Calcination Temperature Effects on the Architecture, Morphology and Discharge Properties of SnO2 Electrode Materials Ge Performances of a Lini0.8Co0.2O2 Electrode Material
Received: 07-Oct-2017 / Accepted Date: 13-Oct-2017 / Published Date: 16-Oct-2017 DOI: 10.4172/2155-9872.1000382
Abstract
A SnO2 electrode material was successfully synthesized using the Pechini method and is of interest for potential use in lithium batteries. In this work, the effects of the calcination temperature on the fabrication of the SnO2 electrode material were investigated in detail. The architecture, morphology and crystal phase of the SnO2 electrode material were analyzed using SEM and XRD. It was found that a calcination temperature of 400°C produced a crystalline phase, and at 600°C, an excellent crystalline structure was produced. The grain size of the SnO2 electrode material is approximately 40 nm, as observed by XRD and SEM. The capacity of the SnO2 electrode material also increases with increasing calcination temperature and can reach a discharge capacity of 1800 mAh/g and a charge capacity of 710 mAh/g at 600°C. Furthermore, the capacity of the SnO2 electrode material remains at 40% after 12 chargedischarge cycles.
Keywords: Nanoparticles; Calcination temperature; Pechini method; Lini0.8Co0.2O2; Discharge capacity
Introduction
SnO2 as a candidate for lithium ion batteries has attracted considerable attention due to its excellent properties [1-3]. Much effort has been devoted to doping modification and composite modification in order to produce an excellent energy storage system [4-10], where doped metal ions affect the electrochemical properties and surface modification affects the discharge capacity. For example, Ponrouch et al. [4] reported a facile synthetic route to obtain SnO2-carbon composites. The SnO2 nanoparticles were deposited on the surface of graphene. Good cyclability was achieved in Li ion rechargeable batteries, showing a capacity of 545 mAh/g after 50 cycles. Zhou et al. [5] fabricated a SnO2@N-RGO hybrid material that exhibits high specific area and high rate capability. Zeng et al. [6] synthesized SnO2/α-FeM2O3 composite nanotube arrays using a stainless-steel substrate. The result shows a large capacitance and good reusability. Hassan et al. [7] researched a carbon-coated SnO2-NiO nanocomposite synthesized via the molten salt route. Hou et al. [8] explored an effective method to prepare a hybrid material consisting of rutile SnO2 nanoparticles and TiO2 nanorods, which overcomes the problem of poor cycling stability and rate capacity for SnO2 materials in lithium batteries. Xing et al. [9] reported WO3 nanorods uniformly coated with SnO2 nanoparticles using a facile wet-chemical method. The reversible capacity of the fabricated core-shell SnO2/WO3 nanorods is higher than that of bare WO3 nanorods or SnO2 nanostructures. Our group [10] also researched SnO2/CNT (CNT: Carbon Nano Tube) composite electrode materials, which were successfully prepared using the Pechini method. In this paper, SnO2 nanoparticles were embedded in the CNT matrix or dispersed homogeneously on the outer walls of the CNTs. Certain studies have also focused on changing the synthetic route of the SnO2 electrode material. Liu et al. [11] prepared, for the first time, large-area flexible metallic substrates using the hydrothermal process. The substrates were considered as high-performance electrode materials for lithium batteries.
Conversely, the crystal structure and thermal properties are also crutial factors for the application performance [12]. The calcination temperature of the SnO2 electrode material is also an important factor. However, few researchers have performed studied on the effect of calcining temperature on the SnO2 electrode material for application in lithium batteries.
The aim of this work is to identify the optimal calcination conditions to improve the discharge capacity of the SnO2 electrode material. To achieve this goal, we investigated the effects of various calcination temperatures on the architecture, morphological changes and discharge capacity of SnO2.
Experimental
SnO2 powders were synthesized using the Pechini method. First, citric acid was dissolved in DI (deionized) water. The dissolved citric acid was added into α-hydroxypropyl cellulose to obtain aqueous solution A. SnCl4 was dissolved in anhydrous ethanol to obtain solution B. Solution A and solution B were mixed with magnetic stirring for 2 h. Then, the mixed colloidal solutions were heated under reflux for 3 h at 65°C. The oxide sol was dried at 120°C until it became a gelatin. Finally, the obtained products were calcined at different temperatures for 4 h.
The as-prepared products were characterized by X-ray diffraction (XRD, PANalytical, Cu Kα λ=1.5406 Å) and scanning electron microscopy (SEM, TFSEM-6330). Charge-discharge measurements (LAND CT2001A) were performed between 0.25 and 3.00 V versus Li/Li+. A three-electrode system was used in this experiment. The electrolytes contain ethylene carbonate (EC) and diethyl carbonate (DEC, 1:1 v/v).
Results And Discussion
Figure 1 shows the XRD patterns of the SnO2 powders calcined at different temperatures. The peaks appear clearly at 400°C, where the characteristic peaks of 26.6°, 33.9° and 51.0°correspond to the (110), (101) and (211) lattice planes of SnO2 (JPCDS 88-0287), respectively [13]. When the SnO2 powders were annealed at 600°C, the intensities of the characteristic peaks were the strongest, which indicates that an excellent crystalline structure was produced. However, when the calcination temperature was increased to 700°C, the peak intensities decreased, suggesting that the crystallization in the SnO2 powders have been destroyed.
Typical SEM images of the SnO2 powders calcined at various temperatures for 4 h are shown in Figure 2. As seen in Figure 2a, the granular powder product was not formed at this temperature. This indicates that the product still contains organics due to the incomplete calcination at low temperature.
Organics are removed after calcinating at 500°C, as shown in Figure 2b, and the powders begin to emerge. When the calcination temperature is 600°C and 700°C, the shape of the product is oval. The particle size is approximately 40 nm. The crystallinity of the product is obvious. These results agree with those of the XRD analysis.
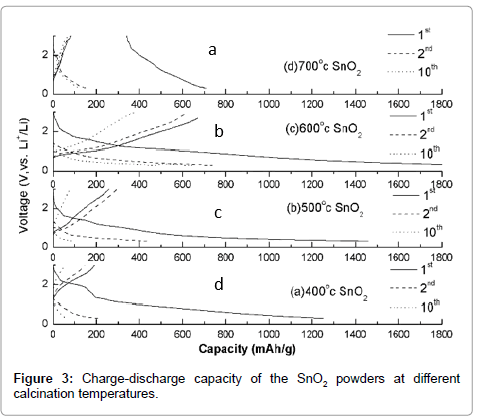
Figures 3 and 4 are the results of the charge-discharge tests. A threeelectrode system was used (the samples were the working electrodes, an Ag/AgCl electrode was the reference, and a platinum electrode served as the counter electrode). The electrolytes contained ethylene carbonate (EC) and diethyl carbonate (DEC, 1:1 v/v). Figure 3 shows the charge and discharge capacity of the SnO2 powders at different calcination temperatures. The charge platform was observed between 0.8 V and 0.9 V. With increasing calcination temperature, the charge platform is not more and more obvious. The charge capacity is the highest at 600°C, where a discharge capacity of 1800 mAh/g and a charge capacity of 710 mAh/g were obtained for the first cycle. This enhanced charge capacity could be attributed to the formation of nanosized particles, which favor lithium ion diffusion. Moreover, the enhanced charge capacity could also be attributed to an increase in the contact area of the active material. As the calcination temperature further increased, the charge capacity decreased, which may be due to the formation of large grains in which lithium ions cannot easily enter.
In Figure 4, the performance of the SnO2 powders is shown for the first 12 cycles. Considering the capacity, the charge capacity is the highest at 600°C for 12 cycles. However, it is also the lowest reversible capacity with 40% capacity retention.
During the second charge-discharge cycle, the SnO2 powders exhibited a reversible capacity with 99% capacity retention at a calcination temperature of 500°C. Even in the twelfth charge-discharge cycle, the reversible capacity still retains 81% capacity, which can greatly improve the lithium battery life. Moreover, better diffusion was obtained at this calcination temperature. This result may indicate that the proper calcination temperature not only induces a proper crystal phase but also leads to a host matrix for Li to increase the useful life.
Conclusion
In summary, SnO2 was synthesized by the Pechini method. The effects of the calcination temperature on the formation of the SnO2 electrode material were investigated in detail. SnO2 electrode materials were analyzed using SEM and XRD. The grain size of the SnO2 electrode material is approximately 40 nm, and the completed crystal phase is obtained at 600°C. The discharge capacity of the SnO2 electrode material can reach 1800 mAh/g and the charge capacity reaches 710 mAh/g at 600°C. Furthermore, the capacity of the SnO2 electrode material remains at 40% after the 12th charge-discharge cycle. This work contributes to a better understanding of the physical and chemical properties of SnO2 materials.
Acknowledgements
The generous financial support of the PhD Program of Harbin University of Commerce of China (Grant No. 14 LG13) is gratefully acknowledged.
References
- Zhang HK, Song HH, Chen XH (2012) Preparation and electrochemical performance of SnO2@carbon nanotube core–shell structure composites as anode material for lithium-ion batteries. Electrochim Acta 59: 160-167.
- Jia TK, Chen J, Deng Z, Fu F, Zhao JW, et al. (2014) Facile synthesis of Zn-doped SnO2 dendrite-built hierarchical cube-like architectures and their application in lithium storage. H Mater Sci Eng BH 189: 32-37.
- Dirican M, Yanilmaz M, Fu K, Lu Y, Kizil H, et al. (2014) Carbon-enhanced electrodeposited SnO2/carbon nanofiber composites as anode for lithium-ion batteries HJ. Power Sources H 264: 240-247.
- Ponrouch A, Sevilla M, Marchante E, Palacin MR, Fuertes AB (2012) Facile synthesis of graphitic carbons decorated with SnO2 nanoparticles and their application as high capacity lithium-ion battery anodes. Journal of Applied Electrochemistry 42: 901-908.
- Zhou X, Wan L, Guo Y (2013) Binding SnO2 nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Advanced Materials 25: 2152-2157.
- Zeng W, Zheng F, Li R, Zhan Y, Li Y (2012) Templates synthesis of SnO2/α-Fe2O3 nanotube array for 3D lithium ion battery anode with large areal capacity. Nanoscale 4: 2760-2765.
- Hassan MF, Rahman MM, Guo Z, Chen Z, Liu H (2010) SnO2-NiO-C nanocomposites as a high capacity anode material for lithium-ion batteries. J Mater Chem 20: 9707-9712.
- Hou X, Wang X, Liu B, Wang Q, Wang Z (2014) SnO2@TiO2 heterojunction nanostructus for lithium-ion batteries and self-powered UV photodetectors with improved performances. Chemelectrochem 1: 108-115.
- Xing LL, Deng P, He B, Nie YX, Wu XL (2011) Assembly of FeWO4-SnO2 core-shell nanorods and their high reversible capacity as lithium-ion battery anodes. Nanotechnology 22: 395702-395702.
- Liu R, Yang WD, Fang HY (2016) Performance of SnO2/carbon nanotube composite electrode materials synthesised by the Pechini method. Micro & Nano Letters 11: 54-56.
- Liu J, Li Y, Huang X, Ding R (2009) Direct growth of SnO2 nanorod array electrodes for lithium-ion batteries. Journal of Materials Chemistry 19: 1859-1864.
- Wu G, Li Z, Wu W, Wu M (2014) Effects of calcination on the preparation of carbon-coated SnO2/graphene as anode material for lithium-ion batteries. Journal of Alloys & Compounds 615: 582-587.
- Bonino CA, Ji L, Lin Z, Toprakci O, Zhang X, et al. (2011) Electrospun carbon-tin oxide composite nanofibers for use as lithium ion battery anodes. ACS Appl Mater Inter 3: 2534-2542.
Citation: Liu R, Yang WD, Song YJ, Liu C (2017) Calcination Temperature Effects on the Architecture, Morphology and Discharge Properties of SnO2 Electrode Materials Ge Performances of a Lini0.8Co0.2O2 Electrode Material. J Anal Bioanal Tech 8:382. DOI: 10.4172/2155-9872.1000382
Copyright: ©2017 Liu R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4863
- [From(publication date): 0-2017 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 4183
- PDF downloads: 680