Research Article Open Access
Can We Improve The Birth Weight Prediction? The Effect of Normal BMI Using A Multivariate Model
| Vila-Candel R1*, Martin-Moreno JM2, Alamar S3, Soriano-Vidal FJ4 and Naranjo de la Puerta FG1 | |
| 1Department of Obstetrics and Gynaecology, Hospital Universitario de la Ribera, Spain | |
| 2Department of Preventive Medicine and Public Health, Universitat de Valencia, Spain | |
| 3Department of Nursing, Universidad Católica de Valencia, Spain | |
| 4Department of Obstetrics and Gynaecology, Hospital LLuis Alcanyis, Spain | |
| Corresponding Author : | Vila-Candel R Department of Obstetrics and Gynaecology Hospital Universitario de la Ribera, Spain Tel: 34962001010 E-mail: RVila@Hospital-Ribera.com |
| Received: December 04, 2014; Accepted: April 19, 2015; Published: April 22, 2015 | |
| Citation: Vila-Candel R, Martin-Moreno JM, Alamar S, Soriano-Vidal FJ, Naranjo de la Puerta FG (2015) Can We Improve The Birth Weight Prediction? The Effect of Normal BMI Using A Multivariate Model. J Preg Child Health 2:154. doi: 10.4172/2376-127X.1000154 | |
| Copyright: © 2015 Vila-Candel R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Objective: The construction of a predictive model that improves the estimation of the fetal weight (EFW). Study Design: A comparative, descriptive study. One hundred forty pregnant women were recruited at two-stage sample in health department in Spain. They were classified in four groups depending on the pre-gestational BMI. Fetal weight was estimated by ultrasound at 35-40 weeks (EFW40w) by one gynecologist. A regression model was created with the variables that reacted to the newborn´s weight, symphysis-fundal height (SFH), EFW40w, gestational age (GA), ferritin level and cigarettes smoked. Results: A multivariate model was created for the NW group to estimate the fetal weight (EFWme), resulting in R2=0.727 (p<0.001). The differences of the averages obtained between EFW40w and EFWme, with the newborn´s weight were significant (p<0.001). EFWme underestimates birth weight by 0.07 g (mean error 0.53%), and EFW40w overestimates it by 300.89 g (mean error 10.12%). In order to evaluate the predictive model and verify the predictions we used the Bland-Altman analysis. The average error in estimating the birth weight with EFWme was 1.94% underestimating the result, whereas the ultrasound error overestimated the result 10.93%. Conclusion: The multivariate model created for the NW group improves the accuracy of the ultrasound.
| Keywords |
| Birth weight; Pregnancy; Ultrasound; Anthropometry; Multivariate analysis |
| Introduction |
| The analysis of birth weight must be addressed from a multifactor perspective [1]. Unfortunately, birth weight is unknown until birth takes place [2]. The use of ultrasound fetal measurements has been extended and the measurements have been combined to estimate fetal weight by regression analysis or physically methods [3]. Fetal weight estimation is inaccurate, with poor sensitivity for prediction at term [4]. It is already known that the absolute error average in predicting birth weight varies from 6 to 12% of the actual weight. Several authors [4-6] have shown that the levels of intra/inter observer variability in fetal measurement as well as the impact of errors on growth assessment are unacceptable. Different studies [7-9] have compare, with discrepancies, the accuracy between clinical and ultrasound methods in order to estimate fetal weight in the third trimester. Birth weight depends on many factors i.e. Maternal, genetic and environmental ones [10]. |
| This study raises the hypothesis that some factors are not distributed randomly, but according to a profile that determines the weight of a newborn at birth. It could let us create a better predictive model of infant weight at birth, rather than the actual birth weight estimation by third trimester routine sonogram, when applied to women depending on their pre-gestational body mass index (BMI). |
| Materials and Methods |
| We performed an observational and prospective study. Based on the WHO ranges, pregnant women were allocated in four different groups depending on their pre-gestational BMI: underweight (UW <18.5 Kg/ m2), normal weight (NW 18.5-24.9 Kg/m2), overweight (OW 25.0-29.9 Kg/m2), obese (OB >30 Kg/m2). A sample of 159 pregnant women was collected from February 2011 to March 2012. |
| A two-stage sampling study was performed. In the first stage, two surgeries (Carlet and Benimodo) were chosen using a simple random probability sampling from among all Primary Care Centres of La Ribera Health Department (Spain). In the second stage, pregnant women were selected using a probability sampling with random start and systematic monitoring depending on the number of pregnancies per year obtained in both of them. |
| Inclusions criteria were based on maternal age between 18 and 36 years, first prenatal appointment between 5 and 12 weeks of pregnancy and single-fetus pregnancy with no fetal deformities. Exclusions criteria included refusal to participate in the study, language barrier, an adverse obstetric history during previous pregnancies, medical conditions that modify fetal growth, maternal infection or any other maternal chronic pathology. |
| We estimated that for a 95% Confidence Interval (CI) and a 4% precision, we needed a minimum sample size of n=147. This study was performed according to the basic principles for all medical research set out in the Declaration of Helsinki. The study was previously evaluated and approved by the Research Committee of the Ribera University Hospital. |
| Six categories of variables were selected: anthropometric, demographic, hematologic, ultrasound, obstetric-neonatal, and toxic variables. Anthropometric variables included in the study were prepregnancy weight and height, BMI and symphysis-fundal height (SFH). Pre-pregnancy weight and height were self-reported and recorded during the initial prenatal examination after enrolment. Pre-pregnancy BMI was calculated as weight in kilograms divided by the squared height in meters (kg/m2). SFH was measured in centimetres with nonelastic measurement tape from the upper border of the symphysis pubis to the top of the uterine fundus, or reversed direction. |
| Demographic variables gathered during the study were maternal age, marital status, education and occupation. |
| Haematological variables collected included haemogram and serum ferritin. They were measured in each trimester of pregnancy (<12, 24 and 34 weeks). Ultrasound variables collected included biparietal diameter (BPD), femur length (FL), and abdominal circumference (AC) of the ultrasound done in the third trimester, between 33 and 35 weeks. They were collected in order to calculate a standardized method7 used to estimate the birth weight at 40 week (EFW40w). We used the equation devised by Hadlock II, for carrying out their routine obstetric sonograms. |
| Obstetric and neonatal variables collected were parity and gestational age in weeks (obtained from last menstrual period remembered by women). Regarding to the newborn, we recorded gender and weight at birth. Toxic variables collected were pre-gestational tobacco consumption and the number of cigarettes smoked per day in each trimester of pregnancy. Basic descriptive statistics are presented comparing pre-gestational BMI groups. Afterwards, it was found normal for each of the continuous variables with the Kolmogorov- Smirnov test. The defined level of statistical significance was p<0.05. |
| In the bivariate analysis, the Student t-test was used to compare the means of two quantitative, normalized variables. Each variable was calculated and compared between the group of pre-gestational BMI test using χ2, and the analysis of variance (Scheffe’s honestly significant differences test). In order to estimate the birth weight, a multivariate regression equation (EFWme) using only variables which statistical significance, was used. Correlation between both estimation methods (EFW40w and EFWme) with birth weight were adjusted by gestational age (38-42 weeks). Accuracy of birth weight estimation was determined by calculating the absolute error of each estimation method ([estimated fetal weight - actual birth weight] / actual birth weight). The Student t test was used to determine if this mean was significantly different from zero. Differences between both methods in the mean absolute error were assessed by the paired t test. The mean error represents the sum of the positive (over-estimation) and negative (under-estimation) deviations from the actual birth weight, approximating zero in a method with very low or no systematic error. In order to evaluate the difference between EFW40w and EFWme an analysis of the individual differences proposed by Bland-Altman [11] was used. Then bias (mean absolute error) and precision (SD percentage error) were obtained. Data were analyzed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL), Version 15.0, and Analyse-it 3.7. |
| Results |
| A total of 140 pregnant women were approached for inclusion in the study. A comparison of demographic and clinical variables among the four groups showed significant differences in occupation, type of work, social status, and parity (Table 1). Multivariate models of maternal categories UW, OW and OB showed no statistically significant differences with respect to EFW40w in predicting birth weight, and therefore were eliminated. |
| The variables that showed statistical significance with birth weight in the NW were: SFH 35-40 weeks (R=0.74, p<0.001), EFW40w (R=0.63, p<0.001), GA (R=0.47, p<0.001), Ferritin (R=-2.84, p=0.007) and number of cigarettes smoked at third trimester (3T) (R=-2.82, p=0.006). The difference between the groups (ANOVA) was not significant. Linear regression analysis between birth weight and NW group with these five predictors explains its 72% variance. This and multivariate regression equation are shown in Table 2. |
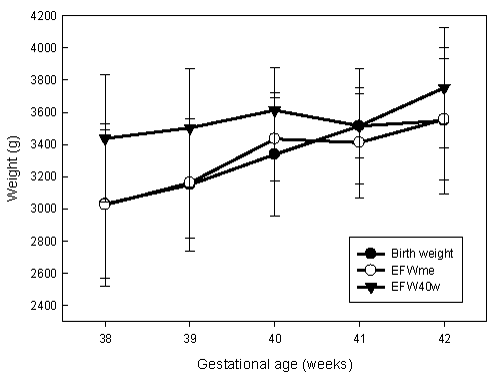
| Then it was decided to study the differences for the actual weight of the newborn between EFW40w and EFWme. T-test was applied for the samples related and the differences were statistically significant (p<0.001) between both. EFW40w, adjusted by gestational age, had a correlation of .59 (p=0.01) at 40 weeks, and 0.69 (p=0.002) for EFWme. Comparing the mean differences between EFW40w, EFWme and birth weight, we observe that the weight of ultrasound overestimates all birth weights. In contrast, the estimation of the multivariate equation underestimates the birth weights at weeks 38 and 41, and overestimates it at week 39, 40 and 42 (Figure 1). |
| The differences in averages obtained from both EFW40w and EFWme with birth weight were statistically significant (p<0.001). The EFWme underestimated birth weight by 0.07 g, and the EFW40w overestimated it by 300.89 g. Therefore, prediction absolute error was 0.53% (95% CI: -2.19-1.12) compared to 10.12% (95% CI: 12.81-7.43). |
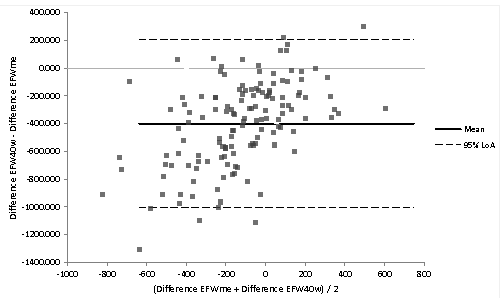
| In order to evaluate the predictive model, an observational and retrospective study was designed. From the initial one, 138 NW pregnant women who met criteria were selected. Next, differences between EFW40w and EFWme as well as the absolute error with respect to birth weight were calculated. To verify the consistency of the predictions we used the Bland-Altman analysis (Figure 2). The birth weight values provided by EFW40w are higher than the EFWme, with a difference of 398.6 g (95% CI: 450.5-346.7) (Table 3). The average error in estimating the birth weight with EFWme was 1.94% (95% CI: 0.8-30.0) underestimating the result, whereas the ultrasound error overestimated the result 10.93% (95% CI: -8.9-12.5). |
| Discussion |
| In our study there were a number of variables related to birth weight in the bivariate analysis. Those were subsequently used to construct the multivariate models. Eventually we have shown, in the NW category, that there is a statistically significant difference in predicting birth weight when it is compared to EFW40w. In a bivariate form, SFH measured between 35-40 weeks was associated with birth weight for the maternal category NW. It got the highest coefficient of determination of all the variables studied, even higher than EFW40w at the third trimester. Rogers et al. [12] correlated SFH with small-forgestational- age (SGA) infants and 73% were detected by measuring 3 cm or even below the average in pregnancy. In normal-weight mother, adjusting the SFH for gestational week Meler et al. [13] obtained a normal curve, and an SFH below 10th percentile was related to a low birth weight (LBW). In contrast, Buchmann et al. [14] described a SFH higher than 40 cm as associated with an increased number of fetal macrosomia, cephalo-pelvic disproportion and/or shoulder dystocia. In our case, fundal height measured between 35 and 40 weeks, and in the presence of the other variables in the multivariate model, indicates that birth weight increases 109.21 g for every centimeter of uterine height (95% CI: 77.6-140.6). |
| EFW40w was associated with birth weight for the maternal category of NW. In presence of the other variables, the coefficient of determination was higher than the obtained by Ben-Haroush [5]. The use of ultrasound as a diagnostic method is well documented [5,14-17]. Depending on the formula used, predicted weight differs in its accuracy [5-16]. In our multivariate model, and in the presence of the other adjusted variables, for each gram of target weight at 40 weeks in the third trimester ultrasound, birth weight increases 0.35 g (95% CI: 0.15-0.54). |
| GA showed statistically significant correlation with birth weight. The average delivery GA was 278 days in primiparous mothers and 279 days for multiparous mothers. Our multivariate analysis showed that for every extra day, there is a fetal weight gain of 13.81 g. This is slightly higher than data obtained by Nahum et al. [16] with 9.66 g and 9.15 g for boys and girls respectively, but it is lower than Carvalho et al. [18] with 28.21 g. |
| The smokers’ ratio before pregnancy was 35.0%, and 20.7% in the last trimester, similar to other studies reviewed [19-21]. Our results specially indicate that smoking during the third trimester of pregnancy, is associated with birth weight. It is a negative correlation where increasing numbers of cigarettes consumed decreases weight at birth. Consequently, smoking during the third trimester seems to have the greatest impact on birth weight. In fact, it is known that women who gave up smoking in the third trimester have babies with birth weights similar to those of nonsmokers [21]. This matches with our results, as smoking in the first two trimesters showed no statistical significance in the adjusted model. The newborn with low birth weight becomes important with this toxic habit, and there is a possible relationship with the children’s health deterioration because of the cytotoxic effect [22]. Petridou et al. [23] described reduced newborn weight, by 190.8 g respectively, as compared to the newborns of non-smoking mothers. Our results are somewhat lower: birth weight is reduced about 21 g for every cigarette smoked; the average number of cigarettes smoked per day was 5, so total decrease was about 105 g, the same results obtained by Gupta et al. [24]. |
| The amount of ferritin in the third trimester had an inverse relationship with birth weight in NW category mothers, so that the less ferritin, the higher the birth weight. In studies reviewed, we found the opposite effect in both cases: high ferritin levels were associated with preterm birth, LBW and premature rupture of membranes [25,26]. Other authors tried to explain a possible association between high levels of ferritin and fetal growth restriction, [27] arguing that ferritin may be a vascular response to both infectious and non-infectious inflammatory diseases. Further studies are needed to confirm this. Hämäläinen et al. [28] observed that anemia and low ferritin level during the first trimester was associated with LBW, while anemia in the second and third trimester was not associated with preterm birth, fetal loss or risk of perinatal complications. The effect found in our study could be explained as a relation between depletion of maternal iron stores and increase of iron transfer to the fetus, although this increase may be limited [29]. The depletion of iron in the second and third trimester of pregnancy in the NW category women physiologically declines from the first trimester. At the same time, iron-carrying capacity increases (transferrin), even when the deficit is eliminated by oral supplementation [30]. In our multivariate model, as a negative relation, for each ferritin unit that dropped (ng/dl), there was a gain of 3.35 g in birth weight. |
| The limitations of the multivariate model (NW) have to do with the accuracy of the ultrasound and the GA at birth, due to the estimated weight, which should be accurate at 40 weeks. All the newborns aged less than 280 days will be overestimated. Nowadays the prediction of the birth weight through ultrasounds (EFW40w) has an absolute error that varies from 6% to 12% [3-5]. Accuracy can be improved in two different ways: first, by controlling the limitations of the technique and second, by adding maternal variables from the multivariate model to the ultrasound measurement. Considering this pattern, error can be reduced up to 1.9%. |
| We decided to implement what can be considered a test of predictive validity, through the use of a multivariate equation to improve the estimation of birth weight in women with a normal pre-gestational BMI. Then, in order to evaluate the equation, the model was used to analyze the correlation with another different group of pregnant women in a retrospective study. In this case, 138 pregnant women, belonging to the BMI group of NW, and meeting all inclusion/exclusion criteria of the initial study, were selected. The average error with our multivariate model underestimated the birth weight, whereas the ultrasound at the third trimester overestimated the result. |
| Thus, we positively evaluate the multivariate model obtained, and so that we suggest the study has an important practical application. Therefore, we should continue by extending this study to the rest of the maternal pre-gestational BMI groups, which showed no statistical significance, to develop a new model for each one. |
| Conclusion |
| The SFH is the variable, which most affects the prediction of weight at birth. The multivariate model created improves the ultrasound measurement by 8.99%. The accuracy of the clinical method must be determined in situations which can alter the evaluation of weight birth in atypical women, and it should be studied in future ways of investigation. |
| Acknowledgements |
| This study was supported by the La Ribera University Hospital, Spain. The authors wish to thank all the study participants for sharing their data. The authors declare no conflict of interest. |
References
- Kramer MS (1987) Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ 65: 663-737.
- Valero De Bernabé J, Soriano T, Albaladejo R, Juarranz M, Calle ME, et al. (2004) Risk factors for low birth weight: a review. Eur J ObstetGynecolReprodBiol 116: 3-15.
- Dudley NJ (2005) A systematic review of the ultrasound estimation of fetal weight. Ultrasound ObstetGynecol 25: 80-89.
- Oliver M, McNally G, Leader L (2013) Accuracy of sonographic prediction of birth weight. Aust N Z J ObstetGynaecol 53: 584-588.
- Ben-Haroush A,Yogev Y, Hod M, Bar J (2007) Predictive value of a single early fetal weight estimate in normal pregnancies. Eur J ObstetGynecolReprodBiol 130: 187-192.
- Sherman DJ,Arieli S, Tovbin J, Siegel G, Caspi E, et al. (1998) A comparison of clinical and ultrasonic estimation of fetal weight. ObstetGynecol 91: 212-217.
- Chauhan SP, Hendrix NW, Magann EF, Morrison JC, Kenney SP, et al. (1998) Limitations of clinical and sonographic estimates of birth weight: experience with 1034 parturients. ObstetGynecol 91: 72-77.
- Mehdizadeh A,Alaghehbandan R, Horsan H (2000) Comparison of clinical versus ultrasound estimation of fetal weight. Am J Perinatol 17: 233-236.
- Chien PF, Owen P, Khan KS (2000) Validity of ultrasound estimation of fetal weight. ObstetGynecol 95: 856-860.
- Barker DJ, Eriksson JG, Forsén T, Osmond C (2002) Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31: 1235-1239.
- Anderson NG,Jolley IJ, Wells JE (2007) Sonographic estimation of fetal weight: comparison of bias, precision and consistency using 12 different formulae. Ultrasound ObstetGynecol 30: 173-179.
- Rogers MS, Needham PG (1985) Evaluation of fundal height measurement in antenatal care. Aust N Z J ObstetGynaecol 25: 87-90.
- Meler E, Peralta P, Figueras F, Eixarch E, Coll O, Puerto B, et al. [Uterine height: normality curves and diagnostic value for low birth weight]. ProgObstetGinecol 2005; 48 (10): 480-6.
- Buchmann E,Tlale K (2009) A simple clinical formula for predicting fetal weight in labour at term--derivation and validation. S Afr Med J 99: 457-460.
- Lee W, Deter RL, Ebersole JD, Huang R, Blanckaert K, et al. (2001) Birth weight prediction by three-dimensional ultrasonography: fractional limb volume. J Ultrasound Med 20: 1283-1292.
- Nahum GG, Stanislaw H (2003) Ultrasonographic prediction of term birth weight: how accurate is it? Am J ObstetGynecol 188: 566-574.
- Halaska MG,Vlk R, Feldmar P, Hrehorcak M, Krcmar M, et al. (2006) Predicting term birth weight using ultrasound and maternal characteristics. Eur J ObstetGynecolReprodBiol 128: 231-235.
- CarvalhoPadilha PD,Accioly E, Chagas C, Portela E, Da Silva CL, et al. (2009) Birth weight variation according to maternal characteristics and gestational weight gain in Brazilian women. NutrHosp 24: 207-212.
- Delgado Peña YP, Rodríguez Martínez G, Samper Villagrasa MP, Caballero Pérez V, Cuadrón Andrés L, et al. (2012) [Socio-cultural, obstetric and anthropometric characteristics of newborn children of mothers who smoke in Spain]. An Pediatr (Barc) 76: 4-9.
- Cano-Serral G, Rodríguez-Sanz M, Borrell C, Pérez Mdel M, Salvador J (2006) Socioeconomic inequalities in the provision and uptake of prenatal care. GacSanit 20: 25-30.
- DiFranza JR,Aligne CA, Weitzman M (2004) Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 113: 1007-1015.
- Barker DJ1 (2002) Fetal programming of coronary heart disease. Trends EndocrinolMetab 13: 364-368.
- Petridou E,Panagiotopoulou K, Katsouyanni K, Spanos E, Trichopoulos D (1990) Tobacco smoking, pregnancy estrogens, and birth weight. Epidemiology 1: 247-250.
- Gupta PC,Subramoney S (2004) Smokeless tobacco use, birth weight, and gestational age: population based, prospective cohort study of 1217 women in Mumbai, India. BMJ 328: 1538.
- Bhargava M,Iyer PU, Kumar R, Ramji S, Kapani V, et al. (1991) Relationship of maternal serum ferritin with foetal serum ferritin, birth weight and gestation. J Trop Pediatr 37: 149-152.
- Jansson L, Holmberg L, Ekman R (1979) Variation of serum ferritin in low birth weight infants with maternal ferritin, birth weight and gestational age. ActaHaematol 62: 273-277.
- Bánhidy F,Acs N, Puhó EH, Czeizel AE (2011) Iron deficiency anemia: pregnancy outcomes with or without iron supplementation. Nutrition 27: 65-72.
- Hämäläinen H,Hakkarainen K, Heinonen S (2003) Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. ClinNutr 22: 271-275.
- Scholl TO,Hediger ML (1994) Anemia and iron-deficiency anemia: compilation of data on pregnancy outcome. Am J ClinNutr 59: 492S-500S discussion 500S-501S.
- Milman N (2012) Oral iron prophylaxis in pregnancy: not too little and not too much! J Pregnancy 2012: 514345.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13078
- [From(publication date):
April-2015 - Dec 18, 2024] - Breakdown by view type
- HTML page views : 8749
- PDF downloads : 4329
