Research Article Open Access
Cadmium Removal from Aqueous Solutions Using Dried Banana Peels as An Adsorbent: Kinetics and Equilibrium Modeling
Prashant D Deshmukh*,Gajanan K Khadse, Vilas M Shinde and Pawankumar LabhasetwarCSIR, National Environmental Engineering Research Institute, Nagpur, India
- *Corresponding Author:
- Prashant D Deshmuk
CSIR, National Environmental Engineering Research Institute
Nagpur
India
Tel: +919503033716
E-mail: prashantd999@gmail.com
Received date: March 30, 2017; Accepted date: April 21, 2017; Published date: April 24, 2017
Citation: Deshmukh PD, Khadse GK, Shinde VM , Labhasetwar P (2017) Cadmium Removal from Aqueous Solutions Using Dried Banana Peels as An Adsorbent: Kinetics and Equilibrium Modeling. J Bioremediat Biodegrad 8: 395. doi: 10.4172/2155-6199.1000395
Copyright: © 2017 Deshmukh PD, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The use of Dried banana peels as an adsorbent for removal of cadmium ions from aqueous solutions has been studied. Batch experiments have been conducted at different concentrations to evaluate the maximum adsorption capacity of Dried banana peels. The influence of pH, contact time, adsorbent dose was investigated at room temperature. Langmuir and Freundlich adsorption isotherm models used to test equilibrium of adsorption. The process of adsorption was found to be fast and equilibrium has been reached in within 2 hours. The maximum adsorption capacity of cadmium on Dried banana peels is 5.91 mg/g, evaluated by Langmuir adsorption isotherm model. Pseudo-first-order and pseudo-second-order kinetic models applied to evaluate rate constants. FTIR spectra of adsorbent showed the presence of hydroxyl, carboxylic and amine groups in dried banana peels. This study shows that banana peels has great potential for removal of cadmium ions and can be used as a good adsorbent for removal of cadmium from water and wastewater at very low concentration.
Keywords
Adsorption; Cadmium ions; Banana peels; Langmuir isotherm; Kinetic study
Introduction
Heavy metals are considered as primary pollutants due to its toxicity and mobility in natural water system. Among the various heavy metals, cadmium is considered as extremely toxic and carcinogenic to human beings. Cadmium is non-biodegradable heavy metal persists in environment for a long time causing harmful effects to aquatic ecosystem even at a very low concentration in water [1,2]. In natural water bodies, the major anthropogenic sources of cadmium contamination that include discharge of wastes and effluent from industries such as metallurgical processes, electroplating, plastics manufacturing, battery manufacturing, pesticide and fertilizer industry and mines [3,4]. Past events like Itai-Itai disease at Toyama Prefecture in Japan showed the extent of health effects due to the contamination of cadmium in water [5]. The cadmium contamination also causes the health effects for instance high blood pressure, bone fraction, destruction of RBCs, reproductive toxicity, hepatic effects and immunological effects [6,7]. The permissible limit of cadmium in drinking water is 0.003 mg/L as guided by World Health Organization (WHO) [8].
Several treatment technologies are available for removal of cadmium from water like chemical precipitation, reverse osmosis, ion exchange, membrane filtration, electrolysis etc. [9] but these treatment technologies have found to be expensive, high chemical demanding and least efficient to remove cadmium at low initial concentration. In recent years, biosorption is found to be a promising method than that of other methods as it is non-expensive, easily operational, regenerable and ecofriendly method [10-13]. Removal of cadmium at low initial concentration has wide applications for treatment of cadmium contaminated water and can effectively acts as a finishing treatment in effluent treatment plants to reduce cadmium level bellow discharge limits of stipulated standards.
Various types of agricultural waste materials like rice husk, sugarcane bagasse, saw dust, brazil nutshell, grape stack, mango peels and coconut copra meal etc. are studied as an adsorbent for removal of cadmium and other heavy metal ions from water and waste water [5,12,14]. In literature, it is reported that functional groups like carboxyl, hydroxyl, phosphate, thio and amino present on the walls of agricultural waste biomass play an important role for binding of heavy metals. These functional groups bind metal through ion exchange by transferring hydrogen ions or through complex formation by sharing electron pair [15]. Most of these agricultural materials showed significant adsorption capacities than Granular Activated Carbon [13]. Cadmium removal by using banana peels has been studied previously in multicomponent system [10] and they achieved adsorption capacity of 5.71 mg/g for cadmium removal onto dried banana peels.
The present investigation is undertaken to study the removal of cadmium as a single metal from aqueous solutions by using Dried banana peel as an adsorbent. Banana is one of the major fruit consumes worldwide in large quantity. Discarded peels of banana fruit from market area and household garbage generates waste in bulk quantity. Banana peels constitutes cellulose, hemicelluloses, lignin and pectin in its biomass containing functional groups like carboxyl, hydroxyl and amine. These functional groups are reported as important for binding of metal ions on biosorbents [14,16,17].
In this study batch experiments have been carried out for optimization of various process parameters like initial pH, contact time, adsorbent dose and initial metal ion concentration. The maximum adsorption capacity of Dried banana peels has been evaluated by using Langmuir adsorption isotherm equilibrium and intensity of adsorption was evaluated by Freundlich adsorption isotherm. Kinetic study conducted by using pseudo-first-order and pseudo-secondorder kinetic models. The intraparticle diffusion model is applied to test whether intraparticle diffusion is rate limiting factor or not. The adsorbent material was characterized by FTIR spectroscopy to identify affinity of functional groups present in adsorbent material for removal of cadmium ions.
Materials and Methods
Preparation of adsorbent material
Banana peels from Banana fruits was collected from local market of Nagpur, India. Banana peels were dried in sunlight for 3 days and cuts into small pieces then washed with distilled water and dried it in oven at 70°C for 5 hours. Oven dried pieces were grounded and sieved using American standard 60 mesh sieve.
Preparation of the stock solution of cadmium nitrate
Stock cadmium solution (1000 ppm) was prepared by dissolving of dried analytical grade cadmium nitrate Cd (NO3)2 .4H2O (Sigma- Aldrich) of = 99% purity into double distilled water. Working solutions of concentrations 1, 5, 10, 15, 20, 25, 30, 40, 50 ppm were prepared by using appropriate amount of stock cadmium solution.
Apparatus
The concentration of cadmium was measured by using Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) (Thermo Scientific iCAP 6300) at a wavelength of 228.8 nm at 0.5 ml/min pump speed. Argon gas was used to generate plasma. pH meter-1500 (Eutech Instrument) was used to adjust the pH of solution. FTIR spectrum of adsorbent material was analyzed by Bruker Vertex 70 FTIR Spectrometer (2:100 adsorbent: KBr ratio). Remi Orbital Shaker was used for agitation of the solutions.
Batch study for process parameters
Parameters selected for study was contact time, adsorbent dose, pH, initial concentration. Effect of these parameters was studied by keeping one parameter constant and alternatively changing other parameters.
The amount of cadmium adsorbed onto dried banana peels was calculated by using mathematical mass balance equation.
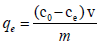 (1)
(1)
Where, qe is amount of cadmium adsorbed on adsorbent; c0 is the initial metal ion concentration in solution; ce is the metal concentration in solution at equilibrium; v is volume of solution in liter and m is the mass of adsorbent in grams.
Study of adsorption isotherm
To study adsorption isotherm, various concentrations of cadmium 5, 10, 15, 20, 25, 30, 40 and 50 mg/L were prepared by using cadmium stock solution (1000 mg/L). Initial pH of solution was in the range of 2-3 and adjusted to 6-7 using 0.1 N HCl or NaOH solution. 0.5 gm of Dried banana peels added to each flask containing 100 ml cadmium solution. Whole content was agitated on orbital shaker for the time of 120 min at room temperature, 25°C. During agitation, flasks were covered with aluminum foil to avoid evaporation. After completion of contact time, contents of flask were filtered through whatman filter paper No. 41 and filtrate was analyzed for the residual cadmium concentration in solution by using ICP-OES instrument. Recovered adsorbent was kept for drying for further material characterization.
The Langmuir and Freundlich adsorption isotherm models describes well the equilibrium between cadmium ions adsorbed onto the surface of adsorbent and residual metal ion concentration in solution at constant temperature [10,18].
Langmuir adsorption isotherm: Experiments carried out at various initial concentration ranging from 5 ppm to 50 ppm at constant temperature of 25°C. Langmuir adsorption isotherm plotted by using standard straight line equation which considers monolayer coverage of adsorbent. Monolayer adsorption capacity was evaluated using equation [19];
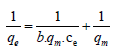 (2)
(2)
Where qe (mg g-1) is the amount of metal adsorbed, and ce (mg L-1) is concentration at equilibrium, qm (mg g-1) is the maximum adsorption capacity of adsorbent and b (Lg-1) is the Langmuirs constant. The graph of 1/ce vs. 1/qe was plotted to evaluate the values of qm and b from the slope and intercept of graph.
Freundlich adsorption isotherm: Freundlich adsorption isotherm equations were used to model adsorption behavior of cadmium by Dried banana peels. Freundlich (Eq. 3) adsorption isotherms was plotted by using standard straight-line equation and corresponding two parameters KF and n was calculated from graph of log ce verses log qe [20].
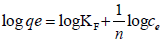 (3)
(3)
qe (mg g-1) is the amount of metal adsorbed, and ce (mg L-1) is concentration at equilibrium KF and n are Freundlich isotherm parameters which indicates adsorption capacity and adsorption intensity, respectively.
Study of adsorption kinetics
It is important to know the rate of batch sorption kinetics for the optimization of contact time and for design of adsorption unit in water treatment system. Kinetics experiments were carried out using 250 ml conical flasks. Into each flask 100 ml of solution was taken which spiked with cadmium (II) concentration to 1 ppm and 10 ppm. Then added 0.5 g Dried banana peels to each flask and kept for shaking on mechanical orbital shaker at shaking speed of 85 rpm. Contact time of different time interval (5 to 200 min) were given upto reach equilibrium. After specific contact time, the whole content was filtered through whatman filter paper No. 41 and separated adsorbent from solutions. Filtrate was analyzed for residual cadmium concentration.
All the experiments were carried out at 25°C. A blank solution without adding adsorbent was processed for same process in all the experiments to correct experimental errors. All the experiments were carried out for three times, out of which two identical readings were taken into consideration.
Results and Discussion
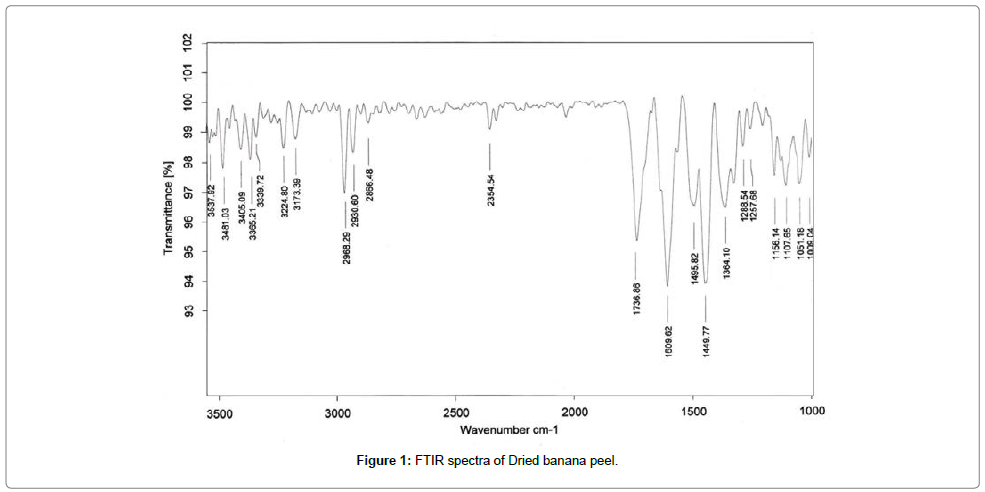
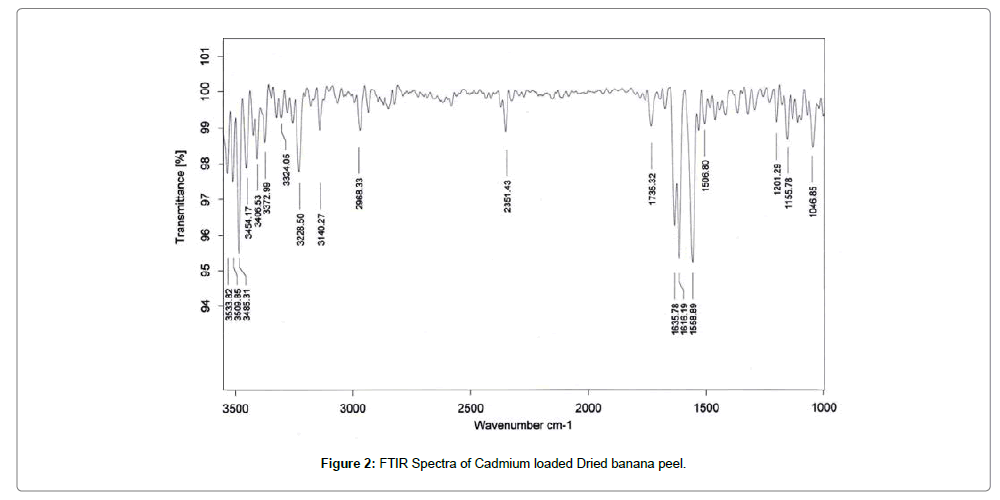
Fourier transform infrared (FTIR) spectroscopy of adsorbent
The FTIR spectra of biosorbent revealed that Dried banana peel contains number of functional groups in its biomass indicating complex nature of biosorbent. Two separate samples of adsorbent i.e., raw adsorbent and cadmium loaded adsorbent were analyzed for FTIR. Spectra of adsorbent (Figures 1 and 2) shows transmittance peaks at various frequencies indicated presence of different functional groups. In the raw adsorbent, strong peak due to O-H stretching at the frequency 3485, and 3365 cm-1 indicated that the existence of free hydroxyl group of polymeric compounds such as lignin or pectin that containing the functional groups of alcohols, phenols and carboxylic acids. A broad range of frequency (3600 to 2800 cm-1) is assigned to free hydroxyl group indicating the presence of polymeric compounds [21,22]. The peak due to N-H bending vibrations of primary amines was observed at 1609 cm-1. A peak at 1736 cm-1 is due to C=O stretching vibrations of carboxylic groups (-COOH, -COOCH3) which can be attributed to carboxylic acids or their esters [23]. Difference in transmittance at these peaks of cadmium loaded adsorbent after adsorption indicated that carboxylic group is involved in binding mechanism. The peak at 1465cm-1 may be due to aromatic ring vibration of lignin. Peaks at the wave number 1288 and 1364 cm-1 can be attributed to C-H bending of crystalline cellulose and C-H bending of cellulose, hemicelluloses or lignin polymer and the peak at 1107 cm-1 assigned to stretching vibrations of C-N bond of aliphatic amines. FTIR spectra of raw and cadmium loaded Banana peel indicate that Banana Peels composed functional groups like hydroxyl, carboxyl and amine groups. Lowering in transmittance and small deflection in band frequencies after adsorption at these peaks shows the participation of these functional groups in adsorption [14,23,24].
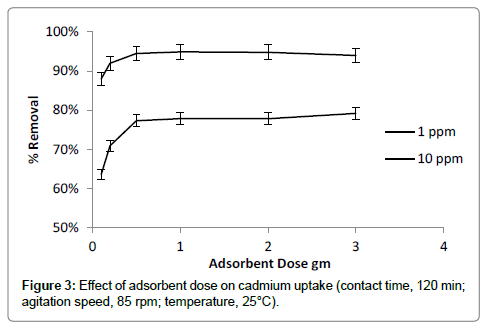
Effect of adsorbent dose
The adsorption of cadmium was studied with increasing of adsorbent quantity of adsorbent in the range of 0.1 to 3 g/100 ml (Figure 3). Result showed that adsorption efficiency of banana peels increased with increase of adsorbent dose. Increasing adsorbent dose makes available more active surface sites for binding of cadmium. Maximum removal was observed at adsorbent dose of 0.5 g/100ml. Increase in adsorbent dose beyond optimum quantity of 0.5 g/100 ml, the removal percent of cadmium not increased considerably because of reduction in available effective surface area [25].
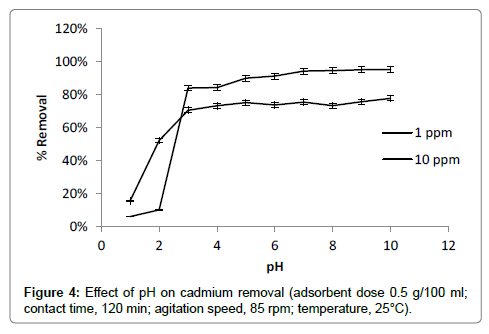
Effect of pH
pH is one of the most important factors affecting adsorption process for binding of cadmium. pH of solution studied in the range of 1-10 (Figure 4). In acidic pH range cadmium ions compete with H+ ions for binding on available surface sites therefore lower adsorption was observed in the pH range of 1 to 4. Highly increase in rate of metal removal was observed at pH range of 4-7. At pH greater than 4 deprotonation of functional groups like carboxylic group (-COOH) may occur [17]. It makes available more negatively charged active sites on adsorbent. It results into strong attraction of metal ion on surface of adsorbent. In alkaline pH range above 8, the adsorption seems to be better and greater removal was observed. In alkaline pH range precipitation of metal hydroxides may occur. Metal removal may not involve complete adsorption in this pH range therefore pH range 7-8 was selected for further adsorption study [17,26].
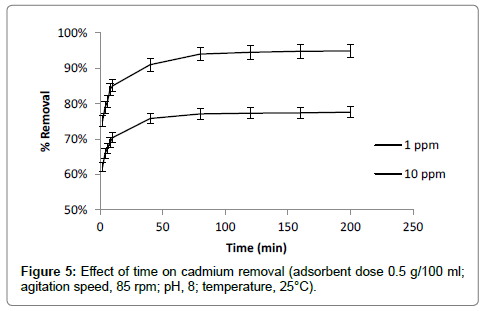
Effect of contact time and shaking speed
Contact time is also one of the most important factors affecting the adsorption of cadmium. The effect of contact time was evaluated for 1 ppm and 10 ppm initial cadmium concentration at different time interval (5-200 min) by keeping pH, adsorbent dose and shaking speed constant. The increased adsorption of cadmium was observed with increasing of contact time. The result showed that maximum adsorption (85% removal) of cadmium was occurred at first 10 min of contact time. Adsorption rate gradually increased up to of 50 min of contact time. After 50 min less increase in adsorption rate was observed and remained almost constant after 120 min (Figure 5). Less increase in adsorption rate after 50 min may be due to lowering of driving force for adsorption as less difference between initial concentration and solid liquid interface occur after 50 min [27]. Optimum contact time for cadmium adsorption was selected as 120 min for further experiments. To prevent settling of adsorbent material at bottom of flask and to give more contact with maximum active adsorption surface, optimum shaking speed of 85 rpm was observed.
Adsorption Isotherms
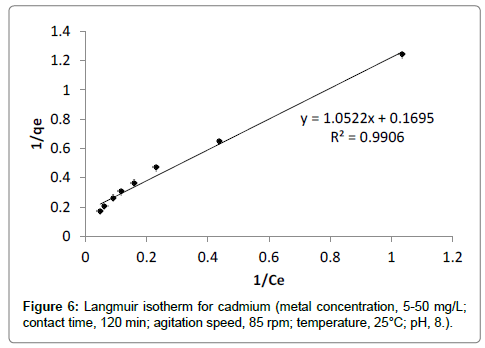
Langmuir adsorption isotherm: The sorption data of different initial concentration was well fitted into Langmuir adsorption isotherm with R2value 0.99 (Figure 6). The maximum adsorption capacity qm (mg/g) for cadmium was found to be 5.91 mg/g. The Langmuir’s parameter ‘b’ indicated bond energy for binding of metal with adsorbent material by complexation reaction. The essential characteristics of Langmuir adsorption isotherm can be explained by dimensionless separation factor ‘r’. The value of ‘r’ describes the type of adsorption isotherm as follows;
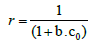 (4)
(4)
Where b is the Langmuir’s constant and co is the initial concentration of cadmium.
For each initial concentration, the calculated value of separation factor ‘r’ found in the range of 0.573 to 0.118 which lies in between 0 and 1, it indicates that the adsorption is favorable [18,27]. The maximum adsorption capacity of banana peels to adsorb cadmium evaluated from Langmuir adsorption isotherm reported in literature is presented in Table 1. It is observed that banana peels have significant adsorption capacity. The maximum adsorption capacity evaluated by this study is matches the results obtained previously in literature [10].
| SI No | Material used | qm mg/g | Reference |
|---|---|---|---|
| 1 | P. ruscipoliawood | 7.40 | [3] |
| 2 | A. donaxL. | 5.70 | [3] |
| 3 | Brazil nuts shell | 19.4 | [3] |
| 4 | Sugarcane bagasse | 10.7 | [3] |
| 5 | Peels of banana | 5.71 | [10] |
| 6 | Fungal P.tenuiculus | 11.4 | [33] |
| 7 | Mango peel waste | 68.92 | [12] |
| 8 | Raphanus sativuspeels | 19.82 | [28] |
| 9 | Moringa oliferabark | 39.41 | [27] |
| 10 | Nauclea diderichiiseed biomass | 6.30 | [32] |
| 11 | Castor seed hull | 6.98 | [18] |
| 12 | Coconut Copra meal | 2.59 | [34] |
| 12 | Grannular activated carbon | 3.70 | [18] |
| 13 | Dried banana peels | 5.92 | Present study |
Table 1: Adsorption capacity of cadmium onto banana peel and other agro based biosorbents.
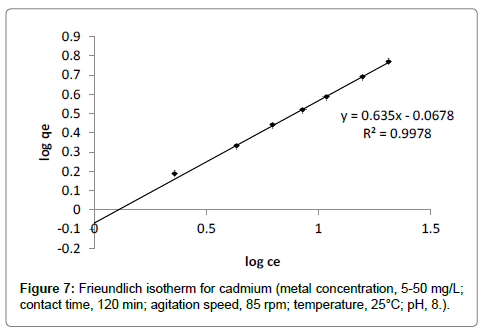
Freundlich adsorption isotherm: Freundlich adsorption isotherm for cadmium is presented in Figure 7 and the corresponding parameters KF (the ultimate adsorption capacity) and n are calculated from the graph of log ce vs log qe. The value of n indicates the measure of adsorption intensity of cadmium on banana peels (Table 2). If the value of n=1, the adsorption is linear; if n<1, the adsorption is a chemical process; if n>1, adsorption is a physical process [12]. The value of n was observed to be 1.57 which indicates the cadmium adsorption onto banana peels involves physical process. The value of R2 0.99, indicated that isotherm holds good for cadmium adsorption.
| Langmuir Isotherm Parameters | |||
|---|---|---|---|
| Metal | R2 | qm (mg g-1) | b (L g-1) |
| Cd | 0.99 | 5.92 | 0.149 |
| Freundlich Isotherm Parameters | |||
| Metal | R2 | n | KF |
| Cd | 0.99 | 1.57 | 0.857 |
Table 2: Langmuir and Freundlich isotherm parameters.
Kinetics study
Kinetic study was carried out to examine adsorption capacity of adsorbent and to evaluate the rate constant. Adsorption kinetics describes the rate of solute uptake by adsorbent with increasing contact time of 2, 4, 6, 8, 10, 40, 80, 120, 160 and 200 min. Various types of kinetics models were used in different studies of heavy metal removal using adsorbent but out of that pseudo first order, second order and intraparticle diffusion kinetics models are widely used for the adsorption of metal ions (Figures 8-10). Applicability of models expressed by correlation coefficient (R2) [18,28,29].
Pseudo first-order-kinetic model: Lagergrens pseudo first-orderkinetic model is used to describe adsorption rate of cadmium based on the adsorption capacity of adsorbent. Lagergrens equation is expressed as follows:
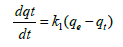 (5a)
(5a)
Where, qe is the amount of cadmium adsorbed (mg g-1) at equilibrium, qt is the amount of cadmium adsorbed (mg g-1) at any time t (min), k1 is equilibrium rate constant of pseudo first order adsorption (l min-1). Integrating the equation (5a) for boundary layer conditions t=0 to t=t and q=0 to q=t gives,
 (5b)
(5b)
This equation (5b) represents integrated rate law for pseudo first order reaction. The linear form of equation is expressed as follows,
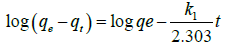 (5c)
(5c)
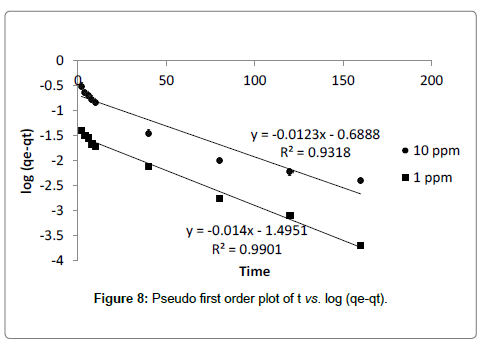
Where, k1 is the rate constant. The correlation coefficient (R2) value calculated from the plot of log (qe-qt) vs. t is found to be high (Figure 4). But the calculated values of qe from plot not matches with experimental values for both the 10 ppm and 1 ppm concentrations (Table 3) It indicates that adsorption of cadmium onto dried banana peels does not follow first order kinetics.
| C0 mg/L | 1ppm | 10ppm |
|---|---|---|
| Pseudo first-order | ||
| qe(exp)/mg g-1 | 0.189 | 1.552 |
| qe(cal.)/mg g-1 | 0.031 | 0.205 |
| k1(min-1) | 0.032 | 0.0276 |
| R2 | 0.99 | 0.93 |
| Pseudo second-order | ||
| qe(cal)/mg g-1 | 0.190 | 1.557 |
| K2(min-1) | 4.83 | 0.641 |
| h (mg g-1 min-1) | 0.174 | 1.56 |
| t1/2 | 1.08 | 1.0 |
| R2 | 1.0 | 1.0 |
| Intraparticle diffusion | ||
| BL(cal)/ mg g-1 | 0.15 | 1.30 |
| kd(g mg-1min-1) | 0.002 | 0.021 |
| R2 | 0.84 | 0.80 |
Table 3: Comparison of various factors at different initial concentration.
Pseudo second-order-kinetics model: The linear form of pseudo second order kinetic model is given by equation [30],
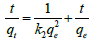 (6a)
(6a)
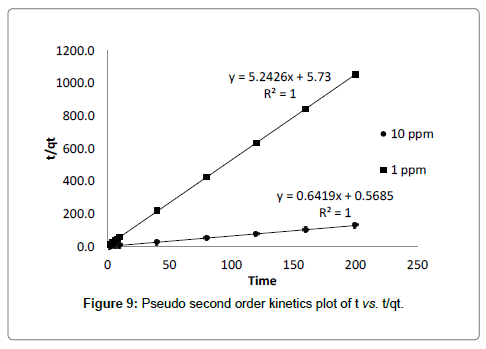
Where, k2 (g mg-1 min-1) is the rate constant of pseudo second order equation. qe is the adsorption capacity of adsorbent at equilibrium, qt is the amount of cadmium adsorbed by adsorbent at time t. The value of qe and k2 can be calculated from the slope and intercept of the linear plot of t vs. t/qt which is presented in Table 3. The experimental value of qe matches to predicted values of qe from the plot with correlation coefficient value (R2=1) which suggested that, second order kinetic model fits better to describes the kinetics of adsorption [28].
As experimental data fits, well in second order kinetics (Figure 4), the values of initial sorption rate ‘h’ and half adsorption time ‘t1/2’ can be calculated from the following equations (9) and (10), respectively which are based on equation of second order kinetics.
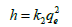 (6b)
(6b)
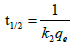 (6c)
(6c)
Intraparticle diffusion model
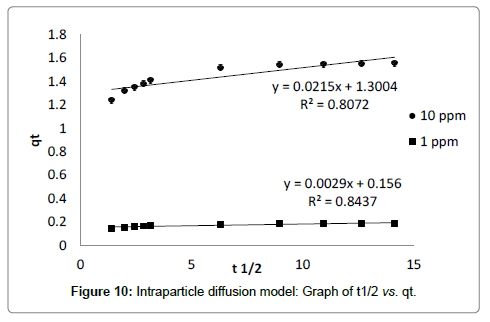
Intraparticle diffusion model proposed by Weber and Morris [31] has been widely used by researchers for the analysis of adsorption kinetics. The process of metal adsorption onto adsorbent mainly involves three steps including bulk diffusion, film diffusion and pore diffusion or intraparticle diffusion. Out of these, intraparticle diffusion process is slow process and it may be a rate limiting process. Adsorption of metals generally controlled by intraparticle diffusion or liquid phase mass transport rates. In this study, experiments were carried out by batch mode with rapid stirring of the contents therefore intraparticle diffusion may be the rate limiting step [29]. Intraparticle diffusion model is applied to test whether it is a rate limiting step or not. The intraparticle diffusion rate constant (Kd) is calculated from the relationship between qt and the square root of time t1/2 is expressed in following equation (7) provided by Weber and Morris [31].
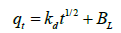 (7)
(7)
Where, Kd (mg g-1 min-1/2) is intraparticle diffusion rate constant, BL (mg g-1) is proportional to thickness of boundary layer. The values of Kd and BL are calculated from the slope and intercept of plot of qt vs. t1/2 (Table 3). The plot of adsorption capacity of adsorbent (qt) versus square root of time (t1/2) is found to be linear with R2 value of 0.80 and 0.84 for 10 ppm and 1 ppm initial concentrations, indicated considerable linearity representing that intraparticle diffusion is involved in adsorption process (Figure 5). The slope of graph (Kd) is 0.021 and 0.002 mg g-1 min-1 for 10 ppm and 1 ppm initial concentration respectively, indicating that some of boundary layer may be involved in adsorption. If the line of linear graph passing through origin, it indicates that intraparticle diffusion may rate limiting factor but in the graph line is not passing through origin, having intercept value greater than zero [32-34]. It suggested that intraparticle diffusion may not rate limiting factor and surface adsorption along with intraparticle diffusion may play important role in adsorption of cadmium onto dried banana peel [18,28].
Conclusion
Dried banana peels show a good efficiency for the removal of cadmium ions from aqueous solution. FTIR spectra of banana peel indicates that the presence of functional groups like hydroxyl and carboxyl. Adsorption capacity evaluated by Langmuir adsorption isotherm is found to be 5.91 mg/g which is slightly greater than previous studies. Experimental data has well fitted into second-order kinetic model suggesting second-order nature of the process. Intraparticle diffusion suggested that intraparticle diffusion is not the rate limiting factor for adsorption. The present study concludes that easily available, nonhazardous agricultural waste material like banana peels can be successfully used as an adsorbent material for removal of cadmium from aqueous solutions even at low concentration.
Acknowledgment
Authors are thankful to Director, CSIR-National Environmental Engineering Research Institute, Nagpur-440020, India for providing all the facilities to carry out the present work. We also acknowledge support and guidance from the staff of Water Technology and management Division, CSIR-NEERI, Nagpur.
References
- Bargagli R, Nelli L, Ancora S, Focardi S (1996) Elevated cadmium accumulation in marine organisms from Terra Nova Bay Antarctica. Polar Biol 16: 513-520.
- Frazier JM (1979) Bioaccumulation of cadmium in marine organisms. Environ Health Perspect 28: 75-79.
- Basso MC, Cerrella EG, Cukierman AL (2002) Lignocellulosic materials as potential biosorbents of trace toxic metals from wastewater. IndEngChem Res 41: 3580-3585.
- Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59: 203-216.
- Rao KS, Mohapatra M, Anand S, Venkateshwarlu P (2010) Review on cadmium removal from aqueous solution.Int J EngSciTechnol 2.
- Drasch GA(1993) Increase of cadmium body burden for this century. Sci Total Environ 67:75-89
- Waalkes MP(2000) Cadmium carcinogenesis in review. J of InorgBiochem79: 241-244.
- World Health Organization (2011) Guidelines for drinking water quality(4thedn
- Jose TM, Qiming Y, Gavin MW (1999)Biosorption of cadmium (II) From aqueous solutions by pretreated biomass of marine alga DurvillaeaPotatorum. Wat Res 33: 335-342.
- Anwar J, Shafique U, Zaman W, Salman M, Dar A (2010) Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. BioresTechnol 101: 1752-1755.
- Brierley CL (1991) Bioremediation of metal-contaminated surface and groundwaters. GeomicrobiolJ8: 201-223.
- Iqbal M, Saeed A, Iqbal ZS(2009) FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J Hazard Mater164: 161-171.
- Nguyen TH, Ngo HH, Guo WS, Zhang J, Liang S, et al. (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. BioresTechnol 148: 574-585.
- Osvaldo KJ, Leandro VAG, Julio CPM, Vagner RB,Tania MSM, et al.(2007) Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse.BioresTechnol98: 1291-1297.
- Kumar J, Balomajumder C, Mondal P (2011) Application of Agro-Based Biomasses for Zinc Removal from Wastewater - A Review. Clean Soil Air Water 39: 641-652.
- Romero-Gonzalez ME, Williams JC, Philip HE, Gardiner(2001) Study of the mechanisms of cadmium Biosorption by dealginated seaweed waste. Environ SciTechnol 35: 3025-3030.
- Munusamy T, Yi-Ling L, Ling-Chu L, Jiunn-Fwu L (2010) Cellulose-Based Native and Surface Modified Fruit Peel for the Adsorption of Heavy Metal Ions from Aqueous Solution: Langmuir Adsorption Isotherms. J ChemEng Data 55: 1186-1192.
- Sen TK, Mohammod M, Maitra S, Dutta BK (2010) Removal of Cadmium from Aqueous Solution Using Castor Seed Hull: A Kinetic and Equilibrium Study. Clean Soil Air Water 38: 850-858.
- Langmuir I(1918) The adsorption of gases on surfaces of glass, mica and platinum. J Am ChemSoc 40: 1361-1403.
- Freundlich H (1907)Uber die adsorption in loesunggen. Z PhysChem 57: 385-470.
- Alemdar A, Sain M(2008) Isolation and characterization of nanofibers from agricultural residues: wheat straw and soy hulls. BioresTechnol 99: 1664-1671.
- Gnanasambandam R, Protor A(2000) Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem 68: 327-332.
- Li FT, Yang H, Zhao Y, Xu R(2007) Novel modified pectin for heavy metal adsorption. ChinaChemLett 18: 325-328.
- Dowell F, Wang D (2013) Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques. Appl Energy 104: 801-809.
- El Nemr A (2007) Pomegranate husk as an adsorbent in the removal of toxic chromium from wastewater. ChemEcol 23: 409-425.
- Perez-Marin AB, Meseguer ZV, Ortuno JF, Aguilar M, Saez J,et al.(2007) Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazard Mater B 139: 122-131.
- Reddy DHK, Lee SM, Seshaiah K (2012) Removal of Cd(II) and Cu(II) from Aqueous Solution by Agro Biomass: Equilibrium, Kinetic and Thermodynamic Studies. Environ Eng Res 17: 125-132.
- Ashraf MA, Rehman MA, Alias Y, Yusoff I (2013) Removal of Cd(II) onto Raphanussativus peels biomass: equilibrium, kinetics, and thermodynamics. Desalination Water Treat 51: 4402-4412.
- Hameed BH, EI-Khaiary MI(2008) Sorption kinetics and isotherm studies of a cationic dye using agricultural waste: Broad bean peels. J Hazard Mater 154: 639-648.
- Ho YS, Mackay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34: 735-742.
- Weber Jr, WJ, Morris JC(1963) Kinetics of adsorption on carbon from solution. J SaintEng 98: 31-59
- Omorogie MO, Babalola JO, Unuabonah EI, Gong JR (2012) Kinetics and thermodynamics of heavy metal ions sequestration onto novel Naucleadiderrichii seed biomass. BioresTechnol 118: 576-579.
- Grassi DA, Galicio M, Fernández CA (2011) A homogeneous and low-cost biosorbent for Cd, Pb and Cu removal from aqueous effluents. ChemEcol 27: 297-309.
- Augustine EO,Yuh-Shan H (2008) Kinetic biosorption study of cadmium onto coconut copra meal as biosorbent.Int J EnvironPollut 34: 1-4.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 39228
- [From(publication date):
May-2017 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 37614
- PDF downloads : 1614