Research Article Open Access
Can Non-invasive Ventilation Settings Predicts Functional and Survival Outcome in ALS Patients?
Braga ACM1*, Pinto S1 and Pinto A1,21Translational and Clinical Physiology Unit, Molecular Medicine Institute, Faculty of Medicine, University of Lisbon, Lisbon, Portugal
2Department of Physical Medicine and Rehabilitation, Hospital Santa Maria-CHLN, Lisbon, Portugal
- Corresponding Author:
- Anna Caroline Braga
Translational and Clinical Physiology Unit
Institute of Molecular Medicine
Faculty of Medicine, University of Lisbon
1649-028, Lisbon, Portugal
Tel: +351-217805000
E-mail: carolineaero@hotmail.com
Received Date: April 07, 2017; Accepted Date: April 27, 2017; Published Date: April 28, 2017
Citation: Braga ACM, Pinto S, Pinto A (2017) Can Non-invasive Ventilation Settings Predicts Functional and Survival Outcome in ALS Patients?. J Community Med Health Educ 7:521. doi:10.4172/2161-0711.1000521
Copyright: © Braga ACM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Introduction: The lack of more specific tools, with low costs, that may be associated with the ALSFRS-R (Amyotrophic Lateral Sclerosis Functional Scale-Revised) score to assist in analyzing the prognosis, is a constraint factor in the follow-up of ALS ventilated patients.
Objective: we analyzed the potential predictors of ALSFRS-R functional decline related to Non-Invasive ventilation (NIV) settings, Nocturnal Pulse Oximetry (NPO), and Pulmonary Function Test (PFT).
Methods: Prospective, comparative trial of 60 consecutive ALS patients, compliant to NIV, during 5 years of follow-up. Subjects were assigned to Group 1 (not-survivors) or Group 2 (survivors) at end of study. Data from ALSFRS-R, NPO, PFT and NIV settings were collected once each three months.
Results: No clinical or laboratory differences were observed between groups for any variable at admission. Disease duration from onset as well as Total use of NIV presented non-significant differences at end of study. However, these 2 variables were correlated positively with Expiratory Positive Airways Pressure (EPAP), Inspiratory Positive Airways Pressure (IPAP) and backup breath rate (all parameters of NIV), maximal inspiratory pressure (MIP-PFT), and SpO2mean (NPO). Multivariate Cox regression analysis showed that data from NIV settings and PFT were predictors of functional decline.
Conclusions: For the first time, determinants of functional decline are significantly related to NIV equipment settings as well as to compliance data.
Keywords
Amyotrophic lateral sclerosis; Functional decline; Noninvasive ventilation; Prognosis; Survival
Abbreviations
ALS: Amyotrophic Lateral Sclerosis; NIV: Noninvasive Ventilation; Bipap: Bilevel Positive Airway Pressure; PEEP: Positive End Expiratory Pressure; PFT: Pulmonary Function Test; % Spontaneous Cycles: Percentage of Spontaneous Cycles Made by the Patient; I:E Ratio: Inspiration: Expiration Ratio; NHU/D: Number of Hours of Non-invasive Ventilation Use per Day; BRmean: Breath Ratemean; IPAP: Inspiratory Positive Airways Pressure; EPAP: Expiratory Positive Airways Pressure; IS: Inspiratory Sensitive; ES: Expiratory Sensitive; RT: Rise Time; BRbackup: Breath Rate back up; SpO2mean: Saturation of Oxygenmean; HR: Heart Rate; Time SatO2<90%: Time of Saturation of Oxygen less than 90%; FVC: Forced Vital Capacity; MIP: Maximal Inspiratory Pressure; MEP: Maximal Expiratory Pressure; P.01: Mouth Occlusion Pressure at 100 milliseconds of MIP; PaO2: Partial pressure of Oxygen; PaCO2: Partial pressure Carbon Dioxide; SatO2: Oxygen Saturation in analysis blood gas
Introduction
Respiratory insufficiency (RI) can occur at different clinical stages of amyotrophic lateral sclerosis (ALS), and is the most frequent cause of death [1]. According to current clinical guidelines [2,3] and a recent Cochrane systematic review [4], non-invasive ventilation (NIV) is the treatment of choice on the management of RI in ALS. There is evidence that NIV intervention plays a critical role in prolonging life [5], as well as its adherence, which is frequently described by the number of hours of use by day (nhu/d). Therefore, the disease management implies the need to obtain full adherence to NIV. Others variables beyond number of hours/day, namely the ventilation settings of NIV equipment, need be evaluated about their potential role on functional and survival outcomes, and it has never been published. In addition, recent studies have suggested other causes of death in ALS [6-11], which reinforces the need to clarify whether respiratory disturbances and discomfort with NIV are present near the time of death, and whether they could possibly be amenable to corrections. We aimed to investigate the prognostic factors for functional decline among ALS patients under NIV, considering all variables recorded by the equipment software [12] from initial adaptation until to death or end of study. We hypothesized which differences between patients alive or dead in the same time frame would contribute to indicate the relevant prognostic factors for ALS ventilated patients.
Methods and Materials
Our study followed an exploratory, observational, and prospective design. We included 60 ALS subjects regularly followed in our ALS clinic. The time of follow-up was 5 years. The sample was diagnosed with definitive or probable disease on the revised El-Escorial criteria with age ranging from 18 to 75 years. It was divided in two groups: G1 (n=29) subjects who died over the follow-up period; and G2 (n=31) subjects who were alive at end of the study.
Functional assessment
All patients were evaluated in each 3 months with the revised ALS Functional Rating Scale (ALSFRS-R) [13]. This tool rates the ability to perform activities of daily living from 0 (total inability) to 48 points (no limitation) and incorporates respiratory items (dyspnea, orthopnea, respiratory insufficiency).
Non-Invasive Ventilation criteria for initiation: All patients were ventilated based on percutaneous nocturnal pulsed oximetry [14,15], Pulmonary function test and abnormal phrenic nerve response as well as the presence of respiratory symptoms (in according with respiratory sub-score of the Amyotrophic Lateral Sclerosis Functional Scale- Revised [13] [ALSFRS-R]<12), and blood gases daytime.
Nocturnal pulse oximetry (NPO) was measured continuously during sleep, by fingertip infra-red pulse oximeter. Mean oxygen saturation (SpO2mean) overnight, Time of SpO2<90% (percentage of time which SpO2 recording was below 90%). Nocturnal mean oxygen saturation assessed the need for start of nocturnal NIV. A compliance minimum of 6 h of nocturnal pulse oximetry recording was accepted in this study. Regarding the Pulmonary function test (PFT), it was performed using standard equipment according to the American Thoracic Society [16] (ATS) recommendations. Predicted values of conventional pulmonary function parameters were calculated by normalizing to the reference values proposed by the European Community for Steel and Coal [17]. We used Forced Vital Capacity (FVC) percentage of the predicted value (<75%FVC), Maximum Inspiratory pressure (MIP), Maximum Expiratory Pressure (MEP), and inspiratory pressure 100 milliseconds into an occluded inspiratory effort (P0.1). All these examinations are part of our usual routine care. Subjects with gastrostomy, cognitive impairment, or other medical condition, as heart or lung disorders, oncology diseases or previous history of stroke, were excluded. Regular follow-up included ALSFRSR score, PFT and NPO each three months.
All ALS subjects were ventilated with a Bipap Goodknight 425-ST bi-level device (Tyco™ Healthcare Group LP, California, USA) at hospital setting, in our unit by a Rehabilitation Physician. ALSFRS-R scores and PFT were collected by a physician blinded to study. It was considered an effective ventilation whether patient presented fingertip pulse oximetry>96%, and heart rate between 60-80 bpm under NIV during the medical consultation, with further analyses of satisfactory parameters of nocturnal percutaneous pulse oximetry as aforementioned. The compliance was evaluated as described elsewhere (number of hours of use/day [nhu/d]>4 h, percentage of Spontaneous Cycles [%SC] <60%, and Breath Rate [BR]<15 bpm) [18,19]; and we recorded data of compliance and each ventilation settings changes throughout NIV use [20]. All samples were fully compliant to NIV. This study was approved by our ethical committee, and participants signed informed consent.
Statistical analysis
Kolmogorov-Smirnov test was used to analyzing the normality of sample. It was used means plus/minus standard deviation for continuous variables. Categorical variables (gender, onset and group) were transformed from dummy variables to metric variables to be submitted to Cox-regression Models.
We used Cox regression models to identify predictors of functional decay (monthly). The independent variables were: NIV settings: Inspiratory Positive Airways Pressure (IPAP), Expiratory Positive Airways Pressure (EPAP), percentage of Spontaneous Cycles (%SC), Breath Ratemean (BRmean), Inspiration: Expiration ratio (I:E), Inspiratory sensitivity (IS), Expiratory Sensitivity (ES), Rise time (rT), Breath Rateback-up (BR back-up), Number of hours usage/day (nhus/ day); NPO measurements: Heart Rate (HR), Time of Oxygen Saturation lower than 90%(SatO2<90%), %Oxygen Saturationmean (%SpO2mean); PFT: Maximum Inspiratory Pressure (MIP), Maximum Expiratory Pressure (MEP), Pressure of occlusion on first second (P. 01), Forced Vital Capacity [%FVC predicted]; %Oxygen Saturation (SatO2); Daytime Blood Gas Analyses: Partial pressure of CO2 (PaCO2), and Partial pressure of Oxygen (PaO2).
Partial correlations were tested between all independents variables and Total Disease duration (from symptoms onset to death or end of study) and Total use NIV (from adaptation until death/end of study). Kaplan Meier curve estimate was performed to analysis the survival regarding the Total NIV use. The sample size was calculated with G*Power, version 3.1.19. (Effect size d=0.8). The statistical significance level was p ≤ 0.05.
Results
Sixty subjects fully compliant to NIV were included in this study (43 males and 17 females divided in two groups: G1 (n=29; non-survivors); and G2 (n=31; survivors). Both groups had a majority of male subjects as well as spinal onset, with affected limbs equally distributed (Upper/ Lower). Clinical characteristics at NIV adaptation had non-significant difference between groups (Table 1, Demographic and clinical characteristics of groups at NIV adaptation†).
| Variables | G1 (n=29) | G2 ( n=31) |
|---|---|---|
| Male/female ratio | 24/5 | 19/12 |
| Age of Onset | 63.4(± 13.2) | 67.2(± 10.7) |
| Type of Onset(S/B)ratio | 22/7 | 23/8 |
| Limbs(Upper/Lower)ratio | 15/14 | 16/15 |
| Evolution up NIV (days) | 764.5(± 599.6) | 564.7(± 316.0) |
| ALSFRS-R (mean ± S.D) | ||
| Total | 31.86(± 6.09) | 33.52(± 3.69) |
| Spinalscore | 18.04(± 6.97) | 20.07(± 3.76) |
| Bulbarscore | 11.00(± 2.96) | 11.138(± 2.59) |
| Respiratoryscore | 10.72(± 1.75) | 10.71(± 1.73) |
| Respiratory Function Test (mean ± S.D) | ||
| Forced vital Capacity (FVC), %predicted | 82.7(± 19.58) | 92.7(± 19.8) |
| Maximum Inspiratory Pressure | 55.3 (± 24.0) | 53.15(± 23.4) |
| Maximum Expiratory Pressure | 68.8(± 26.2) | 72.8(± 19.93) |
| Mouth Occlusion Pressure | 91.9(± 31.3) | 89.0(± 32.8) |
| Nocturnal Pulse Oximetry (mean ± S.D) | ||
| %SpO2 | 93.64(± 2.7) | 93.75(± 1.24) |
| Time Sat<90%(%) | 5.86(± 15.03) | 3.15(± 3.53) |
| Heart rate | 73.34(± 14.08) | 68.10(± 11.05) |
Table 1: Demographic and clinic characteristics of groups at NIV adaptation with non-statistical significance.
At end of study significant differences were found among groups regarding functional score (ALSFRS spinal, bulbar and total scores) and NPO data (Heart rate and Time SatO2<90%) as expected. However, we also found significant differences on data from Bipap ventilation settings which are summarized at Table 2: Clinical characteristics at end of study (t-test)).
| Functional Scores and Slope | Mean ± S.D value (G1) | Mean ± S.D value (G2) | P |
|---|---|---|---|
| ALSFRS(Bulbar) | 6.04(± 4.44) | 9.08 (± 4.21) | 0.016* |
| ALSFRS(Spinal) | 7.13 (±5.86) | 13.92 (±8.15) | 0.006* |
| ALSFRS(respiratory) | 7.128(±2.81) | 7.32(±1.86) | 0.762 |
| ALSFRS(Total) | 11.54 (± 7.84) | 20.28(± 9.11) | 0.001* |
| ALSFRS bulbar slope/month | 0.245(± 0.28) | 0.084(± 0.19) | 0.025* |
| Nocturnal Pulse Oximetry | |||
| %Time Sat O2<90% | 10.30(± 20.75) | 1.20(± 1.96) | 0.034* |
| Heart Rate | 77.53(± 16.39) | 66.39(± 9.21) | 0.005* |
| Bipap Ventilation Settings | |||
| Breath Ratemean | 13.97(± 1.66) | 12.88(± 1.42) | 0.011* |
| Breath Ratebackup | 12.76(± 1.39) | 11.92(± 1.40) | 0.028* |
| IPAP | 18.46(± 2.59) | 17.76(± 3.42) | 0.40 |
| EPAP | 4.7 (± 0.48) | 4.4(± 0.68) | 0.07 |
| IS | 1.3(± 0.8) | 1.3(0.8) | 0.73 |
| ES | 2.0(± 1.7) | 1.7(± 1.4) | 0.76 |
| %Spontaneous Cycles | 30.0(± 23.2) | 28.6(± 20.1) | 0.9 |
| I:E ratio | 0.4(± 0.08) | 0.05(± 0.05) | 0.7 |
| Rise Time | 1.4 (± 0.7) | 1.3(± 0.7) | 0.5 |
| Clinical Characteristics | |||
| Disease Duration from Onset(days) | 1482.7(± 895.1) | 1340.3(± 518.6) | 0.46 |
| Total Use NIV (days) | 769.9(± 554.9) | 698.3(± 501.0) | 0.60 |
Table 2: Clinical characteristics at end of study (t-test).
Disease duration from symptoms onset and Total use NIV were slightly higher in G1, but overall with non-significant results. Both groups started the NIV use at approximately 50% of disease duration. Statistical significant correlations were found between the following variables: Disease Duration (from symptoms onset) and Total Use of NIV (days) with ventilation settings (IPAP, EPAP, BRmean and BRbackup), and NPO (SpO2mean) and PFT (MIP) (Table 3). The variable of NIV with more significant result that influenced both Disease duration (R=0.293, p=0.041) and Total use NIV (R=0.54, p<0.001), was the IPAP (Table 3: Partial correlation analyses between NIV settings, PFT, and NPO data with Disease duration and Total use of NIV).
| Partial Correlations | |||
|---|---|---|---|
| Variables | Pearson Correlations (R) |
Disease Duration | Total Use of NIV |
| NIVsettings | |||
| IPAP | R Sig.(2-tailed) |
0.293 0.041* |
0.540 <0.001** |
| EPAP | R Sig.(2-tailed) |
0.236 0.10 |
0.328 0.021* |
| BRmean | R Sig.(2-tailed) |
0.447 0.001** |
0.269 0.62 |
| BRbackup | R Sig.(2-tailed) |
0.297 0.038* |
0.341 0.016* |
| PFT | |||
| MIP | R Sig.(2-tailed) |
0.371 0.020* |
0.231 0.15 |
| NPO | |||
| SpO2mean | R Sig.(2-tailed) |
0.326 0.017* |
0.250 0.69 |
**Correlation is significant at the 0.01 level(2 tailed)
Table 3: Partial correlation analyses between NIV settings, PFT, and NPO data with Disease duration and Total use of NIV.
Functional slope per month
We carried out a Multiple Cox regression adjusted by type of disease onset to identify predictors of ALSFRS-R Total slope. The variables with relevant explanatory power on ALSFRS-R Total slope among the NIV parameters data were: percentage of spontaneous cycles (%SC) (β=0.45; p=0.006) inspiratory and expiratory ratio [I:E] (β=21.3; p=0.02), rise time (RT) (β=8.58; p=0.03), inspiratory sensitivity (IS) (β=-4.62; p=0.002), expiratory sensitivity (ES) (β=1.36; p=0.01), and IPAP (β=-1.15; p=0.05), with a significant model: -2-Log LL=57.5; χ2=60.92; p<0.0001; and effect size f2: 0.56 (Table 4: Predictors of ALSFRS-R total slope among NIV settings, NPO, PFT, and clinical characteristics).
| Variables | B | SE | Wald | Sig |
|---|---|---|---|---|
| NIV settings | ||||
| %Spontaneous Cycles | 0.457 | 0.165 | 7.633 | 0.006* |
| I:E ratio | 21.360 | 9.383 | 5.182 | 0.02* |
| Number of HoursUse/day | 0.319 | 0.222 | 2.073 | 0.15 |
| BR mean | -2.177 | 1.357 | 2.572 | 0.10 |
| IPAP | -1.153 | 0.604 | 3.649 | 0.05* |
| EPAP | -1.147 | 1.532 | 0.560 | 0.45 |
| IS | -4.624 | 1.475 | 9.826 | 0.002* |
| ES | 1.369 | 0.582 | 5.536 | 0.01* |
| RT | 8.580 | 3.949 | 4.720 | 0.03* |
| BRbackup | 2.819 | 1.669 | 2.852 | 0.09 |
| Nocturnal Pulse Oximetry | ||||
| SpO2mean | 1.313 | 0.873 | 2.264 | 0.13 |
| Time SatO2<90% | -0.046 | 0.167 | 0.078 | 0.78 |
| HR | 0.047 | 0.079 | 0.352 | 0.55 |
| Pulmonary Function Test | ||||
| % FVC | -0.120 | 0.089 | 1.829 | 0.17 |
| MIP | -0.195 | 0.117 | 2.795 | 0.09 |
| MEP | 0.331 | 0.163 | 4.116 | 0.04* |
| P01 | -0.189 | 0.075 | 6.377 | 0.01* |
| PaO2 | 0.075 | 0.103 | 0.536 | 0.46 |
| PaCO2 | -0.239 | 0.208 | 1.328 | 0.24 |
| SatO2 | 3.178 | 1.391 | 5.222 | 0.02* |
| Clinical Characteristics | ||||
| Gender | -15.901 | 7.000 | 5.160 | 0.02* |
| Evolution until NIV | -0.015 | 0.005 | 8.042 | 0.005* |
| Age at onset | -0.116 | 0.133 | 0.762 | 0.38 |
| Disease duration | 0.012 | 0.004 | 9.011 | 0.003* |
Table 4: Predictors of ALSFRS-R total slope among NIV settings, NPO, PFT, and clinical characteristics.
• Multiple Cox regression model.
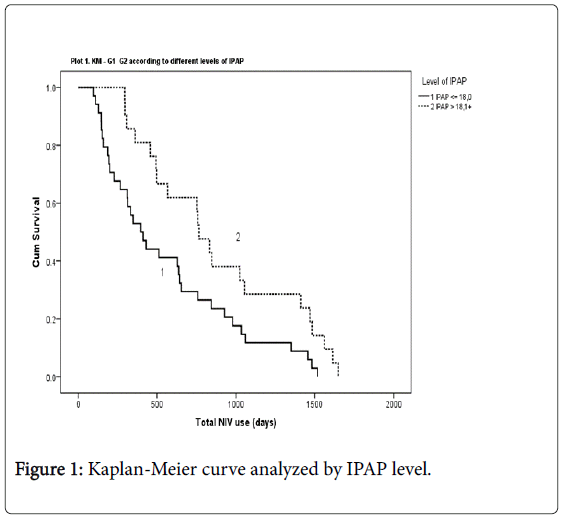
Kaplan-Meier curve estimate stratified by IPAP level
A one sample T-test showed an IPAP mean value of 18 cm H2O (18 ± 3), range (12.5-27), and p ≤ 0.001. We analyze the survival regarding the average of IPAP used by the sample. We dichotomize this variable (using the visual binning tool on SPSS), and we considered this variable dichotomized (IPAP level-18 cm H2O<IPAP>18 cm H2O) as an independent variable to investigate prognostic factors for survival. We performed a Kaplan-Meier curve estimate stratified by IPAP level that showed total NIV use in days were significantly higher on patients using higher levels of IPAP (L-R: χ2=6.28; p=0.012) (Figure 1: Kaplan- Meier curve analyzed by IPAP level).
Discussion
Several studies have highlighted the use of NIV in ALS patients, and have documented benefits on survival and quality of life [9,14,21]. Recent evidence shows the need to improve comfort and synchrony for a better NIV compliance. As NIV allows a large range of ventilation parameters and settings, it is mandatory to have accurate information about these issues to better understand the interplay between patient and ventilator [22]. Currently the usual criteria to follow-up these ventilated patients has only considered compliant those patients under NIV which have used the equipment more than 4 hours/day. There is a total lack of studies regarding the influence of NIV settings on functional decline.
This study shows for the first time the importance of analyzing the compliance data and ventilation settings as independent predictors of ALSFRS-R slope, and suggesting that the variables that affecting the respiratory comfort of patient are indeed a prerequisite to a better NIV compliance. And this can influence the functional decline.
The limited available literature on Patient-Ventilator Asynchrony in neuromuscular patients suggest that severe asynchronies may be associated with increased work of breathing and sleep disruption [23,24]. Improve sleep quality results in improved quality of life [25-27]. Patient-ventilator interaction is complex and multifactorial, as it is dependent upon respiratory system conditions, various disease states, neural function, and clinical input. When optimized, patient-ventilator interaction can provide patient comfort during positivepressure breaths [28]. Depending upon a number of factors, mechanical augmentation of Volume Tidal reduces the intensity and duration of inspiratory muscle contractions, thus lowering patient work of breath [29]. A study carried out by Vitacca et al. [30] reported discrepancies between Bilevel mechanical ventilator set to optimize work of breath and settings chosen by COPD patients to maximize comfort. The NIV settings identified as predictors in this study reinforce the importance of sleep quality and comfort of ventilated patients to reach a better compliance. The percentage of spontaneous cycles activated by patient influences on the respiratory muscle work, and this can lead patients to performing unnecessary inspiratory effort. In general longer inspiratory times improve oxygenation by increasing the mean airway pressure (longer period of high pressure increases mean airway pressure over the entire respiratory cycle), allows redistribution of gas from more compliant alveoli to less compliant alveoli. But by other hand can increase risk of gas trapping, intrinsic PEEP and barotrauma by reducing expiratory time and usually are less well tolerated by the patient. It can also decrease peak pressure by decreasing inspiratory flow. Regarding the role of the Rise time (RT) as ALSFRS-R slope predictor, it determines the speed of rise of flow (volume control mode) or pressure (pressure control and pressure regulated volume control modes). Very short rise times may be more uncomfortable for the patient, and long rise times may result in a lower tidal volume being delivered (pressure control mode) or higher pressure being required (volume control and pressure regulated volume control modes). The last NIV parameter identified were the trigger sensitivity (IS; ES). These determine how easy it is for the patient to trigger the ventilator to deliver a breath. Triggering may be flow-triggered or pressure triggered. Flow triggering is generally more sensitive. The smaller the flow or the smaller the negative pressure the more sensitive the trigger. In general increased sensitivity is preferable in order to improve patient-ventilator synchrony, but excessively high sensitivity may result in false or auto- triggering. The study performed by Gonzales [31] described the adjustment of cycling criteria and rise time could potentially impact breathing frequency, work of breathing, trigger timing, and patient-ventilator synchrony, with potential consequences for the respiratory comfort and compliance of patient. ALS patients usually presenting respiratory symptoms associated to muscle weakness which certainly carrying out to hypoxic events, hence a full adherence of NIV can help to minimize this situation. ALS natural history is closely related to hypoxia, and motor neurons are particularly vulnerable to hypoxic conditions because of their high oxygen consumption and poor antioxidant enzymatic defenses [32].
Non-invasive ventilation unfortunately will not prevent a fatal event at final stages of disease as death by respiratory failure [33]. However, respect the limits of tolerance of patients as well as validate their complaints, recognize symptoms of discomfort related to mask-such as face/nose pain, nasal congestion, aerophagia and sleep disruption can influence a proper compliance [34]. The ventilation settings and its changes must help to provide improvements on respiratory symptoms as well as on daytime arterial blood gas levels and nocturnal oximetry measurements. Currently, the use of capnography measurements showed to be an efficient tool both for assessing nocturnal hypoventilation and compliance to NIV treatment of ALS patients [35]. Although important, in this present study we did not evaluate it mainly due to cost. To our knowledge, this is the first study carried out to analyze the behavior of NIV settings and its use on ALS patients. The main findings in this study were related to predictor role of NIV settings, and Nocturnal pulse oximetry data. Indeed, minor changes in nocturnal pulse oximetry (NPO) data (SatO2<90%; HR; and SpO2mean), became significantly different close to end of clinical evolution or end of study, and they were related to disease duration from onset. This suggests that a status of eupnea, which provides a heart rate and breath rate closer to resting pattern during the night, must be achieved as soon and longer as possible. In addition, oximetry studies in the follow-up can help to identify and correcting changes on abnormal pattern of sleep-even the minors.
We recognize the limitations impacted by the type of study, and emphasized that these results may have been influenced by sample size and ALS center biased. Therefore, more studies should be conducted on a fully randomized trial to validate these findings.
In the future, the need for an individualized approach based on closer clinical management should be part of treatment attitude for ALS ventilated patients. It is necessary to pay special attention to the respiratory comfort of ALS patients. Problems that can affect compliance deserve closer attention, and should be addressed timely. Compliance plays a very important role in clinical decision-making, and can make the difference on the survival in these patients, and it suggests that NIV can have a modifier role play on follow-up, whether and when a more rigorous management is achieved.
Acknowledgements
The authors are grateful to Linde Healthcare Co for technical support.
Financial Support
This work was funded by Fundação para a Ciência e Tecnologia- Portugal: SFRH/BD/78413/2011
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Authorship
Anna Braga and Anabela Pinto were responsible for the study design, follow-up of the patients (respiratory care) and supervised data collection, analysis and manuscript preparation; Susana Pinto was responsible for the clinical and blinded evaluation, the follow-up of patients, and helped with data collection and revised the manuscript; Anabela Pinto was MD responsible for the respiratory care (prescription, respiratory care and equipment’s), and collaborated by obtaining local ethics committee approval, patient informed consent, advising and helping in all phases of the project, including analysis and manuscript preparation.
Ethics
The study was approved by the Ethical Committee at Lisbon University, and performed in accordance with the ethical standards stated in the 2000 Helsinki Declaration. Written informed consent was obtained from all participants.
References
- Chiò A, Calvo A, Moglia C, Gamna F, Mattei A, et al. (2012) Non-invasive ventilation in amyotrophic lateral sclerosis: A 10 year population based study. J NeurolNeurosurg Psychiatry 83: 377-381.
- Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, et al. (2012) EFNS guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol 19: 360-375.
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, et al. (2009) Practice parameters update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review). Neurology 73: 1218-1226.
- Radunovic A, Annane D, Jewitt K, Mustfa N (2009) Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 4: CD004427.
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, et al. (2009) Practice parameters update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review): Report of the quality standards subcommittee of the American Academy of Neurology. Neurology 73: 1218-1226.
- Kurian KM, Forbes RB, Colville S, Swingler RJ (2009) Cause of death and clinical grading criteria in a cohort of amyotrophic lateral sclerosis cases undergoing autopsy from the Scottish motor neurone disease register. J NeurolNeurosurg Psychiatry 80: 84-87.
- Masami T (2002) The causes of death in patients with amyotrophic lateral sclerosis-Analysis in National Hospital Organization. Neurological Medicine 63: 170-174.
- Corcia P, Pradat P, Salachas F, Bruneteau G, ForestierNl, et al. (2008) Causes of death in a post-mortem series of ALS patients. Amyotrophic Lateral Sclerosis 9: 59-62.
- Spataro R, Lo Re M, Piccoli T, Piccoli F, La Bella V (2010) Causes and place of death in Italian patients with amyotrophic lateral sclerosis. ActaNeurolScand 122: 217-223.
- Sancho J, Servera E, Dıaz JL, Bañuls P, Marín J (2011) Home tracheotomy mechanical ventilation in patients with amyotrophic lateral sclerosis: Causes, complications and 1-year survival. Thorax 66: 948-952.
- Asai H, Hirano M, Udaka F, Shimada K, Oda M, et al. (2007) Sympathetic disturbances increase risk of sudden cardiac arrest in sporadic ALS. J NeurolSci 254: 78-83.
- Almeida JP, Pinto A, Pinto S, Ohana B, DeCarvalho M (2012) Economic cost of home-telemonitoring care for BiPAP-assisted ALS individuals. Amyotroph Lateral Scler 13: 533-537.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, et al. (1999) The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J NeurolSci 169: 13-21.
- Pinto A, de Carvalho M, Evangelista T, Lopes A, Sales-Luís L (2003) Nocturnal pulse oximetry: A new approach to establish the appropriate time for non-invasive ventilation in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord 4: 31-35.
- Andersen PMea (2012) EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol 19: 360-375.
- American Thoracic Society (1995) Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J RespirCrit Care Med 152: 1107-1136.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, et al. (1993) Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European Community for steel and coal. Official Statement of the European Respiratory Society. EurRespir J Suppl 16: 5-40.
- de Almeida J P, Pinto AC, Pereira J, Pinto S, de Carvalho M (2010) Implementation of a wireless device for real-time telemedical assistance of home-ventilated amyotrophic lateral sclerosis patients: A feasibility study. Telemed J E Health 16: 883-888.
- Pinto A, Almeida JP, Pinto S, Pereira J, Oliveira AG, et al. (2010) Home telemonitoring of non- invasive ventilation decreases healthcare utilization in a prospective controlled trial of patients with amyotrophic lateral sclerosis. J NeurolNeurosurg Psychiatry 81: 1238-1242.
- Vitacca M, Assoni G, Pizzocaro P, Guerra A, Marchina L, et al. (2006) A pilot study of nurse-led, home monitoring for patients with chronic respiratory failure and with mechanical ventilation assistance. J TelemedTelecare 12: 337-342.
- Piepers S, Van den Berg JP, Kalmijn S, van der Pol WL, Wokke JH, et al. (2006) Effect of non-invasive ventilation on survival, quality of life, respiratory function and cognition: A review of the literature. Amyotroph Lateral Scler 7: 195-200.
- Rabec C, Rodenstein D, Leger P, Rouault S, Perrin C, et al. (2011) Ventilator modes and settings during non-invasive ventilation: Effects on respiratory events and implications for their identification. Thorax 66: 170-178.
- Jolliet P, Tassaux D, Vignaux L (2009) Patient-ventilator interaction during non-invasive ventilation. In: Yearbook of intensive care and emergency medicine. Springer Verlag, Berlin, USA.
- Epstein SK (2011) How often does patient-ventilator asynchrony occur and what are the consequences? Respir Care 56: 25-38.
- Banks S, Dinges DF (2007) Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med 3: 519-528.
- Scharf SM, Maimon N, Simon-Tuval T, Bernhard-Scharf BJ, Reuveni H, et al. (2010) Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 6: 1-12.
- Crescimanno G, Canino M, Marrone O (2012) Asynchronies and sleep disruption in neuromuscular patients under home noninvasive ventilation. Respir Med 106: 1478-1485.
- Gentile MA (2011) Cycling of the mechanical ventilator breath. Respir Care 56: 52-60.
- Kallet RH, Diaz JV (2009) The physiologic effects of noninvasive ventilation. Respir Care 54: 102-115.
- Vitacca M, Nava S, Confalonieri M, Bianchi L, Porta R, et al. (2000) The appropriate setting of noninvasive pressure support ventilation in stable COPD patients. Chest 118: 1286-1293.
- Gonzales JF, Russian CJ, Gregg Marshall S, Collins KP (2013) Comparing the effects of rise time and inspiratory cycling criteria on 6 different mechanical ventilators. Respir Care 58: 465-473.
- Moreau C, Gosset P, Kluza J, Brunaud-Danel V, Lassalle P, et al. Deregulation of the hypoxia inducible factor-1α pathway in monocytes from sporadic amyotrophic lateral sclerosis patients. Neuroscience 172: 110-117.
- Gordon PH, Corcia P, Lacomblez L, Pochigaeva K, Abitbol JL, et al. (2009) Defining survival as an outcome measure in amyotrophic lateral sclerosis. Arch Neurol 66: 758-761.
- Berry RB, Chediak A, Brown LK, Finder J, Gozal D, et al. (2010) Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med 6: 491-509.
- Kim SM, Park KS, Nam H, Ahn SW, Kim S, et al. (2011) Capnography for assessing nocturnal hypoventilation and predicting compliance with subsequent noninvasive ventilation in patients with ALS. PLoS One 6: e17893.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 4010
- [From(publication date):
April-2017 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 3250
- PDF downloads : 760