Can Signaling Molecules in Fibroblasts Detect Prodromal Alzheimer's Disease?
Received: 30-Apr-2020 / Accepted Date: 09-Jun-2020 / Published Date: 16-Jun-2020 DOI: 10.4172/2161-0460.1000490
Abstract
Ras/ERK, /p-38, /JNK and PI3K/AKT pathways may mediate amyloid-β-peptide toxicity, neuron death and cognitive decline, modulating oxidative stress and inflammation in AD. Although it is a neurodegenerative disorder, AD affects different systemic molecular mechanisms that are present in patients’ peripheral tissues, too. We investigated possible MAPK and PI3K-AKT alterations in fibroblast primary cultures from sporadic AD, MCI and control subjects, testing these molecular pathways as possible AD-related trait markers.
By western blot and phospho-Elisa, we evaluated their epigenetic status and some of their downstream pathways that might be involved in preclinical and early AD stages: the alpha-APP-levels, as a marker of the alpha-secretase activity, the expression of Bax, as an apoptosis and mTOR-autophagy regulator and the phospho-p70S6K status, that modulates protein synthesis, Tau hyper-phosphorylation and mTOR-cell signaling.
While the expression of phospho-ERK1/2 decreased in fibroblasts from mild/moderate AD compared to severe patients’ and controls’ cells, it did not shown difference in fibroblasts from seven sporadic Parkinson Disease patients compared to HC. Moreover, phospho-ERK1/2 expression and AD severity correlated and including MCI-converter patients to AD cases, this statistical significance was further confirmed. The expression of phospho-AKT also increased in the cells of severe AD patients compared to the fibroblasts of patients with mild/moderate AD, MCI and control subjects and it correlated with the disease severity, too. Phospho-p38- and phospho-JNK-SAP Kinases as well as phospho-p70S6K and Bax increased in cells from AD patients but did not correlate with disease severity, while alpha- APP levels decreased in patients’ cells and it only correlated to phospho-ERK1/2 levels in control fibroblasts, indicating the ERK involvement in the physiological metabolism of APP.
Then ERK1/2 and AKT pathways could be suitable to check the disease molecular mechanisms in these peripheral cells from the disease prodromal stage, in particular, ERK-modulation might help to discriminate converter and nonconverter MCI. Furthermore, since fibroblasts show molecular dysfunctions similar to those observed in autopsy brains, these cells might also be useful peripheral targets to investigate and modulate Aβ-related APP metabolism, Tau hyper-phosphorylation and mTOR-cell signaling, preventing the AD progression.
Keywords: ERK and AKT; MMSE; MCI-Converter; Alpha-APP; P70S6Kinase; Tau synthesis; Apoptosis; mTOR-pathway; Personalized drugs
Abbreviations
AD: Alzheimer's Disease; MCI: Mild Cognitive Impairment; HC: Healthy Control; MAPK (JNK, p38 and ERK): Mitogen- Activated Protein Kinase; ERK: Extracellular Signal-Regulated Kinase; JNK: cJun N-terminal Kinase; AKT: Protein Kinase B; PI3K: Phosphatidylinositol-3-Kinase; p-ERK, -JNK,-P38 and -AKT: Phospho-ERK, Phospho-JNK, Phospho-p38 and Phospho-AKT; Aβ: Amyloid Beta Peptide; ROS: Reactive Oxygen Species; MMSE: Mini-Mental State Examination; APP: Amyloid Precursor Protein; CNS: Central Nervous System
Introduction
During the different phases of Alzheimer's disease (AD), a variety of growth factors and mitogen compounds are modified, mediating their cellular effects through activation of MAPK (JNK, p38 and ERK1/2) and PI3K cascade [1,2]. These pathways regulate many cellular processes that are involved in cognitive functions, too [3-8].
JNK and p38 are activated by a variety of environmental stresses, inflammatory cytokines, growth factors and G-protein-coupled receptors agonists. Furthermore, ERK plays an important role in regulating cell growth and proliferation, and modulating stress and inflammation signals through the activation of p53 and autophagy by mTOR [8,9]. ERK regulates the activities of several transcription factors, such as ELK1 and CREB that play a kay role in neuronal differentiation and long-term memory formation, too.
PI3K-AKT signaling has great importance in the cell cycle process, promoting survival and growth in response to extracellular signals and also fulfills various biological functions including angiogenesis, apoptosis and autophagy, through activation of downstream responses. It also phosphorylates MAP-kinase-kinase-kinases that are the upstream protein regulators of MAPK. Currently, these pathways have been involved in several molecular mechanisms responsible for the AD pathogenic processes, even if the underlying mechanisms and interactions are not yet outlined.
Furthermore, ERK1/2 and AKT may regulate the phosphorylation of p70S6-kinase and its phosphorylation at threonine 389 has been correlated with autophagy modulation, too.
ERK1/2 pathway has also involved in Tau-hyper-phosphorylation, which is evident before the deposition of Aβ fibrils, through p70S6- kinase activation, a downstream pathway of PI3K/AKT [4,7]. Recently, Li observed harpagoside, a natural anti-inflammatory, improves in rats the Aβ-induced cognitive impairment via up-regulating BDNF expression, modulating MAPK and PI3K pathways [8,10].
JNK, p38 and ERK1/2 activation has been demonstrated in AD neurons and dystrophic neuritis and in CNS from patients at early disease stages [11-16]. In AD neocortical pyramidal neurons, the neurofilament hyperphosphorylation is accompanied by ERK1/2 activation, that is associated with age-dependent amyloid plaque deposition and increased oxidative stress and the modulation of secretase in AD animal model [17-19]. Pei and colleagues specify that ERK1/2 active forms have been found both in tangle-neurons and in AD neurons that did not show abnormal hyperphosphorylation of Tau [20]. In human CSF, ERK1/2 correlates with total- and phospho-Tau levels and elevated pTau-levels and reduced Aβ1-42 concentration are described as molecular biomarkers [21].
ERK1/2 is related to Aβ accumulation, with consequent memory dysfunction in Tg2576 mice and phospho-ERK1/2 is involved in synaptic plasticity and memory formation through CREB-activation [6,22,23]. Pharmacological or molecular inhibition of ERK1/2 and/ or CREB function leads to memory impairment, whereas their genetic overexpression and activation contribute to memory enhancement [5,24]. Rilapladib, an oral inhibitor of lipoprotein associated phospholipaseA2 that can have pro-inflammatory and oxidative properties, is also activated by ERK1/2 phosphorylation and it is currently investigated in phase II trials for AD [25].
In animal model, p38 and JNK activation has also associated with Aβ deposition and tau-hyperphosphorylation and the link between intracellular Aβ and JNK phosphorylation has been confirmed in cortical neurons from sporadic and PS1-linked AD patients[26-28].
Moreover, Aβ toxicity mediates MAPK and PI3K signaling in animal models, neuroblastoma cell line and primary human fibroblast cultures [8,29-31].
In bradykinin-treated fibroblasts from sporadic and familial patients, dysfunctions in ERK1/2 signaling cascade have also detected and correlated to dementia duration [31,32].
Activated ERK1/2 and PI3K/AKT have been shown to directly phosphorylate GSK3β and GSK3β-genetic variants are associated to total- and phospho-Tau and Aβ levels, in AD patients [33,34]. Moreover, fibroblasts are a useful cell model to investigate Tau modifications [35].
To investigate the complex interactions among Aβ, Tau and MAPK and PI3K/AKT signaling, in patients with different disease severity, we compared these parameters in primary fibroblasts obtained from mild and moderate/severe patients, Mild Cognitive Impairment (MCI) and Healthy (HC) subjects [36,37]. Although some studies show modulation of these pathways in the autopsy brain of the patients and/or in pathology models, our results indicate a specific ERK and AKT relationship to severity of the disease in patient's fibroblasts and their involvement in Aβ-dependent mTOR signaling [38]. We suggest that these signaling pathways, playing a key role for the disease, may help to select and target clinical investigations, suggesting some personalized therapeutic strategies [21,39]. Thus, they might help to identify individuals at an early stage or high risk of the disease.
Materials and Methods
Population study
Twenty AD patients (six severe, eight moderate and six mild), 7 MCI subjects and 13 age-related controls took part into the study. Their socio-demographic and clinical features are shown in Table 1. Criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) were applied for the diagnosis of probable AD or MCI [40,41]. All patients presented with progressive cognitive impairment predominantly affecting memory and were assessed with brain Computed Tomography (CT) or nuclear magnetic resonance imaging (NMRI), blood tests for excluding secondary dementia (thyroid hormones, folic acid, vitamin B12, VDRLTPHA and erythrocyte sedimentation rate) and a comprehensive neuropsychological battery. AD patients were considered severe if they had a MMSE score < 12, moderate for a score between 13 and 19 and mild for a score between 20 and 24. Exclusion criteria were psychiatric disorders such as major depression, mania and schizophrenia, a past or present history of substance abuse, neoplastic or haematologic disorders, recent infections or surgery, severe hepatic or renal insufficiency, myocardial infarction or head injury within the last six months and ongoing antiplatelet, anti-inflammatory, anti-neoplastic, corticosteroid or immunosuppressive treatments. No patient had a familial history of dementia. For HC, active medical illness, personal or family history of neurological or psychiatric disorders and alcoholism or drug abuse were ruled out by clinical interview. Absence of cognitive impairment was defined by a MMSE score>26/30. Experiments were undertaken with written consent of each participant and the study was approved by the local Ethics Committee of San Gerardo Hospital, Monza, Italy.
| Subjects | Number | Men/Women | Age ± SD | MMSE ± SD |
|---|---|---|---|---|
| AD | 20 | 09-Nov | 74.2 ± 1.99 | 16.63 ± 5.09 |
| HC | 13 | 06-Jul | 69.4 ± 1.63 | 29.22 ± 0.47 |
| MCI | 7 | 04-Mar | 74.6 ± 2.04 | 27.64 ± 1.03 |
Table 1: Demographic characteristics and neuropsychological score of tested subjects. APO-Eε4 total distribution in AD (20) and MCI (7) is 11/27. For Age, One-way analysis of variance is: p=0.35, for MMSE, AD vs HC p< 0.01 and AD vs MCI p<0.05 and HC vs MCI p>0.05.
Skin biopsy and primary cell culture
From HC and MCI subjects and patients skin biopsies were performed and cultured into Dulbecco’s Modified Eagle’s Medium (DMEM, Euroclone, Italy), supplemented with 10% heat inactivated Fetal Calf Serum (FCS), 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. The obtained fibroblasts were plated in dish and they were expanded until they reached the sub-confluence. Then they were collected in cold PBS within 12th trypsin treatment, as previously described [30].
To obtain dry pellets, fibroblasts were centrifuged at 250 x g at 4ºC for 10 min, then they were preserved at -20ºC, until the protein extraction.
Western Blot analysis (WB)
Sub-confluent cell cultures of fibroblasts were collected in cold PBS and lysated in Cell Extraction Buffer, containing protease and phosphatase inhibitors (CEB, Invitrogen, UK) [30]. Proteins of cytosol (25 ug) were separated by electrophoresis on 10-12% SDS polyacrylamide gels and they were blotted on nitrocellulose filter. Blots were blocked 1 h at room temperature on a shaker in 5% no-fat dried milk in TBS-T buffer (50 mM Tris-HCl pH 7.6, 200 mM NaCl, 0.1% Tween 20).
Anti-p38, -JNK, -ERK1/2, -AKT, -p70/S6 and -Bax immunoblot (WB)
Blots were incubated overnight, on a shaker, at 4ºC with the primary rabbit antibodies. They were: Anti-phosphorylated p38 [pTpY180/182] and anti-total p38, anti-phosphorylated JNK [pTpY183/185] and anti-total JNK, anti-phosphorylated ERK1/2 [pTpY185/187] and anti-total ERK1/2 (1:500 polyclonal), monoclonal anti-phospho-AKT (1:500), anti-phosphorylated p70 S6-Kinasi [T389] (1:300), anti-phospho-Tau (1:1000, [Ser400/404- Thr403 ]) and mouse anti-Bax (1:200, Merck-Millipore Italy). All antibodies (Cell Signaling, The Netherlands) were diluted in 5% nofat dried milk in TBS-T buffer. Mouse anti-actin antibody (1:20000, Merck, Italy) was internal standard.
Peroxidase-linked anti-rabbit/mouse (1:5000/7000 or 1:15000 for actin antibody, Merck, Italy) IgG secondary antibody were incubated for 1 h, at room temperature, on an orbital shaker in TBS-T buffer, containing 3% no-fat dried milk. The presence of signals was detected by chemiluminescent reagents such as ECL Plus Kit (Merck, Italy). The protein signal was capture with a photographic film and imaging densitometer (BioRad, USA) was employed to scan films, which were analyzed by Bio-Rad Multi-Analyst/TM software. The value of signals in WB was expressed as the ratio between the target and actin signals and/or between phosphorylation and total form of the specific kinase, in our experimental condition, no difference was shown in the trend of the two different ratios. Experiments were performed in duplicate or tripled, on different gels.
Total- and phospho-ELISA for MAPK and AKT
In order to detect and quantify the levels of these kinases in protein lysates of fibroblasts, we used the Immunoassay Kits (phospho-ELISA kits, BioSource International, Inc, USA). Total levels of p38, JNK, ERK1/2 and AKT are independent of phosphorylation status, so we used total ELISA kits (BioSource International, Inc, USA) to normalize the phosphorylated p38, JNK, ERK1/2 and AKT amount in the samples.
Cytosol protein extraction was performed in CEB buffer, containing 1 mM PMSF, protease and phosphatase inhibitor cocktail (Merck, Italy, 1:200 and 1:100), for 30 min, on ice. Then lysates were centrifuged at 12000 x g for 10 min at 4ºC.
According to the Bio Source assay method, different dilutions of samples were tested for each phosphorylated or total protein detection. The protein absorbance was determined by plate reading at 450 nm (BioRad, USA). The concentrations were calculated comparing the tested absorbance to the specific standard curve values, for each MAPK phosphorylated status and expressed with respect to specific MAPK total status.
Levels of human alpha-APP in cell lysates
In fibroblasts form recruited subjects, APP levels were detected in cytosol protein extracts. By Immunoassay Kit (Merck-Invitrogen, Italy), alpha-APP (αAPP) levels were tested. This kit detects soluble αAPP which is cleaved by alpha-secretase, but not soluble beta-APP which is cleaved by beta-secretase, as described in commercial data sheet.
Statistical analysis
By Graph Pad Prism program statistical analysis was performed. All results were expressed as mean ± Standard Deviation (SD). Oneway ANOVA analysis, followed by Bonferroni post-test, was used to determine the significance of differences among the values obtained in the subgroups of recruited subjects. By Pearson’s test, possible correlations between protein-kinase expressions and MMSE score or αAPP levels were analyzed. By Student-t test, we evaluated the statistical analyses between patients’ and HC group.
Results
Stress-activated protein kinase in AD fibroblasts
The phosphorylation state of p38 and JNK was assessed in fibroblasts from AD, MCI and age-matched HC.
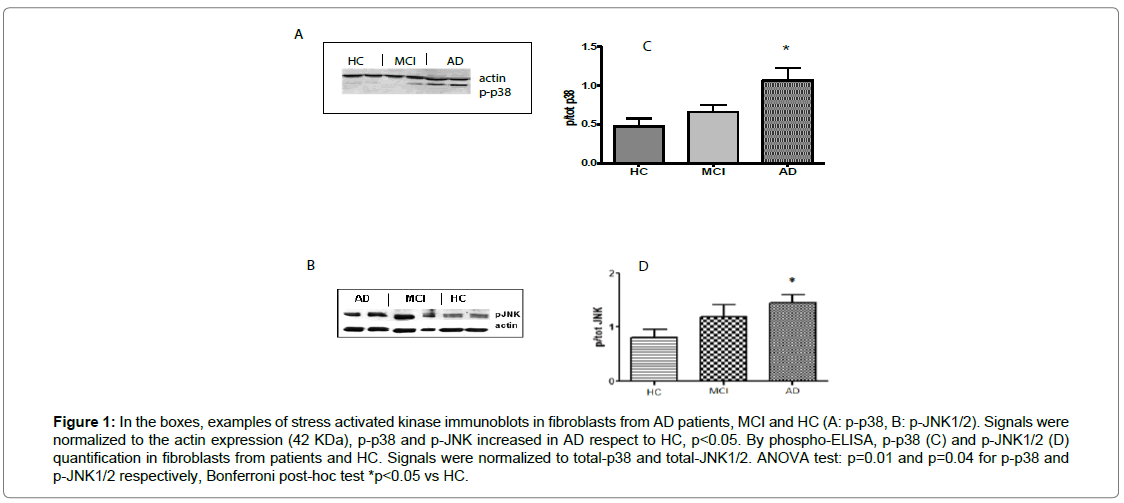
By western blot and phospho-ELISA assays, comparable results were shown (Figures 1A and 1B). A significant difference (p=0.03) between patients (1.07 ± 0.36, ratio value in WB) and HC (0.47 ± 0.23, ratio value in WB) was observed, indicating a 100% increase of p38-phosphorylation in AD subjects, from the earliest stages of the disease. A trend to p-p38 increase was shown in MCI subjects (0.66 ± 0.20, ratio value in WB, p=0.21 vs HC and p=0.06 vs AD), too. By WB, anti-phospho-JNK antibody displayed specific and 100% increased immunoreactivity in AD (1.45 ± 0.32, ratio value in WB) compared to control fibroblasts (0.78 ± 0.28, p<0.05) and in MCI fibroblasts (1.20 ± 0.42, p>0.05) (Figure 1C). By phospho-ELISA, comparable results were shown (Figure 1D). There is no correlation between p-JNK or p-p38 and disease severity.
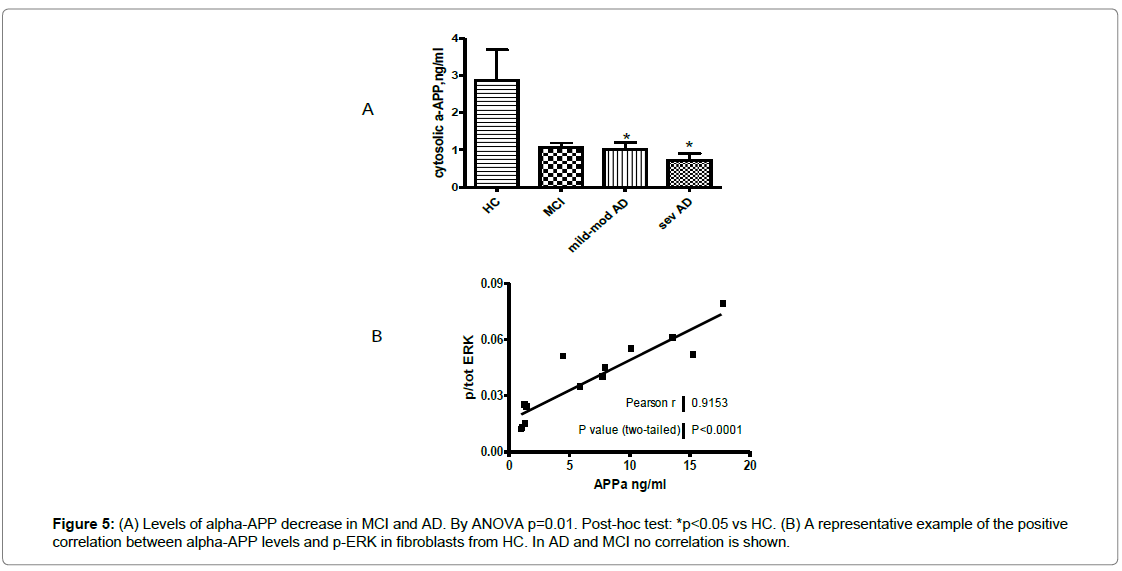
Figure 1: In the boxes, examples of stress activated kinase immunoblots in fibroblasts from AD patients, MCI and HC (A: p-p38, B: p-JNK1/2). Signals were normalized to the actin expression (42 KDa), p-p38 and p-JNK increased in AD respect to HC, p<0.05. By phospho-ELISA, p-p38 (C) and p-JNK1/2 (D) quantification in fibroblasts from patients and HC. Signals were normalized to total-p38 and total-JNK1/2. ANOVA test: p=0.01 and p=0.04 for p-p38 and p-JNK1/2 respectively, Bonferroni post-hoc test *p<0.05 vs HC.
The RAS-ERK and PI3K-mTOR pathways and disease severity in AD fibroblasts
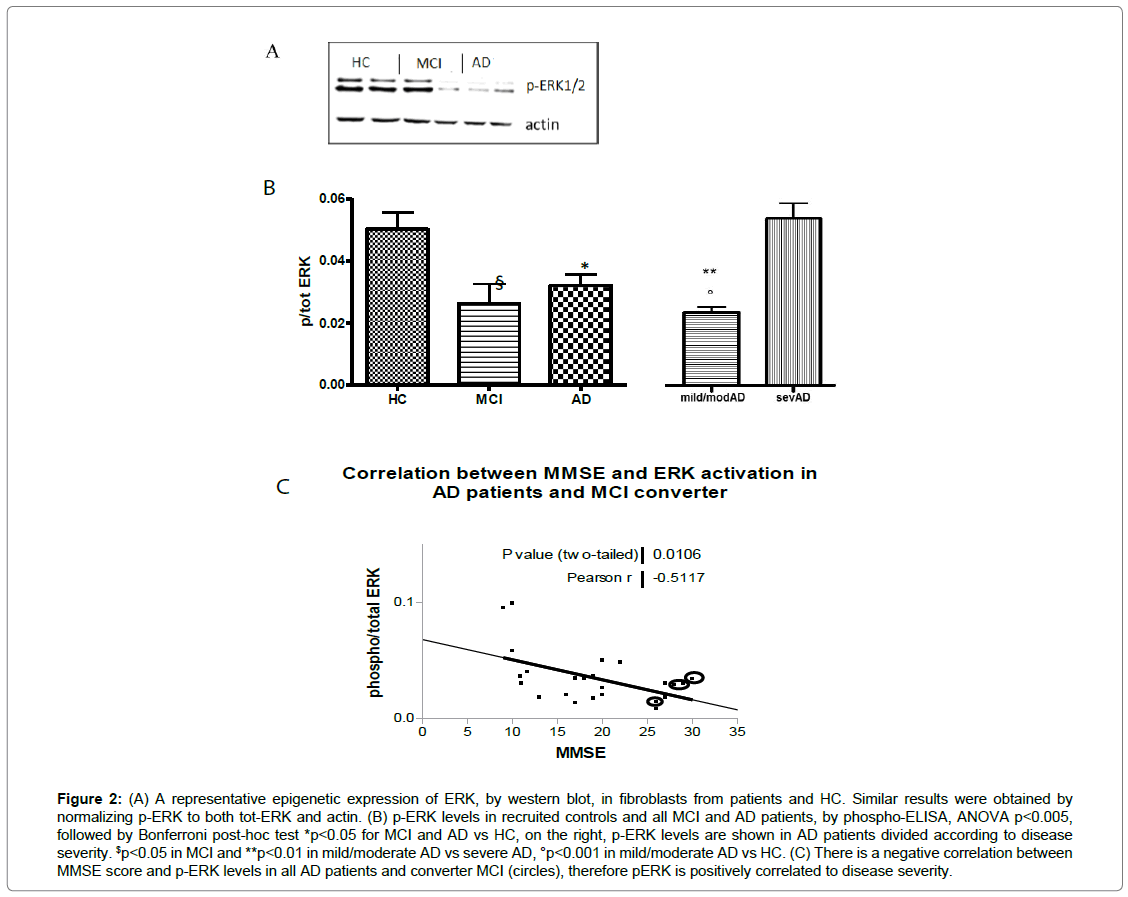
By WB, the examples of p-ERK modulation were shown in fibroblasts from MCI and AD compared to control fibroblasts (Figure 2A). By phospho-ELISA assay, ERK1/2 phosphorylation reduced by approximately 50% in patients, precisely in fibroblasts from mild and moderate AD (0.021 ± 0.006 p/tot, p<0.001) compared to HC cells (0.050 ± 0.020 p/tot). Furthermore, there was a statistically significant increase of p-ERK1/2 in cells from severe AD patients (0.053 ± 0.012 p/tot) compared to fibroblasts from mild and moderate patients (p<0.01). No difference was observed between severe AD and HC, while p-ERK1/2 decreased in MCI fibroblasts, too (0.026 ± 0.017 p/tot, p <0.05 vs HC) (Figure 2B). We also observed a significant inverse correlation between p-ERK1/2 and MMSE score (r= -0.596, p=0.01), indicating a direct correlation with disease severity. Including the p-ERK values and corresponded MMSE score of four converters MCI, the correlation was maintained (Figure 2C). Moreover, in non-converter-MCI subjects, no correlation between p-ERK levels and MMSE score was shown.
Figure 2: (A) A representative epigenetic expression of ERK, by western blot, in fibroblasts from patients and HC. Similar results were obtained by normalizing p-ERK to both tot-ERK and actin. (B) p-ERK levels in recruited controls and all MCI and AD patients, by phospho-ELISA, ANOVA p<0.005, followed by Bonferroni post-hoc test *p<0.05 for MCI and AD vs HC, on the right, p-ERK levels are shown in AD patients divided according to disease severity. $p<0.05 in MCI and **p<0.01 in mild/moderate AD vs severe AD, °p<0.001 in mild/moderate AD vs HC. (C) There is a negative correlation between MMSE score and p-ERK levels in all AD patients and converter MCI (circles), therefore pERK is positively correlated to disease severity.
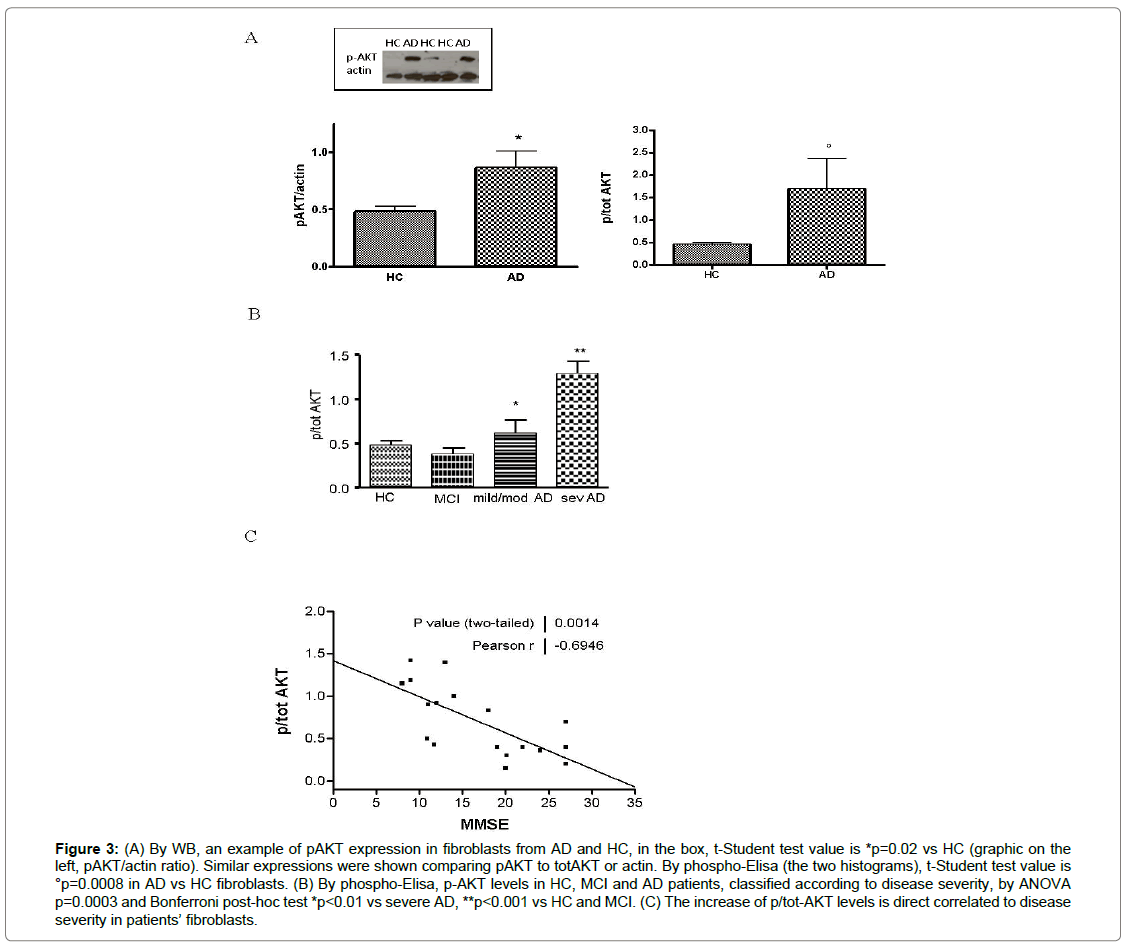
As well as ERK1/2, AKT kinase is involved in cell survival and it modulates mTOR-p70S6K pathway. In patients’ fibroblasts, p-AKT increased by 40% or 50% in western blot (p=0.02) or ELISA (p=0.0008), respectively vs HC cells (Figures 3A-B). In MCI, p-AKT status was reduced compared to severe AD (p<0.001) and it was decreased with respect to mild/moderate patients (p<0.01), too. In addition, there is a direct correlation between phospho-AKT expression and disease severity, evaluated by MMSE score (r=-0.63, p=0.03) (Figure 3C).
Figure 3: (A) By WB, an example of pAKT expression in fibroblasts from AD and HC, in the box, t-Student test value is *p=0.02 vs HC (graphic on the left, pAKT/actin ratio). Similar expressions were shown comparing pAKT to totAKT or actin. By phospho-Elisa (the two histograms), t-Student test value is °p=0.0008 in AD vs HC fibroblasts. (B) By phospho-Elisa, p-AKT levels in HC, MCI and AD patients, classified according to disease severity, by ANOVA p=0.0003 and Bonferroni post-hoc test *p<0.01 vs severe AD, **p<0.001 vs HC and MCI. (C) The increase of p/tot-AKT levels is direct correlated to disease severity in patients’ fibroblasts.
Regulation of Tau metabolism and apoptosis in patients’ fibroblasts
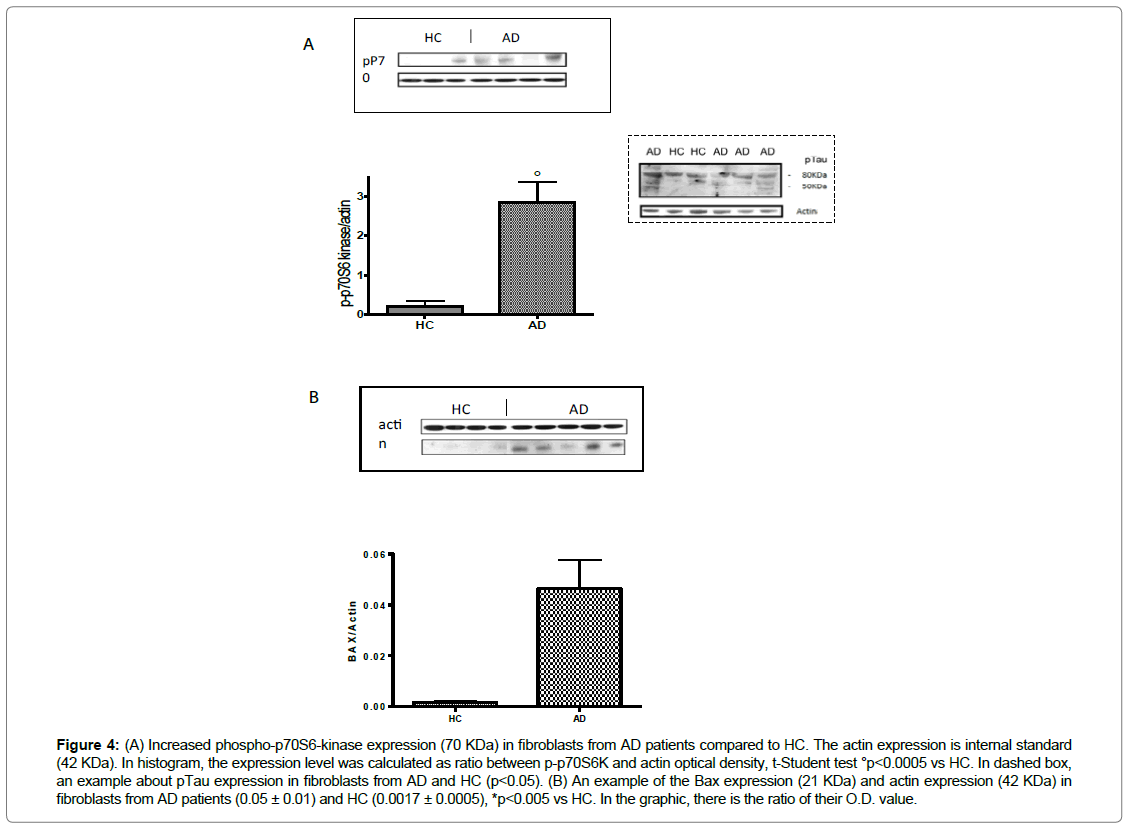
We also tested the phosphorylation of p70S6-kinase that is regulated by ERK1/2 and AKT pathway and its phosphorylation at threonine 389 has been correlated with autophagy inhibition, too. In particular, the 70kDa isoform regulates the activation of Tauprotein synthesis and its phosphorylation, as described in CNS cells. While the 85KDa nuclear isoform modulates the transcriptional mechanisms.
In fibroblasts, our results showed an increasing trend of phosphop70S6K in AD patients (p<0.001 vs HC) and it is interesting to note that the predominant isoform is the cytosolic one (70KDa) (Figure 4A). Our results also indicated the increase of pTau- Ser400/Thr403/Ser404 in fibroblasts from AD patients compared to the cells of HC (p<0.05) (dashed box) (Figure 4).
Figure 4: (A) Increased phospho-p70S6-kinase expression (70 KDa) in fibroblasts from AD patients compared to HC. The actin expression is internal standard (42 KDa). In histogram, the expression level was calculated as ratio between p-p70S6K and actin optical density, t-Student test °p<0.0005 vs HC. In dashed box, an example about pTau expression in fibroblasts from AD and HC (p<0.05). (B) An example of the Bax expression (21 KDa) and actin expression (42 KDa) in fibroblasts from AD patients (0.05 ± 0.01) and HC (0.0017 ± 0.0005), *p<0.005 vs HC. In the graphic, there is the ratio of their O.D. value.
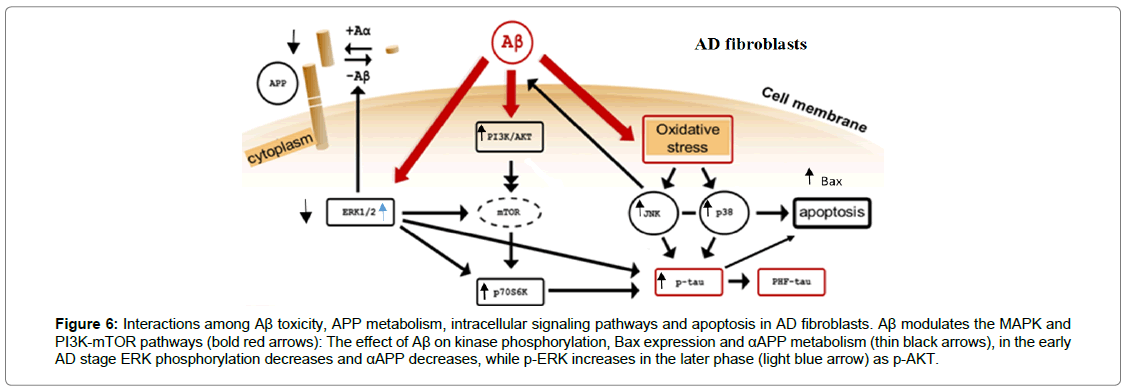
Moreover, in a subgroup of patients and HC, it was detected an increase (p<0.05) of the cytoplasmic levels of Bax (Figure 4B). This result indicates the triggering of the apoptotic mitochondrial pathway [42]. Bax is also a downstream pathway that both ERK1/2 and AKT regulate, in fact, it modulates apoptosis and autophagy signaling.
ERK and APP metabolism
Since the ERK1/2 signaling might be linked to APP metabolism, we also tested the cytosolic αAPP levels in fibroblasts from recruited subjects [43]. A 60% reduction of the αAPP levels were observed in AD patients, in particular in fibroblasts from severe patients compared to HC (p=0.003) (Figure 5A). In MCI fibroblasts, αAPP distribution was heterogeneous. Moreover, phospho-ERK1/2 and αAPP correlation was very significant in HC (r=0.915), while in MCI subjects and AD patients this correlation was lost (Figure 5B).
Discussion
Various research groups have turned their attention to the signal transduction pathways involved in the pathway of GSK-3β, such as MAP kinase, PI3K/AKT and PKC. They play a role in mediating the toxic effect of Aβ in the regulation of Tau hyper-phosphorylation, this toxicity may also modulate the mechanisms of synaptic and neuronal degeneration [34,44-48].
It knows that several systemic mechanisms and pathways may be investigated in fibroblasts, reflecting the biochemical and molecular alterations that have been observed in CNS [30-33,35]. Since in the brain of AD patients, MAPK and PI-GSK3/AKT modulations seem related to the alterations and deposition of Aβ and Tau, we investigated these specific pathways in primary cultures from human fibroblasts. In this way, we considered systemic but specific molecular mechanisms involved in AD, particularly in the early phase of disease.
Since fibroblasts are independent of the postmortem changes, they can be suitable for pharmacological and molecular studies, too [49]. Moreover, in these cells the pathogenic mechanisms, involved in AD progression, may be investigated as possible therapeutic targets [32]. Following autopsy, the same PKC alterations have been described in both fibroblasts and brain, therefore it thinks these kinase-linked molecular changes and/or their epigenetic modifications may also be maintained in these peripheral cells [31].
In this work, the data show affected MAPK and AKT pathways, in fibroblasts from AD patients and MCI subjects compared to HC, highlighting the role of these pathways in Aβ induced-oxidative stress, APP metabolism, Tau phosphorylation and their involvement in the etiopathology and progression of disease, modulating inflammation and mTOR-signaling.
In particular, since the early stages of AD, the stress activated phosphokinases p38 and JNK increased their phosphorylation and a clear upward trend has also shown in MCI. These modifications may be a response to cell bio-energetic impairment but they did not correlate to the disease severity. Moreover, the involvement of these stressactivated kinases, in the early stage of disease, might also be responsible for the reduced Energy-Dependent Activity of Glutamate Transporters (EAATs), justifying in part the decreased glutamate uptake, previously observed in fibroblasts from AD patients, in the absence of EAATs modifications both to mRNA and protein expression [39,43].
In addition, the signal transduction pathway mediated by p38 is involved in the alteration of synaptic plasticity caused by Aβ, suggesting a role of this kinase at CNS level, in the decline of the mnemonic functions, features of the disease [39]. Bodles and colleagues hypothesized that activated microglia, observed in the early stage of the disease, is able to phosphorylate JNK and p38, inducing the production of factors that are pro-inflammatory and toxic to neurons [50]. Moreover, these kinases are jointly responsible for Tau phosphorylation that we observed to be dysregulated in AD fibroblasts, too [51].
Different authors also report ERK1/2 activation in AD neurons and dystrophic neuritis [13-15]. Khan described ERK1/2 as a putative peripheral biomarker for AD, in response to the inflammatory signal bradykinin [31]. Furthermore, p-ERK1/2 level increased in CSF from AD patients, as described by Spitzer and colleges [21].
In line with these papers, we also underlined the specific ERK1/2 involvement in AD fibroblasts. In detail, we observed that ERK1/2 phosphorylation depends on the disease severity: ERK1/2 was downphosphorylated in MCI, mild and moderate AD patients with respect to healthy subjects, while p-ERK1/2 levels increased in severe AD cells and they were higher than HC levels. Surprisingly, the inverse correlation between MMSE score and ERK1/2 phosphorylation strengthened the modulation of ERK1/2 in different disease stages. In fact, over at least three years, the four MCI subjects that converted to AD patients have kept this correlation, while it did not show in non-converter- MCI subjects (Figure 2C). For the first time, this correlation has been observed in fibroblasts and we propose this parameter might help to predict the evolution of MCI status. Integrating with neuroimaging investigations, a specific longitudinal study could confirm this hypothesis, suggesting p-ERK as an economical and useful peripheral marker. In fact, we tested p-ERK1/2 levels in fibroblasts from patients with Parkinson’s disease, but no difference showed (p>0.05, n=7 data not shown).
ERK1/2 also modulates inflammatory stimuli thus its phosphorylation, in the later stages of the disease, could account for the involvement of inflammatory mechanisms in AD pathogenesis [31]. This phenomenon could also suggest that a compensatory mechanism may occur in severe AD fibroblasts, resulting in the cell attempt to balance the oxidative stress that Aβ induces from the early stages of disease. On the other hand, an unbalance between kinases and phosphatases system may occur in fibroblasts from severe AD patients. In fact, in fibroblasts from severe patients, Zhao and colleagues demonstrated decreased levels of phosphatase 2A (PP2A), inducing a prolonged ERK1/2 activation [33]. This protein phosphatase and PP1 phosphatase also decrease in the brains of AD patients and they regulate Tau phosphorylation [52]. Moreover, the up regulation of MKP-1, that is an essential negative regulator of MAPKs by dephosphorylating them at both tyrosine and threonine residues, reduces Aβ generation and alleviates cognitive impairments in Alzheimer’s disease models [53].
Owing to the decreased phosphatase activity in the AD brain, the dephosphorylation of these kinases and Tau would be compromised, allowing Tau to be abnormally hyper phosphorylated at multiple sites. In fact, Tau is phosphorylated at more than 21 sites in AD brain and ERK1/2 has been referred to be responsible for Tau phosphorylation, too [7,26]. The p70 S6-kinase is a downstream protein of ERK1/2 pathway and its cytosolic isoform (70kDa) is involved in the activation of Tau-protein synthesis and its phosphorylation, as described in CNS [54]. Since in AD fibroblasts, we observed a specific increased in 70KDa isoform, we hypothesized an involvement in Tau posttranslational regulation for ERK1/2-activated p70S6K. We have tested this hypothesis and our data indicate Tau hyper phosphorylation in AD cells.
The data obtained in our laboratory shown that the pathway regulated by PI3K/AKT is up-regulated in fibroblasts from severe AD patients compared to HC cells, in the early stages of the disease, a trend towards increased p-AKT has also observed. An opposite trend has been showed in MCI. Moreover, a significant correlation between AKT phosphorylation and the disease severity was observed, too. This finding could also be explained by the involvement of AKT in GSK-3β phosphorylation, the key kinase involved in Tau hyper-phosphorylation [55]. Several authors have demonstrated AKT activation in brains from AD and our results are in agreement with these data [33].
The activation of PI3K/AKT signaling pathway would lead to phosphorylation of AKT cascade, i.e. p70S6K, Tau, mTOR, Bax, modulating Aβ-oxidative damage, protein expression and their epigenetic regulation, autophagy and apoptosis pathways. The phosphorylation of p70S6K at threonine 389 has been used as a hallmark of activation by mTOR and correlated with autophagy inhibition in several diseases [34,36-38]. Also in fibroblasts, the increase of p-AKT and p-p70S6K could support the involvement of this signaling pathway in AD pathogenesis, mirroring CNS results.
Moreover, the induction in the cytoplasmic levels of Bax highlights the triggering of apoptotic mitochondrial pathway [43]. In the early stages of the disease, this result could be due to the activation of p38 and JNK (SAP Kinase) that integrate with the modulation of ERK1/2 and AKT signal transduction pathways (Figure 6). The assessment of p-AKT and p-ERK1/2 levels in fibroblasts from patients may be a molecular support to the clinical investigations. It might also be useful to studyi the relationship between the modulation of AKT and ERK1/2 phosphorylation and Tau protein expression [56].
Figure 6: Interactions among Aβ toxicity, APP metabolism, intracellular signaling pathways and apoptosis in AD fibroblasts. Aβ modulates the MAPK and PI3K-mTOR pathways (bold red arrows): The effect of Aβ on kinase phosphorylation, Bax expression and αAPP metabolism (thin black arrows), in the early AD stage ERK phosphorylation decreases and αAPP decreases, while p-ERK increases in the later phase (light blue arrow) as p-AKT.
The secretion of αAPP by a-secretase is a highly regulated second messenger pathway that involves several protein kinases including MAPK, PKC, PKA, etc. [44,45]. Here we showed a decreased αAPP levels in fibroblasts from AD and some MCI, compared to HC.
Furthermore, the correlation between αAPP detection and ERK1/2 phosphorylation in HC fibroblasts evidences a straight ERK1/2 involvement in αAPP metabolism, that is lost during the disease progression, as shown in AD brain [43]. No correlation was observed between SAPKs or AKT and αAPP levels.
Instead, AKT seems assumes a more specific involvement in Tau phosphorylation, autophagy and apoptosis modulation.
On the other hand, recent data have showed the physiologic αAPP function in limiting neuron damage and death, in response to neurotoxic stress conditions: αAPP is able to stimulate AKT activity in wild-type fibroblasts, human neuroblastoma cells and neurons [43,57]. According to these data, we observed a decreased AKT activity and reduced αAPP levels in fibroblasts from patients, right from the earliest disease phases, while in severe AD a different trend appears. All these evidences in our ex-vivo cellular model show a key role for ERK1/2 and AKT, in the disease physiopathology, supporting and clarifying some indications obtained from the brain autopsy of AD patients [57,59].
Conclusion
Our studies support the validity of fibroblasts as peripheral ex vivo model for studying the pathogenesis of AD. They showed the kay involvement of p-ERK1/2 and p-AKT in relation to disease severity and, for the first time, we observed that the phosphorylation status of ERK might differentiate between MCI-converter and MCI non-converter. Moreover, our results underline that the regulation of mTOR signaling in AD also has a kay role in these peripheral cells, facilitating the approach of biomolecular and pharmacological research.
Nowadays, skin fibroblasts are the most suitable source of induced Pluripotent Stem (iPS) cells, too [58,60]. These cells and the signaling pathways, that we have investigated, are considered for the development of cell models for some neurodegenerative diseases and drug screening. From this point of view, our study might be a pioneering approach to investigate any mechanisms related to Alzheimer’s disease and it might also be useful to test possible personalized therapy.
Declarations
Potential conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The research has been given ethical approval in accordance with the Declaration of Helsinki.
Acknowledgement
This work was partially supported by:
The Italian Ministry of University and Research [grant n°CTN01_00177_165430 ALISEI-IVASCOMAR, 2013-2017: Cluster project for Identification, Validation and commercial development of new diagnostic and prognostic biomarkers for complex diseases],
The European Community grant agreement n°212043: NAD, 7°Framework Programme, 2007-2013.
References
- Crutcher KA, Scott SA, Liang S, Everson WV, Weingartner J (1993) Detection of NGF-like activity in human brain tissue: Increased levels in Alzheimer's disease. J Neurosci 13: 2540-2550.
- Gärtner U, Holzer M, Arendt T (1999) Elevated expression of p21ras is an early event in Alzheimer's disease and precedes neurofibrillary degeneration. Neurosci 91: 1-5.
- Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribé E, et al. (2005) Current advances on different kinases involved in tau phosphorylation and implications in Alzheimer's disease and tauopathies. Curr Alzheimer Res 2: 3-18.
- Sadot E, Jaaro H, Serger R, Ginzburg I (1998) Ras-signaling pathways: Positive and negative regulation of tau expression in PC12 cells. J Neurochem 70: 428-431.
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Briggs CA, et al. (2007) Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. JNeurosci 27: 10578-10587.
- Jin P, Choi DY, Hong JT (2012) Inhibition of extracellular signal-regulated kinase activity improves cognitive function in Tg2576 mice. Clin Exp Pharmacol Physiol 39: 852-857.
- Qi H, Prabakaran S, Cantrelle FC, Chambraud BE, Chambraud B, et al. (2016) Characterization of neuronal tau protein as a target of extracellular-signal-regulated kinase. J Biol Chem 291: 7742-7753.
- Li J, Ding X, Zhang R, Jiang W, Sun X, et al. (2015) Harpagoside ameliorates the amyloid-β-induced cognitive impairment in rats via up-regulating BDNF expression and MAPK/PI3K pathways. Neurosci 303: 103-114.
- Wang J, Whiteman MW, Lian H, Wang G, Singh A, et al. (2009) A non-canonical MEK/ERK Signaling pathway regulates autophagy via regulating beclin 1. J Biol Chem 284: 21412-21424.
- Chen Y, Zhu L, Ji L, Yang Y, Lu L, et al. (2018) Silencing the ACAT1 gene in human SH-SY5Y Neuroblastoma cells inhibits the expression of cyclo-oxygenase 2 (COX2) and reduces β-Amyloid-Induced toxicity due to activation of protein kinase C (PKC) and ERK. Med Sci Monit 24: 9007-9018.
- Zhu X, Ogawa O, Wang Y, Perry G, Smith MA (2003) JKK1, an upstream activator of JNK/SAPK, is activated in Alzheimer's disease. J Neurochem 85: 87-93.
- Sun A, Liu M, Nguyen XV, Bing G (2003) p38 MAP kinase is activated at early stages in Alzheimer's disease brain. Exp Neurol 183: 394-405.
- Ferrer I, Blanco R, Carmona M, Puig B (2001) Phosphorylated mitogen-activated protein kinase (MAPK/ERK1/2-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm 108: 1397-1415.
- Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, et al. (1999) Activation of neuronal extracellular receptor kinase (ERK1/2) in Alzheimer disease links oxidative stress to abnormal phosphorylation. NeuroReport10: 2411-2415.
- Webber KM, Smith MA, Lee HG, Harris PL, Moreira P, et al. (2005) Mitogen- and stress-activated protein kinase 1: convergence of the ERK and p38 pathways in Alzheimer's disease. JNeurosci Res 79: 554-560.
- Veeranna T, Kaji B, Boland T, Odrljin T, Mohan P, et al. (2004) Calpain mediates calcium-induced activation of the ERK1, 2 MAPK pathway and cytoskeletal phosphorylation in neurons: Relevance to Alzheimer's disease. Am J Pathol 165: 795-805.
- Webster B, Hansen L, Adame A, Crews L, Torrance M, et al. (2006) Astroglial activation of extracellular-regulated kinase in early stages of Alzheimer disease. J Neuropathol Exp Neurol 65: 142-151.
- Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, et al. (2009) Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr 139: 1987-1993.
- Lee IS, Ryu DK, Lim J, Cho S, Kang BY, et al. (2012) Artesunate activates Nrf2 pathway-driven anti-inflammatory potential through ERK signaling in microglial BV2 cells. Neurosci Lett 509: 17-21.
- Pei JJ, Braakb H, Ana WL, Winblad B, Cowburn RF, et al. (2002) Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer’s disease. Brain Res Mol Brain Res 109: 45-55.
- Spitzer P, Schieb H, Kamrowski-Kruck H, Otto M, Chiasserini D, et al. (2011) Evidence for elevated cerebrospinal fluid ERK1/2 levels in Alzheimer dementia. Int J Alzheimers Dis Pp: 1-9.
- Sun Y, Zhang JR, Chen S (2017) Suppression of Alzheimer's disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp Ther Med 14: 5267-5274.
- Adams JP, Sweatt JD (2002) Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42: 135-163.
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, et al. (1999) The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci 19: 4337-4348.
- Oijen MV, Meer IMVD, Hofman A, Witteman JC, Koudstaal PJ, et al. (2006) Lipoprotein-associated phospholipase A2 is associated with risk of dementia. Ann Neurol 59: 139-144.
- Echeverria V, Ducatenzeiler A, Dowd E, Jänne J, Grant SM, et al. (2004) Altered mitogen-activated protein kinase signaling, tau hyperphosphorylation and mild spatial learning dysfunction in transgenic rats expressing the beta-amyloid peptide intracellularly in hippocampal and cortical neurons. Neurosci129: 583-592.
- Puig B, Gomez-Isla T, Ribe E, Cuadrado M, Torrejón-Escribano B, et al. (2004) Expression of stress-activated kinases c-Jun N-terminal kinase (SAPK/JNK-P) and p38 kinase (p38-P) and tau hyperphosphorylation in neurites surrounding betaA plaques in APP Tg2576 mice. Neuropathol Appl Neurobiol30: 491-502.
- Shoji M, Iwakami N, Takeuchi S, Waragai M, Suzuki M, et al. (2000) JNK activation is associated with intracellular beta-amyloid accumulation. Brain Res Mol Brain Res 85: 221-233.
- Daniels WM, Hendricks J, Salie R, Taljaard JJ (2001) The role of the MAP-kinase superfamily in beta-amyloid toxicity. Metab Brain Dis 16: 175-185.
- Zoia CP, Riva C, Isella V, Proserpio P, Terruzzi A, et al. (2011) Nonfibrillar Aβ 1-42 inhibits glutamate uptake and phosphorylates p38 in human fibroblasts. Alzheimer Dis Assoc Disord 25: 164-172.
- Khan TK, Alkon DL (2015) Peripheral biomarkers of Alzheimer's disease. J Alzheimers Dis 44: 729-744.
- Zhao WQ, Feng C, Alkon DL (2003) Impairment of phosphatase 2a contributes to the prolonged MAP kinase phosphorylation in Alzheimer’s disease fibroblasts. Neurobiol Dis 14: 458-469.
- Tramutola A, Triplett JC, Domenico FD, Niedowicz DM, Murphy MP, et al. (2015) Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): Analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133: 739-749.
- Kettunen P, Larsson S, Holmgren S, Olsson S, Minthon L, et al. (2015) Genetic variants of GSK3B are associated with biomarkers for Alzheimer's disease and cognitive function. J Alzheimers Dis 44: 1313-1322.
- Ibáñez-Salazar A, Bañuelos-Hernández B, RodrÃguez-Leyva I, Chi-Ahumada E, Monreal-Escalante E, et al. (2017) Oxidative stress modifies the levels and phosphorylation state of tau protein in human fibroblasts. Front Neurosci 11: 495-504.
- Fan S, Zhang B, Luan P, Gu B, Wan Q, et al. (2015) PI3K/AKT/mTOR/p70S6K pathway is involved inA25-35-induced autophagy. Biomed Res Int 2015: 161020-161029.
- Talboom JS, Velazquez R, Oddo S (2015) The mammalian target of rapamycin at the crossroad between cognitive aging and Alzheimer's disease. NPJ Aging Mech Dis 1: 15008-15015.
- Liang H, Nie J, Van Skike CE, Valentine JM, Orr ME (2019) Mammalian target of rapamycin at the crossroad between Alzheimer's disease and diabetes. Adv Exp Med Biol 1128: 185-225.
- Jang S-S, Chung HJ (2016) Emerging link between Alzheimer’s disease and homeostatic synaptic plasticity. Neural Plast 2016: 7969272-7969291.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, et al. (2011) The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 263-269.
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, e al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 270-279.
- Renault TT, Manon S (2011) Bax: Addressed to kill. Biochimie 93: 1379-1391.
- Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802: 396-405.
- Götz J, Eckert A, Matamales M, Matamales M, Ittner LM, et al. (2011) Modes of Aβ toxicity in Alzheimer's disease. Cell Mol Life Sci 68: 3359-3375.
- Liang Z, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2007) Down-regulation of cAMP dependent protein kinase by over-activated calpain in Alzheimer disease brain. J Neurochem 103: 2462-2470.
- Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, et al. (2001) Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol 11: 144-158.
- Kwok JB, Loy CT, Hamilton G, Lau E, Hallupp M, et al. (2008) Glycogen synthase kinase-3beta and tau genes interact in Alzheimer’s disease. Ann Neurol 64: 446-454.
- Arif M, Wei J, Zhang Q, Liu F, Basurto-Islas G, et al. (2014) Cytoplasmic retention of protein phosphatase 2A inhibitor 2 (I2PP2A) induces Alzheimer-like abnormal hyperphosphorylation of tau. J Biol Chem 289: 27677-27691.
- Connolly GP (1998) Fibroblast models of neurological disorders: Fluorescence measurement studies. Trends Pharmacol Sci 19: 171-177.
- Bodles AM, Barger SW (2005) Secreted beta-amyloid precursor protein activates microglia via JNK and p38-MAPK. Neurobiol Aging 26: 9-16.
- Munoz L, Ammit AJ (2010) Targeting p38 MAPK pathway for the treatment of Alzheimer's disease. Neuropharmacol 58: 561-568.
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22: 1942-1950.
- Du Y, Du Y, Zhang Y, Huang Z, Fu M, et al. (2019) MKP-1 reduces Aβ generation and alleviates cognitive impairments in Alzheimer’s disease models. Sig Transduct Target Ther 4: 58.
- Zhou XW, Tanila H, Pei JJ (2008) Parallel increase in p70 kinase activation and tau phosphorylation (S262) with Aβ overproduction. FEBS Lett 582: 159-164.
- Baik TK, Kim YJ, Kang SM, Song DY, Min SS, et al. (2016) Blocking the phosphatidylinositol 3-kinase pathway inhibits neuregulin-1-mediated rescue of neurotoxicity induced by Aβ1-42. J Pharm Pharmacol 68: 1021-1029.
- Milosch N, Tanriöver G, Kundu A, Rami A, François JC, et al. (2014) Holo-APP and G-protein-mediated signaling are required for sAPPα-induced activation of the Akt survival pathway. Cell Death Dis 28: e1391.
- Shi C, Zheng DD, Fang L, Wu F, Kwong WH, et al. (2012) Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim Biophys Acta 1820: 453-460.
- Novosadova EV, Grivennikov IA (2014) Induced pluripotent stem cells: From derivation to application in biochemical and biomedical research. Biochemistry (Mosc) 79: 1425-1441.
- Mueed Z, Tandon P, Maurya SK, Deval R, Kamal MA, et al. (2019) Tau and mTOR: The hotspots for multifarious diseases in Alzheimer’s development. Front Neurosci 12: 1017-1031.
- Wren MC, Zhao J, Liu CC, Murray ME, Atagi Y, et al. (2015) Frontotemporal dementia-associated N279K tau mutant disrupts subcellular vesicle trafficking and induces cellular stress in iPSC-derived neural stem cells. Mol Neurodegener 10: 46-59.
Citation: Zoia CP, Compagnoni P, Bazzini C, Ulisse A, Conti E, et al. (2020) Can Signaling Molecules in Fibroblasts Detect Prodromal Alzheimer's Disease? J Alzheimers Dis Parkinsonism 10:490. DOI: 10.4172/2161-0460.1000490
Copyright: © 2020 Zoia CP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2696
- [From(publication date): 0-2020 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1961
- PDF downloads: 735