Campesterol Reduces Tumor Development and Mammary Cellular Proliferation in N-Methyl-N-Nitrosourea- Induced Breast Cancer in Female Mice
Received: 28-Dec-2022 / Manuscript No. bcp-23-84906 / Editor assigned: 02-Jan-2023 / PreQC No. bcp-23-84906(PQ) / Reviewed: 17-Jan-2023 / QC No. bcp-23-84906 / Revised: 23-Jan-2023 / Manuscript No. bcp-23-84906(R) / Accepted Date: 23-Jan-2023 / Published Date: 30-Jan-2023 DOI: 10.4172/2168-9652.1000399 QI No. / bcp-23-84906
Abstract
Background: Breast cancer has remained a big challenge globally due to high numbers of individuals acquiring the disease, whereby it was estimated that in 2020, about 2.3 million women were diagnosed with the disease and 685,000 deaths occurred globally. Due to the high incidence and prevalence of breast cancer, different methods have been employed to illness. Some of the methods that are currently employed in the treatment of breast cancer include surgery, radiotherapy and chemotherapy. However, most of these methods have limitations for example surgery and radiotherapy are expensive and in addition to killing the cancer cells, they also damage the healthy cells.
Aim: The aim of the current study was to determine the antitumor effects of campesterol in N-methyl-Nnitrosourea induced breast cancer in female mice.
Methods: Four groups of female mice had mammary cancer induced and then provided with feeds containing different doses of campesterol. The mice were given feeds either containing 0% campesterol (control), 2% campesterol, 4% campesterol or 6% campesterol for 14 weeks.
Results: The results of the present study showed that the two doses of campesterol (4% and 6% campesterol) caused a significant decrease in tumor volume (p < 0.05) when compared to the control group. The results also indicated that the dose of 4% campesterol reduced the tumor incidence by 25% while that of 6% campesterol reduced the tumor incidence by 50% when compared to the control group. The results further indicated that the three doses of campesterol reduced cellular proliferation in mammary tissues when compared to the control group.
Conclusion: The results of the present study showed that campesterol could reduce tumor size, tumor incidence, number of tumors developed and mammary cellular proliferation in female mice.
Introduction
Many drugs and phytochemicals have been used for cancer treatment [1]. Depending on the kind of cancer, the anticancer agents have been developed for different targets during cancer treatment [1]. In some cases, the anticancer agents have been successful in cancer treatment while others have failed [2]. There are many studies that have been carried out about the antitumor effects of different substances. A study conducted by [3] showed that gensinoside Rg1 had the ability to prevent tumor incidence, the total number of tumors, tumor volume, and tumor burden in Sprague Dawley Rats induced with breast cancer. In this study, it was found out that gensinoside Rg1 reduced tumor incidence by 50% (4/8), and the average tumor size in gensinoside Rg1 treated rats was 10.31 mm3 compared to 20.85mm3 in untreated group. In another study conducted by [4], artonin reduced tumor size in the mice induced with breast cancer. In this study, ten female mice were induced with breast cancer and the average size of the breast tumors was 10.22 mm3 in artonin-treated mice compared to 18.09 mm3 in the untreated group. Another study that intended to establish the anticancer effect of salvianolic acid in rats, the results showed that the substance was able to reduce tumor incidence by 60% (6/10), and tumor size. The average tumor size in salvianolic acid-treated rats was 8.78 mm3 compared to 20.26 mm3 in the untreated group [5].
Furthermore, Annona muricata was found to reduce tumor size but it was not able to reduce tumor incidence in 7, 12-dimethylbenzaanthracene (DMBA)-induced breast cancer in female albino mice. The average tumor size in the Annona muricatatreated mice was 10.12 mm3 compared to 22.66 mm3 in untreated group [6]. In the study conducted by [7] about the chemo-preventive potential of ferulic acid in 7,12-dimethylbenzaanthracene-Induced mammary carcinogenesis in Sprague-Dawley rats, it was found out that ferulic acid reduced tumor size and tumor incidence. The substance reduced tumor incidence by 66% (8/12) and the average tumor size of ferulic acid-treated rats was 11.64 mm3 compared to 20.68 mm3 in the untreated group. In another study conducted by [8], it was found that rosmarinic acid protected Swiss albino mice from 7, 12-dimethylbenz (a) anthracene (DMBA) induced skin carcinogenesis. The results showed that the substance reduced the incidence of tumor development by 50% (5/10) while the average tumor volume in rosmarinic acidtreated rats was 10.60 mm3 compared to 20.38 mm3 in the untreated group. The substance paradol was found to reduce the incidence of 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesis by 70% (7/10) according to the study carried out by [9].The substance morin was found to provide protection against ultraviolet-b radiation-induced skin photocarcinogenesis in mice. The results of the study showed that the substance reduced the tumor incidence by 30% (4/12) and the average tumor volume of the morintreated mice was 11.24 mm3 compared to 20.98 mm3 in the untreated group [10].
Another study was carried out which showed that catechin reduced the incidence of tumor development in mice by 50% (3/6) and the average tumor volume was 10.06 mm3 in catechin-treated mice compared to 18.84 mm3 in the untreated group [11]. Likewise, a study was conducted a study which showed that Ginsenoside Rh2 reduced the incidence of colorectal cancer development in mice by 60% (6/10) [12]. In the study conducted by [13] about the effect of ellagic acid on breast cancer development, the results showed that the substance decreased the incidence of tumor development by 70% (7/10) and the average tumor volume was 11.86 mm3 in ellagic acid-treated rats compared to 22.88 mm3 in untreated rats. In the study about the antitumor effects of cinnamic acid and tanshinone in mice, [14] found out that both substances had a protective effect. Cinnamic acid reduced the incidence of tumor development by 50% (5/10) and the mean tumor volume was 11.34 mm3 in cinnamic acid-treated mice compared to 22.68 mm3 in the untreated group. On the other hand, tanshinone reduced the incidence of tumor development by 60% (6/10) and the average tumor volume was 10.48 mm3 compared to 22.68 mm3 in the untreated group.
Many diseases tend to cause disruption of the body especially at tissue level [15]. Cancer is among the disorders that that affect the normal integrity of the body tissues in the parts which are affected [16]. The anticancer agents tend to reverse the integrity of tissues from the diseased state to a normal during or after recovery [17]. Many studies have been conducted to investigate the effect of different substances on the histomorphology of cancer-affected mammary tissues. In the study conducted by [18], Orobanche crenata methanolic extract was found to improve the histomorphology of the mammary tissue of mice that was invaded by tumors from the diseased form characterized by reduced sizes of cells, cell shrinkage and destruction of the monolayer in the untreated group to the restoration of the normal cell size, cell integrity as well as reconstruction of the monolayer and reduction in cellular proliferation in the Orobanche crenata-treated group. In another study conducted by [19], the aqueous extract of Brewers‘rice was found to improve the histomorphology of mammary tissues in mice from the cancerous form characterized by interlacing bundles of spindle cells that developed from mammary ducts in the untreated group to a nearly normal histomorphology where the cells are in the normal cuboidal structure in the Brewers‘rice-treated group. Still [20] conducted a study in which the methanolic extract of Manilkara zapota leaf was found to be to reduce mammary cellular proliferation and reversed the histomorphology of cancer invaded mammary tissues in albino mice characterized by blende round epithelial cells and elongate spindle cells in the group that never received the extract to normal cuboidal cells of mammary tissues in the Manilkara zapota-treated group. Montanine, an alkaloid was found to improve the histological appearance and reduction in cellular proliferation of mammary tissues in mice invaded by mammary tumors from the cancer form characterized by lobes radiating from the epidermoid tissue in mice that were never treated with the substance to the normal appearance of lobules with normal cuboidal cells in the group treated with montanine [21]. Furthermore, the methanolic extract of Manilkara zapota fruit was found to cause an improvement of the histomorphology of mammary tissues in mice affected by mammary gland carcinoma in which the extract transformed the cancer form characterized by epidermoid formation in the untreated group to normal lobular structures with normal cuboidal cells in the Manilkara zapota-treated group, however, the substance was not found to have any effect on cellular proliferation [22]. In the study to determine the effect of Bidens pilosa on mammary cancer in mice, the aqueous extract of the plant was found not to have any significant effect on the histomorphology of mammary tissues affected by tumors in mice, according to the study conducted by [23]. The cancer form of mammary tissues was characterized by cysts formed by cuboidal epithelium and the epithelial cells were interspersed with spindle cells which gave the epithelial layer a variation in thickness. Another study was carried out by [24] in which the results showed that anthocyanins improved the histomorphology and reduced cellular proliferation of mammary tissues in mice invade by tumors from the cancer form that was characterized by varying degree of cyst formation, keratinization, small-sized cells and paucity of mitotic figures in untreated group to nearly normal ductile structure with cuboidal cells and fibroblasts in the extracellular matrix. Temozolomide and N-(2-hydroxyphenyl) acetamide (NA-2) were found to synergistically transform the histomorphology of mammary tissues invaded by tumors to normal one. The cancer form of mammary tissue was characterized by round and oval nodules, cysts and central necrosis to normal tubular structure, cuboidal cells and fibres in the extracellular matrix, however, no effect on the cellular proliferation was noted [25]. In addition, the cancer form of mammary tissue in mice characterized by hyperplastic nodules made up of a large bunch of dividing acinar cells was transformed by hydroalcoholic extract of Tabernaemontana divaricata to normal lobular structure and the integrity of the mammary tissue extracellular matrix was restored [26]. A study conducted by [27] showed that the aqueous extract of Actinidia eriantha Benth root did not transform the cancer form of mammary tissues in mice characterized by intraacinous and intraduct hyperplasia towards the normal mammary histomorphology and did not have any effect on cellular proliferation. Likewise, the ethyl acetate extract from Selaginella doederleinii Hieron did not significantly transform the cancer form of mammary tissue histomorphology in mice characterized by hyperplastic lesion composed of budding alveolar tissue as well as overgrowth of fine ducts towards the normal mammary tissues histomorphology [28].
Materials and methods
Study design
This was an experimental study that involved the induction of mammary cancer in female mice followed by administration of different doses of campesterol. The mice were obtained from Mbarara University of Science and Technology while N-methyl-N-nitrosourea and campesterol were obtained from Sigma-Aldrich Company, Shangai-China.
Study site
The animal experiment, taking measurements of tumor size dimensions and the histomorphology of mammary tissues was done at College of Veterinary Medicine, Animal Resources and Biosecurity (CoVAB), Makerere University.
Study animals
Sixteen (16) female mice were used in this study, and the animals were divided into four groups.
Feed preparation
The experimental diets were formulated using commercial campesterol (Sigma-Aldrich, Shangai-China). The formulation of experimental diets was done at Nuuma Animal feeds solution-Masaka (U). The experiment involved formulation of four experimental diets that included; one campesterol free diet, and the three that will contain varying quantities of campesterol which were 2%, 4% & 6% campesterol [29]. These concentrations were prepared by adding different calculated amounts of campesterol to the base diet to get a desired percentage. Campesterol free diet comprised of 25, 5, 6, 8, 11.4 and 7% protein, fat, fiber, total minerals, moisture and total vitamins respectively, while carbohydrates constituted the remainder of the diet. The diet containing 2% campesterol was formulated by adding 20g of campesterol to 980g of the base diet to make 1 kg of the feed. Therefore, the diet contained 2% campesterol, 25, 5, 6, 8, 11.4 and 7% protein, fat, fiber, total minerals, moisture and total vitamins respectively, while carbohydrates constituted the remainder of the diet. The diet containing 4% campesterol was formulated by adding 40g of campesterol to 960g of base diet. Therefore, the diet contained 4% campesterol, 25, 5, 6, 8, 11.4 and 7% protein, fat, fiber, total minerals, moisture and total vitamins respectively, while carbohydrates constituted the remainder of the diet. The diet containing 6% campesterol was formulated by adding 60g of campesterol to 940g of base diet. Therefore, the diet contained 6% campesterol, 25, 5, 6, 8, 11.4 and 7% protein, fat, fiber, total minerals, moisture and total vitamins respectively, while carbohydrates constituted the remainder of the diet (Table 1) .
| Group, n=4 | Treatment |
|---|---|
| 1 | No campesterol + cancer induction (control) |
| 2 | 2% campesterol + cancer induction |
| 3 | 4% campesterol + cancer induction |
| 4 | 6% campesterol + cancer induction |
Care of experimental mice
Each group was housed in a cage 0.6m x 0.5m x 0.6m high. The cages had wood shavings placed in them before the mice were introduced there so as to provide a soft ground for the mice. The cages were placed on the raised tables. The wood shavings would be removed after four days and replaced with fresh ones. The mice were protected from any external invaders by locking the laboratory in which the cages were placed as well as locking the cages. Each group was given the respective feed twice a day and adequate fresh water was available all the time.
Induction of mammary cancer and detection of cancer development
Mammary cancer was induced by a single intraperitoneal injection weekly for four weeks as described by [30]. The experimental mice were administered with N-Methyl-N-Nitrosourea (NMU) as a chemical carcinogen. The chemical was prepared into a solution a short time before it is administered and this was done by dissolving it in 4ml of 0.9% Sodium chloride solution. Then the solution was acidified with acetic acid to pH 4. The resulting solution was used within 20 minutes after its preparation and after use; another solution was prepared and used until all animals were covered. The mouse to be administered with the chemical solution was first anesthetized with a mixture of ketamine-HCl and xylazine (150 and 10 mg/kg, respectively) which would be given by intraperitoneal route. The animal would then be positioned on dorsal recumbence on the table. Then the mouse would be injected with 0.2ml of the solution along the ventral midline, half way between the third and the fourth pairs of mammary glands by intraperitoneal route. The development of breast tumors was detected by palpation of the mammary gland in the thoracic, abdominal and inguinal regions on the weekly basis beginning from the fourth week after the injection of the chemical solution. This was then confirmed by histological examination of the masses extracted from the mammary glands.
Animal sacrifice, sample collection and disposal of sacrificed mice
At the end of the experiment, the body weights of the mice were taken using a digital scale before sacrifice. The mice were then sacrificed by cervical dislocation which involved severing the spinal cord. The euthanized mice were placed on dorsal recumbence for tumor dimension measurements.
Determination of tumor size
This was done using Xenograft Tumor Volume Measurement method as described by [31]. The Xenograft Tumor Volume Measurement method involved the use of a Vernier calliper. The dimensions of the tumors were measured by use of a digital Vernier calliper and this was by putting it sufficiently all around the borders of the tumor and later to identify the longest and the shortest sides. The longest side was taken to be the length while the shortest side was taken to be the width. The height of the tumor was considered to be equal to the width. The volume of the tumor was then calculated using the formula;
V= Lx W2/2,
Where,
V= Volume of the tumor
L= length of the tumor
W= Width of the tumor
Determination of histomorphology of mammary tissues
This was done with the procedure as described by [32]. This procedure involved the removal of mammary glands and later placed on the specific glass measuring 70mm × 45mm × 2mm. This was followed by use of 10% phosphate buffered formalin to fix and dehydrate the tissues overnight. It was later followed by use of toluene to clear of the fat, rehydration and then staining using haematoxylin & eosin. The staining was followed by dehydration of whole mounts using alcohol and use of xylene to clear and then finally use of methyl salicylate to bag the tissues. The whole mounts were then sliced to obtain the microscopic lesions that were embedded in paraffin for examination of histological appearance. The histological examination was done using a Plus microscope and the capture of images was done using colour digital camera.
Data analysis
Data collected was sorted and statistically analyzed using graph pad prism software 9.00, 121. The data was analyzed by calculation of the means within the groups and these were written with standard deviation. Statistical significance analysis across the groups was done using one-way ANOVA followed by Turkey’s Post hoc test at a confidence interval of 95%. The data for tumor incidence was analyzed using Microsoft excel, 2010.
Ethical considerations
The research was conducted with respect to the principles of refinement, reduction and replacement. During the period of research, all principles of the Uganda National Council for Science and Technology that intended to promote ethics in research were followed.
Adherence to COVID 19 regulations
It was my responsibility to get vaccinated against COVID 19 before the start of data collection in order to minimize the spread of the disease. Washing of hands all the time and use of alcohol-based sanitizers was ensured, before, during after conduction of a session in the research facility. The face mask was used to cover the nose and mouth while in the research facility. Other personal protective equipment such as gloves and clinical coats were used in addition to wearing closed shoes. It was ensured that all facilities where the research was conducted were sanitized. A social distance of at least two meters was be ensured in case the number went beyond one person in the research facility. All the above standard operating procedures applied to all the personnel who were involved in the study.
Results
Tumor volumes of mice administered with different doses of campesterol
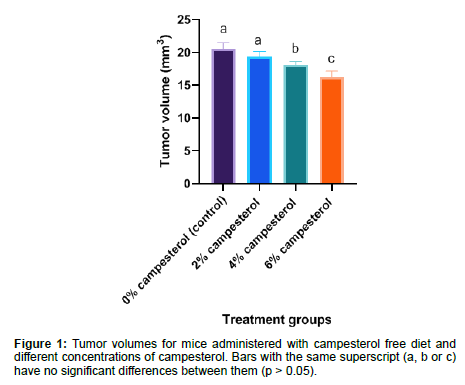
The tumor volumes of mice were (Mean ± SD) 20.53 ± 0.94, 19.41 ± 0.77, 18.15 ± 0.50 and 16.22 ± 0.94 mm3 for groups treated with campesterol free diet, 2% campesterol, 4% campesterol and 6% campesterol respectively. The results in the current study showed that the two doses of campesterol (4% & 6% campesterol) caused a significant reduction (p < 0.05) on the tumor volumes of mice when compared to the control group as shown in (Figure 1).
Number of tumors developed per mouse for groups administered with different doses of campesterol
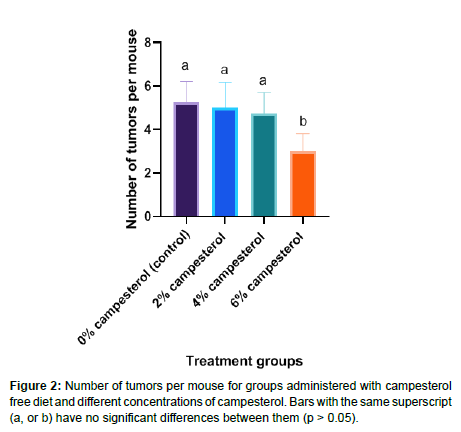
The number of tumors developed per mouse were (Mean ± SD) 5.25 ± 0.96, 5.0 ± 1.15, 4.75 ± 0.96 and 3 ± 0.82 for groups treated with campesterol free diet, 2% campesterol, 4% campesterol and 6% campesterol respectively. The results of the current study indicated that the dose of 6% campesterol caused a significant (p < 0.05) decrease on the number of tumors developed per mouse as shown in (Figure 2).
Tumor incidences of mice administered with different doses of campesterol
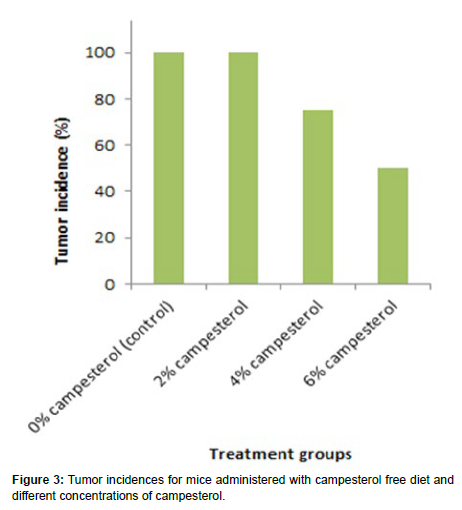
The tumor incidences of mice were 100% (4/4), 100% (4/4), 75% (3/4) and 50% (2/4) for the mice treated with 0% campesterol, 2% campesterol, 4% campesterol and 6% campesterol respectively. The results from the current study indicated that the dose of 4% campesterol reduced the tumor incidence by 25% while the dose of 6% campesterol reduced the tumor incidence by 50% when compared to the control group as shown in (Figure 3).
Mammary histomorphology of mice administered with different doses of campesterol
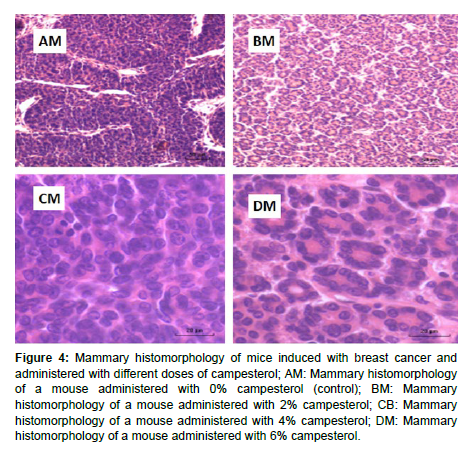
The three doses of campesterol showed a reduction in the proliferation of mammary cells and the reduction was more pronounced with increasing concentration of campesterol as shown in (Figures 4).
Figure 4: Mammary histomorphology of mice induced with breast cancer and administered with different doses of campesterol; AM: Mammary histomorphology of a mouse administered with 0% campesterol (control); BM: Mammary histomorphology of a mouse administered with 2% campesterol; CB: Mammary histomorphology of a mouse administered with 4% campesterol; DM: Mammary histomorphology of a mouse administered with 6% campesterol.
Discussion
Tumor volumes of mice administered with different doses of campesterol
Among the crucial aspects in the staging of breast cancer is the size of the tumor [33]. This aspect of the size of the tumor matters a lot because it determines the chances of spread of the cancer cells given the fact that the tumor size is directly proportional to the cancer cell numbers, and the cell numbers are directly proportional to the chances of metastasis [34]. For the tumor cells to survive and grow it needs stimulation from substances from its microenvironment and these may include cytokines which are among the growth factors [35].
The tumor volumes of mice were (Mean ± SD) 20.53 ± 0.94, 19.41 ± 0.77, 18.15 ± 0.50 and 16.22 ± 0.94 mm3 for groups treated with campesterol free diet, 2% campesterol, 4% campesterol and 6% campesterol respectively. The results in the current study showed that the two doses of campesterol (4% & 6% campesterol) caused a significant reduction (p < 0.05) on the tumor volumes of mice when compared to the control group. These were in agreement with the results obtained by [4, 5]. The results of the current study could be an indication that the concentrations of 4% campesterol & 6% campesterol had a negative effect on the tumor microenvironment [35].
Number of tumors developed per mouse for groups administered with different doses of campesterol
Tumorigenesis which is the transformation from the normal cells to cancerous ones is accompanied with some changes that involve different levels such as cytological level, level of the genes as well as the level of epigenetics, and it is also associated with the abnormality in the division of the parent cells [39]. The dysregulation of the genes responsible for the balance of cellular growth is the determining factor for uncontrollable cellular division. These genes include the protooncogenes that enhance cellular growth and division and the tumor suppressor genes that prevent cellular growth, or on the temporary basis arrest the division of the cell so that DNA can be repaired [40]. Therefore, the transformation of the normal to cancerous cells follows the mutation of these regulatory genes of cellular growth and division [41].
The number of tumors developed per mouse were (Mean ± SD) 5.25 ± 0.96, 5.0 ± 1.15, 4.75 ± 0.96 and 3 ± 0.82 for groups treated with campesterol free diet, 2% campesterol, 4% campesterol and 6% campesterol respectively. The results of the current study indicated that the dose of 6% campesterol caused a significant (p < 0.05) decrease on the number of tumors developed per mouse.
These were in agreement with the results obtained by [3]. The results of the current study could be an indication that the dose of 6% campesterol had a positive effect on the tumor suppressor genes or a negative effect on the proto-oncogenes [41].
Tumor incidences of mice administered with different doses of campesterol
Tumor incidences are determined by the rate of genetic mutations for oncogenesis [36]. On the other hand, some factors may have a contribution on the advancement of the tumor and these may include among others the internal defects of the cells of the immune system such as fatigue of the T cells and the ability to tolerate the binding strength of the antigens located on the tumor cells [37]. Reduction of tumor incidences can also be through; suppression of the tumor by cytokines of the immune system, enhancement of the tumor suppressor genes by stimulating them, and a negative effect on the angiogenesis-stimulating factors so as the prevent the tumors from spreading to distant areas [38].
The tumor incidences of mice were 100% (4/4), 100% (4/4), 75% (3/4) and 50% (2/4) for the mice treated with 0% campesterol, 2% campesterol, 4% campesterol and 6% campesterol respectively. The results from the current study indicated that the dose of 4% campesterol reduced the tumor incidence by 25% while the dose of 6% campesterol reduced the tumor incidence by 50% when compared to the control group.
These were in agreement with the results obtained by [9, 11, 13]. However, the results differed from those obtained by [6]. The results of the current study could be an indication that the concentrations of 4% campesterol and 6% campesterol had a positive effect on the immune system of the mice.
Mammary histomorphology of mice administered with different doses of campesterol
The mammary gland is histologically composed of the ductal - lobular system, stroma and the nipple [42]. The ductal-lobular system is made up of large milk channels called lactiferous ducts. These ducts have their opening at the nipple surface through the sinuses formed by lactiferous ducts. The lactiferous sinuses branch to form ductules that make their termination on to the acini. The acini are combined together to form clusters of lobules [43]. The ductal-lobular system is made up of cuboidal to columnar epithelial lining surrounded by the myoepithelial cells. The myoepithelial cells are variable in appearance but most of them have a flattened structure [44].
The results of the current study showed that three doses of campesterol reduced the proliferation of the tumor-invaded mammary tissues. These were in agreement with the results obtained by [18, 19, 21]. However, the results differed from those obtained by [27, 28]. The results of the current study could be an indication that the three concentrations of campesterol had a negative effect on the cytokines produced by the tumor cells [35].
Conclusion
From the results of the study, it could be concluded that campesterol significantly reduced tumor size, number of tumors developed and tumor incidence. In addition, campesterol reduced proliferation of mammary cells.
Acknowledgement
The authors send thanks to the management of Kampala International University for the outstanding support rendered during this study. Thanks also go to Mr. Kisekka Magidu, the laboratory technician in pathology, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University for the unending support.
Disclosure of Interest
The authors report no conflict of interest
References
- Amelio I, Melino G (2020) Context is everything: extrinsic signalling and gain-of-function p53 mutants. Cell Death Discovery6: 16.
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, et al. (2014) Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Prot of Natio Aca Science 111:283–90.
- Yan C, Wentao G, Kanimozhi GR, Defu Tian B (2020) Ginsenoside Rg1 Induces Apoptotic Cell Death in Triple-Negative Breast Cancer Cell Lines and Prevents Carcinogen-Induced Breast Tumorigenesis in Sprague Dawley Rats. Evid Comple & Alter Med 2: 34-46.
- Etti IC, Abdullah A, Kadir P (2017) Molecular mechanism of the anticancer effect of artonin E in MDA-MB 231 triple negative breast cancer cells. PLoS One 12: 1823-57.
- Deng YM, Yang F, Xu P (2015) Combined salvianolic acid B and ginsenoside Rg1 exerts cardioprotection against ischemia/reperfusion injury in rats. PLoS One 10: 234-245.
- Minari JB, Okeke U (2014) Chemopreventive effect of annona muricata on DMBA-induced cell proliferation in the breast tissues of female albino mice. Egy J Med Human Genet 15: 327.
- Baskaran N, Manoharan S, Balakrishnan S, Pugalendhi P (2010) Chemopreventive potential of ferulic acid in 7,12-dimethylbenzaanthracene-Induced mammary carcinogenesis in Sprague-Dawley rats. Eur J Pharmaco 63: 22.
- Sharmila R, Manoharan S (2012) Anti-tumor activity of rosmarinic acid in 7, 12-dimethylbenz (a) anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. Ind J of Physio Sciences 7: 344-356.
- Suresh S, Manoharan M, Vijayaanand P, Sugunadevi A (2020) Chemopreventive and antioxidant efficacy of (6)-paradol in 7, 12-dimethylbenz (a) anthracene induced hamster buccal pouch carcinogenesis. Pharmacological Reports 62: 1178–1185.
- Anjugam C, Sridevi N, Rajendra Prasad M, Balupillai A (2018) Morin prevents ultraviolet-b radiation-induced photocarcinogenesis through activating thrombospondin-1 in the mouse skin. Asian J Pharma Clin Res 11: 24-34.
- Farhan M, Khan M, Oves H (2016) Cancer therapy by catechin involves redox cycling of copper ions and generation of reactive oxygen species. Toxins 8: 37.
- Li B, Zhao J, Wang C (2011) Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of P53. Cancer Letters 301: 185.
- Ahire V, Kumar K, Mishra, Kulkarni P (2017) Ellagic acid enhances apoptotic sensitivity of breast cancer cells to c-radiation. Nutri & Cancer 69: 904-910.
- Liang F, Li A, Shi R, Liu J, Tang Y, et al. (2018) Anticancer effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in osteosarcoma MG-63 cells: nuclear matrix down regulation and cytoplasmic trafficking of nucleophosmin. Inte J Bioche & Cell 5: 567-574.
- Cowling VH, Cole MD (2017) E-cadherin repression contributes to c-Myc-induced epithelial cell transformation. Oncogene 26: 3582-3586.
- Clark GM (2015) Prognostic and predictive factors for breast cancer. Breast Cancer 2: 79-89.
- Ariazi EA, Ariazi JL, Cordera F, Jordan VC (2016) Estrogen receptors as therapeutic targets in breast cancer. Curr Medicinal Che 6: 181- 202.
- Marwa GA, Amal M, Bassem E (2020) Evaluation of cytotoxic and anticancer effect of Orobanche crenata methanolic extract on cancer cell lines. Tumor Biology 5: 67-74.
- Tan BL, Norhaizan ME, Huynh K (2015) Brewers‘ rice modulates oxidative stress in azoxymethane-mediated colon carcinogenesis in rats. W J Gastroent 21:8826–8835.
- Tan BL, Norhaizan ME, Chan LC (2018) ROS-mediated mitochondrial pathway is required for Manilkara zapota (L.) P. Royen leaf methanol extract inducing apoptosis in the modulation of caspase activation and EGFR/NFkB activities of HeLa human cervical cancer cells. Evid Complem Alternat Med 6: 57-64.
- Govindaraju K, Ingels A, Hasan MN (2018) Synthetic analogues of the montanine-type alkaloids with activity against apoptosis-resistant cancer cells. Bio org Med Chem Lett 28: 589–593.
- Khalek MA, Khatun Z, Habib MR (2015) Antitumor activity of Manilkara zapota (L.) fruits against Ehrlich ascites carcinoma in mice. Biolo gija 61: 145–152.
- Kviecinski MR, Felipe KB, Schoenfelder T (2018) Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol 117: 69–75.
- Fernandes I, Faria A, Azevedo J (2010) Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J Agric Food Chem 58: 3785–3792.
- Hanif F, Perveen K, Jawed H (2014) N-(2-hydroxyphenyl) acetamide (NA-2) and Temozolomide synergistically induce apoptosis in human glioblastoma cell line U87. Cancer Cell Int 14: 133.
- Dantu AS, Shankarguru P, Devi DR (2012) Evaluation of in vitro anticancer activity of hydroalcoholic extract of Tabernaemontana divaricata. Asian J Pharm Clin Res 5: 59–61.
- Wu JG, Ma L, Lin SH (2017) Anti-angiogenic activities of extract from Actinidia eriantha Benth root. J Ethnopharmacol 203: 1–10.
- Sui Y, Li S, Shi P (2016) Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. J Ethnopharmacol 190: 261–271.
- Gemma B, Joan C, Enrique LC, Josep JB, Cristina P(2013) Analysis of hematological parameters in different cancer stages. J Nutri Biochem 24: 39–48.
- Saminathan M, Rai RB, Dhama K, Ranganath GJ, Murugesan V (2014) Histopathology and Immunohistochemical Expression of N-Methyl-N-Nitrosourea (NMU) Induced Mammary Tumours in Sprague-Dawley Rats. Asian J Animal & Veter Adv 9:621-640.
- Mustafa MB, Efe S, Meryem C, Kursat S, Safiye A, et al. (2020) Xenograft Tumor Volume Measurement in Nude Mice: Estimation of 3D Ultrasound Volume Measurements Based on Manual Caliper Measurements. J Basic Clin Health Sci 4: 90-95.
- Tucker DK, Foley JF, Bouknight SA, Fenton SE (2017) Sectioning Mammary Gland Whole Mounts for Lesion Identification. J Breast can 125: 55796.
- Weigelt B, Peterse JL, van‘t Veer LJ (2019) Breast cancer metastasis: markers and models.Nat Rev Cancer 5: 591–602.
- Fidler IJ (2020) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited.Nat Rev Cancer 3: 453–458.
- Hellman (2019) Natural history of small breast cancers.J Clin Oncol 12: 2229–2234.
- Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, et al. (2020) The landscape of cancer genes and mutational processes in breast cancer.Nature 486: 400–404.
- Michaelson JS, Silverstein M, Wyatt J (2017) Predicting the survival of patients with breast carcinoma using tumor size.Cancer 95: 713–723.
- Sivaramakrishna R, Gordon R (2022) Detection of breast cancer at a smaller size can reduce the likelihood of metastatic spread: a quantitative analysis.Acad Radiol 4: 8–12.
- Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, et al. (2021) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups.Nature 486: 346–352.
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, et al. (2020) Mutational heterogeneity in cancer and the search for new cancer-associated genes.J of Oncol499: 214–218.
- Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y (2018) The clonal and mutational evolution spectrum of primary triple-negative breast cancers.Nature486: 395–399.
- Pandya S, Moore RG (2021) Breast development and anatomy.Clin Obstet Gynecol54 91-95.
- Macias H, Hinck L (2022) Mammary gland development.Wiley Interdiscip Rev Dev Biol 1: 533-537.
- Inman JL, Robertson C, Mott JD, Bissell MJ (2019) Mammary gland development: cell fate specification, stem cells and the microenvironment.Development 142:1028-1042.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Godfrey S, Eze ED, Abiola AI, Oluwadare SS, Ponsiano N, et al. (2023) Campesterol Reduces Tumor Development and Mammary Cellular Proliferation in N-Methyl-N-Nitrosourea- Induced Breast Cancer in Female Mice. Biochem Physiol 12: 399. DOI: 10.4172/2168-9652.1000399
Copyright: © 2023 Godfrey S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3034
- [From(publication date): 0-2023 - Nov 19, 2025]
- Breakdown by view type
- HTML page views: 2570
- PDF downloads: 464