 4-6 cycles if the patient retains ECOG PS 0-1 [39]. GemOx regimen utilizes combination of gemcitabine and oxaliplatin for 4-6 cycles, and shows promising results in phase III trials [40]. Paclitaxel, carboplatin with or without etoposide or gemcitabine plus irinotecan are other tested options. Paclitaxel carboplatin yields a response rate of ~40%, median OS of 13 months and 2 year SR of 20%. Addition of etoposide increases the RR to 48% but failed to show increments in survival outcomes, and is deemed more toxic [41]. For poorly differentiated tumors (with or without features of adenocarcinoma, or squamous cell carcinoma, or neuroendocrine features, and with elevated AFP or β- hCG - disseminated GCT) standard BEP is a suitable option [29].
Chest nodules/ Pleural effusion
Women with elevated CA 125 or tumor staining for CA-125, ER, and WT-1 may be offered palliative platinum taxane combinations. Those with ER or PR positivity, should undergo sono-mammography with additional imaging in the form of MRI breast. 27 Men above 40 years with elevated PSA should undergo imaging and sexant prostate gland biopsy. Pleural effusion may be utilized to prepare a cell block which may be subjected to immunostaining and genetic testing. Presence of epidermal growth factor receptor (EGFR) gene mutation suggests a lung adenocarcinoma, and the patient may be offered targeted agents [42,43] Patients with well-preserved organ function having ECOG PS 0-1 who remain with undetermined primary, should go on to receive platinum based chemotherapy, while others are encouraged to enroll in clinical trials.
Neoplastic masses in the mediastinum
Females must undergo sono-mammography and if these imaging remains inconclusive, an MRI breast should be sought. 27 Men above 40 years with elevated PSA should have exploration in the direction of prostate gland primary and offered a trial of anti-androgen therapy, while those less than 50 years having undifferentiated histology should have testicular and scrotal ultrasound examination and may be offered BEP regimen. Men having squamous cell carcinoma (SCC) may be treated as non-small cell lung cancer using nab-paclitaxel or gemcitabine and platinum combinations [44,45].
Retroperitoneal metastases
Patients with disseminated retroperitoneal disease and normal tumor markers are reviewed in MDT setting for possibility of surgical resection with or without radiotherapy. Chemotherapy is reserved for high risk patients, having undifferentiated histology or wide spread metastases. Chromosomal genetic testing for i (12p) may suggest GCT and treated as high risk disease with standard BEP regimen [29].
Liver metastases
Patients’ with child-pugh score A or B should be offered surgical resection, followed by GI tract directed palliative chemotherapy. If liver nodules are multiple, patients may may be treated with liver directed therapy, while for those having diffuse infiltration, palliative chemotherapy should be considered in accordance to histology, keeping clinical presentation in mind [46]. Retrospective data suggests patient tumors having IHC or molecular profile assay consistent with colo-rectal cancers have similar outcomes as primary CRC when treated with standard FOLFOX or FOLFIRI regimens, when compared to empiric therapy for CUPS [47]. Patients IHC panel revealing Hepar 1, CD10+, CD13+ and elevated serum AFP may direct at primary liver cancer and hence, treated in line of hepatocellular carcinoma (HCC) [48].
Bone metastases
Men above 40 years having bone metastases with elevated PSA, prostate specific acid phosphatase and NKX3.1 IHC staining should have a trial of anti-androgen therapy as well as bone directed therapy [31]. Localizing X-rays of painful or weight bearing bones are mandatory and discussed in multi-disciplinary team setting for orthopedic intervention if more than 50% of bone cortex is eroded followed by local irradiation, while prophylactic irradiation alone may be used if the cortex remains preserved. Pain palliation using radioisotope therapy i.e. samarium 153, strontium 90, etc., remains an option where indicated. Bone scintigraphy and PET CT imaging also helps to define extent and bulk of the disease. Women with ER or PR positivity should have breasts examined and imaged, and should have a trial of anti-estrogens apart from bisphosphonates [49].
Brain
Standard staging utilizing PET-CT or CT imaging of head, neck, chest, abdomen and pelvis is mandatory. Neurosurgical intervention for resectable <3 metastases is considered standard, followed by whole brain irradiation, provided, brain is the only site for disease. For unresectable solitary brain metastases, precision sterotactic radiosurgery or sterotactic radiotherapy remains a suitable option [46]. Watchful waiting or histology and IHC directs further exploration, to identify the possible site and tailor therapy accordingly.
Cutaneous metastases
Of skin metastases, around 5% cases remain a CUP [50]. Primary tumors that invade veins are most likely from the lung, kidney, or breast primary. Oro-pharyngeal cancers have also been reported to metastasize to the face and neck. Histology and IHC remain integral and may define the primary site of origin. Treatment depends on the site and extent of skin involvement e.g. resection with negative margins for isolated solitary lesion, electron beam irradiation for extensive lesions confined to a region that may be encompassed in radiation field, while palliative chemotherapy is reserved for widespread skin dissemination.
Disseminated disease
Metastatic CUPS are offered palliative therapy purely to alleviate symptoms and enhance quality of life. Patients having ECOG PS 0-1, normal serum LDH and favorable prognosis may be treated with polychemotherapy, and those with ECOG PS 2, elevated LDH and unfavorable prognosis may be offered single agent chemotherapy. On the other hand those having a PS 3-4 are best treated with supportive and tender loving care. Few centers in the West encourage accrual into clinical trials. Paclitaxel and carboplatin combination is considered “standard”, having a response rate of 39%, median survival of 13 months and a two year of 20% for poor risk or unfavorable prognosis [51,52].
Large prospective study in CUPS looked at origin based on molecular assay and customized site-specific therapies. In 98% of tumors a single tissue of origin was successfully predicted and when treated with site-specific treatment the median survival was 12.5 months [53].
Follow Up for Occult Primaries
No active treatment is offered after completion of planned therapy for patients having isolated solitary metastases or site specific metastasis and ESMO Guidelines do not advise follow-up for asymptomatic patients [51]. Patients may be followed every 2-3 months for first 18 months, and then every 3-4 months for the next 18 months [46]. Diagnostic tests may be required based on symptomatology. Psycho-social support and rehabilitation may be required in these cases. Patients with disseminated disease are monitored more frequently for tolerance to palliative treatment, sequelae of disease and any further oncologic intervention. Palliative chemotherapy may be offered for 6-9 courses till best response, taking into account tolerability, side effects, organ functions, and patients’ preference.
In a Swedish study (1958-2008), 35,168 CUP survivors had significant risk of developing subsequent malignancy with standardized incidence ratio in males (in descending order); breast, genitalia, small bowel, upper aero-digestive tract, and in females; small bowel, thyroid gland and upper aero-digestive tract. It occurred more commonly in age above 70 years and 40% occurred within first year of follow-up. Adenocarcinoma remained the most common histology. The risk of subsequent cancers after index malignancy was attributed to; recurrence of primary neoplasm, intensive medical surveillance after preceding malignancy, immunosuppression from tumor growth or exposure to anti-neoplastic agents and/ or therapeutic radiation [54].
Conclusion
In summary, the treatment of CUPS remains challenging and requires a multidisciplinary team effort to identify the primary site. The most important determinants guiding the treatment alone are IHC, the site of CUPS, the burden of disease and the patients PS. An IHC panel of CK7, CK20, CDX-2, TTF-1 may help to identify the primary and the treatment guided accordingly. However, if the primary anatomical site could not be identified, a combination of IHC, the site of CUP and the extent of disease helps to identify a ‘favorable’ sub-set which may exhibit an extended survival when optimally treated.
If the anatomical site remains un-identified, and the patient does not fall in the ‘favorable’ sub-set, additional evaluation is warranted, which is also directed by the IHC. If a single tissue of origin is suspected, site specific therapy is indicated. However, if the tissue of origin remains elusive, empiric therapy may be indicated in carefully selected patients with a good PS. A platinum combination yields a response rate of 25-53% and a median survival exceeding 12 months. Over the last several years, there has been a small increment in survival in sub-sets of patients; however, overall the prognosis remains dismal. The hope is that we could move away from ‘empiric broad-spectrum’ treatment to ‘diagnose the tissue of origin and offer site-specific treatment’.
References
- Briasoulis E Pavlidis N (1997) Cancer of Unknown Primary Origin Oncologist 2: 142-152.
- Armstrong AC Blackhall FH (2007) Management of cancer from an unknown primary. Expert OpinPharmacother 8: 445-455.
- Cancer: unknown primary site (2014) American Society of Cancer.
- Hainsworth JD, Weiss LM (2014) Carcinoma of an unknown primary site. Cancer.
- Fehri R, Rifi H, Alboueiri A, Malouche D, Ayadi M, et al. (2013) Carcinoma of unknown primary: retrospective study about 437 patients treated at salahazaiez Institute. Tunis Med 91: 205-208.
- Pentheroudakis G Briasoulis E, Pavlidis N (2007) Cancer of unknown primary site: missing primary or missing biology? Oncologist 12: 418-425.
- Centeno BA (2006) Pathology of liver metastases. Cancer Control 13: 13-26.
- Ariza A, Balañá C, Concha Á, Hitt R, Homet B, et al. (2011) Update on the diagnosis of cancer of unknown primary (CUP) origin. ClinTranslOncol 13: 434-441.
- Anderson GG, Weiss LM (2010) Determining tissue of origin for metastatic cancers: meta-analysis and literature review of immunohistochemistry performance. ApplImmunohistochemMolMorphol 18: 3-8.
- Viale G, Mastropasqua MG (2006) Diagnostic and therapeutic management of carcinoma of unknown primary: histopathological and molecular diagnosis. Ann Oncol 17 Suppl 10: x163-167.
- Jackson SB, Strausbauch PH, Finley JL, Laich D, Hewan-Lowe KO (2003) Desmosomes and microvilli mean a lot: diagnosis of neoplasms of unknown origin using electron microscopy. UltrastructPathol 27: 155-161.
- Eyden B, Chakrabarty B, Hatimy U (2009) Carcinoma versus cytokeratin-positive lymphoma: a case report emphasizing the diagnostic role of electron microscopy. UltrastructPathol 33: 33-38.
- Facundo DJ, Quinonez G, Ravinsky E (2003) Transmission electron microscopy of fine needle aspiration biopsies of metastases. Accuracy of both techniques as established by biopsy diagnoses. ActaCytol 47: 457-462.
- Greco FA, Lennington WJ, Spigel DR, Hainsworth JD (2013) Molecular profiling diagnosis in unknown primary cancer: accuracy and ability to complement standard pathology. J Natl Cancer Inst 105: 782-790.
- http://www.rosettagenomics.com/testing-services.
- Pentheroudakis G, Pavlidis N, Fountzilas G, Krikelis D, Goussia A, et al. (2013) Novel microRNA-based assay demonstrates 92% agreement with diagnosis based on clinicopathologic and management data in a cohort of patients with carcinoma of unknown primary. Mol Cancer 12: 57.
- Handorf CR, Kulkarni A, Grenert JP, Weiss LM, Rogers WM, et al. (2013) A multicenter study directly comparing the diagnostic accuracy of gene expression profiling and immunohistochemistry for primary site identification in metastatic tumors. Am J SurgPathol 37: 1067-1075.
- Pentheroudakis G, Pavlidis N (2010) Probing the unknown in cancer of unknown primary: which way is the right way? Ann Oncol 21: 1143-1144.
- Greco FA (2013) Cancer of unknown primary site: improved patient management with molecular and immunohistochemical diagnosis. Am SocClinOncolEduc Book .
- van de Wouw AJ, Jansen RL, Griffioen AW, Hillen HF (2004) Clinical and immunohistochemical analysis of patients with unknown primary tumour. A search for prognostic factors in UPT. Anticancer Res 24: 297-301.
- Pavlidis N Pentheroudakis G (2012) Cancer of unknown primary site. Lancet 379: 1428-1435.
- Kim KW, Krajewski KM, Jagannathan JP, Nishino M, Shinagare AB, et al. (2013) Cancer of unknown primary sites: what radiologists need to know and what oncologists want to know. AJR Am J Roentgenol 200: 484-492.
- Pavlidis N, Briasoulis E, Hainsworth J, Greco FA (2003) Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 39: 1990-2005.
- Culine S, Kramar A, Saghatchian M, Bugat R, Lesimple T, et al. (2002) Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J ClinOncol 20: 4679-4683.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J ClinOncol 5: 649-655.
- Pavlidis N, Pentheroudakis G, Plataniotis G (2009) Cervical lymph node metastases of squamous cell carcinoma from an unknown primary site: a favourable prognosis subset of patients with CUP. ClinTranslOncol 11: 340-348.
- Ettinger DS, Agulnik M, Cates JM, Cristea M, Denlinger CS, et al. (2011) Occult primary. J NatlComprCancNetw 9: 1358-1395.
- Pentheroudakis G, Lazaridis G, Pavlidis N (2010) Axillary nodal metastases from carcinoma of unknown primary (CUPAx): a systematic review of published evidence. Breast Cancer Res Treat 119: 1-11.
- Pentheroudakis G, Stoyianni A, Pavlidis N (2011) Cancer of unknown primary patients with midline nodal distribution: midway between poor and favourable prognosis. Cancer Treat Rev 37: 120-126.
- Pavlidis N, Fizazi K (2009) Carcinoma of unknown primary. Crit Rev OncolHematol 69: 271-280.
- Gurel B Ali TZ, Montgomery EA, Begum S, Hicks J, et al. (2010) NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J SurgPathol 34: 1097-1105.
- Pentheroudakis G, Pavlidis N (2010) Serous papillary peritoneal carcinoma: unknown primary tumour, ovarian cancer counterpart or a distinct entity? a systematic review. Crit Rev OncolHematol 75: 27-42.
- Haug AR, Auernhammer CJ, Wängler B, Schmidt GP, Uebleis C, et al. (2010) 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med 51: 1349-1356.
- Rinke A, Muller HH, Schade BC, Klose KJ, Barth P, et al. (2009) Placebo controlled, double blind, prospective, randomized study of the effect of octreotide LAR in the control of the tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J ClinOncol 27: 4656-4663.
- Rougier P, Mitry E (2000) Chemotherapy in the treatment of neuroendocrine malignant tumors. Digestion 62 Suppl 1: 73-78.
- Kam BL, Teunissen JJ, Krenning EP, de Herder WW, Khan S, et al. (2012) Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging 39 Suppl 1: S103-112.
- Pentheroudakis G, Briasoulis E, Kalofonos HP, Fountzilas G, Economopoulos T, et al. (2008) Docetaxel and carboplatin combination chemotherapy as outpatient palliative therapy in carcinoma of unknown primary: a multicenter Hellenic Cooperative Oncology Group phase II study. ActaOncol 47: 1148-1155.
- Hainsworth JD, Spigel DR, Farley C, Thompson DS, Shipley DL, et al. (2007) Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: the Minnie Pearl Cancer Research Network. J ClinOncol 25: 1747-1752.
- Lee HS, Han HS, Lim SN, Jeon HJ, Lee HC, et al. (2012) Poorly differentiated neuroendocrine carcinoma in a perigastric lymph node from an unknown primary site. Cancer Res Treat 44: 271-274.
- Carlson H, Lenzi R, Raber MN, Varadhachary GR (2013) A phase II study to evaluate the efficacy and toxicity of oxaliplatin in combination with gemcitabine in carcinoma of unknown primary. Int J ClinOncol 18: 226-231.
- Hainsworth JD, Spigel DR, Clark BL, Shipley D, Thompson DS, et al. (2010) Paclitaxel/carboplatin/etoposide versus gemcitabine/irinotecan in the first-line treatment of patients with carcinoma of unknown primary site: a randomized, phase III Sarah Cannon Oncology Research Consortium Trial. Cancer J 16: 70-75.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239-246.
- Furrukh M, Al-Moundhri M, Zahid KF, Kumar S, Burney I (2013) Customised, Individualised Treatment of Metastatic Non-Small-Cell Lung Carcinoma (NSCLC). Sultan QaboosUniv Med J 13: 202-217.
- Socinski MA, Bondarenko IN, Karaseva NA, Makhson A, Vynnychenko I, et al. Results of a randomized, phase III trial of nab paclitaxel and carboplatin compared with cremophor based paclitaxel and carboplatin as first line therapy in advanced NSCLC. J ClinOncol. 28: 18s.
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92-98.
- http://www.nccn.org/patients/clinical/default.aspx.
- Varadhachary GR, Raber MN, Matamoros A, Abbruzzese JL (2008) Carcinoma of unknown primary with a colon-cancer profile-changing paradigm and emerging definitions. Lancet Oncol 9: 596-599.
- Greco FA, Oien K, Erlander M, Osborne R, Varadhachary G, et al. (2012) Cancer of unknown primary: progress in the search for improved and rapid diagnosis leading toward superior patient outcomes. Ann Oncol 23: 298-304.
- Gregoire C, Muller G, Machiels JP, Goeminne JC (2014) Metastatic signet-ring cell carcinoma of unknown primary origin. ActaClinBelg 69: 135-138.
- Koca R, Ustundag Y, Kargi E, Numanoglu G, Altinyazar HC (2005) A case with widespread cutaneous metastases of unknown primary origin: grave prognostic finding in cancer. Dermatol Online J 11: 16.
- Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G; ESMO Guidelines Working Group (2011) Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 22 Suppl 6: vi64-68.
- Golfinopoulos V, Pentheroudakis G, Salanti G, Nearchou AD, Ioannidis JP, et al. (2009) Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev 35: 570-573.
- Hainsworth JD, Rubin MS, Spigel DR, Boccia RV, Raby S, et al. (2013) Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J ClinOncol 31: 217-223.
- Shu X, Liu H, Ji J, Sundquist K, Försti A, et al. (2012) Subsequent cancers in patients diagnosed with cancer of unknown primary (CUP): etiological insights? Ann Oncol 23: 269-275.
Relevant Topics
- Back Pain Diagnosis
- Cardiovascular Diagnosis
- Clinical Diagnosis
- Clinical Echocardiography
- COPD Diagnosis
- Diabetes Diagnosis
- Diagnosis Methods
- Diagnosis of cancer
- Diagnosis of CNS
- Diagnosis of Diabetes
- Diagnostic Products
- Diagnostics Market Analysis
- Heart diagnosis
- Immuno Diagnosis
- Infertility Diagnosis
- Medical Diagnostic Tools
- Preimplementation Genetic Diagnosis
- Prenatal Diagnostics
- Ultrasonography
Recommended Journals
Article Tools
Article Usage
- Total views: 22466
- [From(publication date):
December-2015 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 17454
- PDF downloads : 5012