Research Article Open Access
Building Materials Corrosion Control by Fiber Reinforced Polymers
Singh RK*Department of Chemistry, Jagdam College, JP University, Chapra, Bihar, India
- *Corresponding Author:
- Singh RK
Assistant Professor, Department of Chemistry
Jagdam College, JP University, Chapra-841301
Bihar, India
Tel: 06152 232407
E-mail: rks_jpujc@yahoo.co.in
Received Date: September 01, 2015; Accepted Date: September 17, 2015; Published Date: September 24, 2015
Citation: Singh RK (2015) Building Materials Corrosion Control by Fiber Reinforced Polymers. J Powder Metall Min 4:137.doi:10.4172/2168-9806.1000137
Copyright: © 2015 Singh RK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
This paper highlights the beauty of fiber reinforced polymers (FRP) in the field of corrosion protection reinforced concrete structures (RCS). The fiber reinforced polymers develop anticorrosive barrier on the surface reinforced concrete and minimize the attack of corrosive pollutants. Corrosion is major problems with reinforced concrete. It occurs due to interaction of acids, alkalis, salts, pollutants, particulates, heat, light microorganisms and macro organisms and its own morphology. These substances create hostile environment for building materials of concrete and they produce chemical and corrosion reactions. The other factors also influence the corrosion of materials like acid rain, wind and weathering effects. The industrial effluents, flues gases and wastes provide major role in the field of concrete corrosion. These reactions change their mechanical, physical and chemical properties and tarnish their facial appearance. When building materials of concrete come in contact of corrosive environment, they can develop corrosion cell in presence of electrolyte. The corrosion reactions aggravate with rebar steel of concrete and their deterioration starts. There are several forms of corrosion occurs with iron bar of concrete such as galvanic, pitting, crevice, stress, intergranular, blistering, embrittlement, erosion, cavitations, observes inside and outside of materials. Building materials of concrete also show chemical reaction with corrosive substances and produce dissolving and swelling. Developed Nations expense 4% of their GNP for corrosion protection, parts replacement and repairing and maintenance work. The major sources of corrosive substances are various types of industries, mining, thermal power plants, petroleum refinery, burning of fossil fuel, chemical wastes, biological wastes, human wastes, household wastes, agricultural wastes, animal wastes, food grain wastes, hospital wastes, chimneys flues gases, industrial effluents. These sources release acids, alkalis, salts, organic compounds and metals as a form of effluents, oxides of carbon, oxides of nitrogen, oxides of sulphur, oxides of halogen, hydride of sulphur, hydride of nitrogen, volatile organic compounds as flues gases and different types of wastes. These pollutants substances contaminate water, air and soil. The essential components of RC Structures are sand, stones, cements, iron bars, bricks and water. The above mentioned corrosive substances generate hostile atmosphere for building materials. Due to this corrosive effect life of RC Structures are reduced and disintegration occurs inside and outside of them so their stability, durability and longevity become questionable. There are various techniques for corrosion protection of RC Structures. But these techniques did not give good result for their protection. Hence for this work Fiber Reinforced Polymers will be used to control corrosion of RC Structures.
Introduction
The destructive attack by corrosive substances which come into external contact with the concrete is the main cause for its corrosion. The major cause of corrosive reaction in concrete is water, cement and ballast.
Natural water is suitable for mixing with concrete. Chemical reactions occur with various compounds in presence of water before the cement sets. These are the substances avoided like badly polluted waters, bog waters, industrial effluents which may contain carbohydrates or other organic materials. Chloride ion is very harmful for reinforcement. It is always best to use pure water. The cement [1] itself produces trouble application of chalk, magnesia and sulphate during production. Set concrete shows corrosion due to external Corrosive pollutants.
Chemical attack on concrete and mortar is generally affected by water or aqueous solutions. Corrosive gases and dust particulates can have exhibited destructive effect on concrete and mortar. These pollutants enter inside of concrete and mortar and initiate chemical reaction in this way disbonding produces among them.
Concrete consists of cement and ballast with reinforced steel [2]. The ballast is usually stable against the aggressive media. Whereas, limestone and some other ballasts which are dissolved by acid water as well as ballast containing sheets of mica, in which gypsum can crystallize in the presence of sulphate and crack the concrete structure by swelling. Polymeric materials are used for corrosion protection of reinforced concrete structures but they do not give good results in corrosive atmosphere. Corrosive pollutants penetrate coated polymers and produce disbanding and finally enter inside reinforced concretes and corroding building materials and rebar steel. When it is reinforced with fiber, the attacks of corrosive pollutants decelerate.
Experimentation
The cement is the part of the concrete which is most easily attacked. Portland cement is one of the most important building materials at the present time. It is formed when mixture of limestone and clay are strongly heated. It is mixed with small amount of water, set in few hours to a hard stone like substance. Portland cement [3] is chemically defined as the finely ground mixture of calcium aluminates and silicates of varying compositions, which hydrate when mixed with water to form a rigid solid structure with good compressive strength. Portland cement is a mixture of the following compounds mention in Table 1.
| Formula | Abbreviation | Name |
|---|---|---|
| 2CaO.SiO2 | C2S | Dicalcium silicate |
| 3CaO.SiO2 | C3S | Tricalcium silicate |
| 3CaO.Al3O3 | C3A | Tricalcium aluminate |
| 4CaO.Al2O3.Fe2O3 | C4AF | Tetracalcium alumino ferrite |
| CaSO4.2H2O | CS.2H | Gypsum |
Table 1: Composition of Portland cement.
It is produced from the Portland cement clinker phase by reactions with water. A typical reaction is that of tricalcium aluminate, which forms more than 50% by weight of Portland cement. It illustrates the complicated nature of the hydration of cement;
2(3CaO.SiO2) + 6H2O → 3CaO.2SiO2.3H2O + 3Ca(OH)2
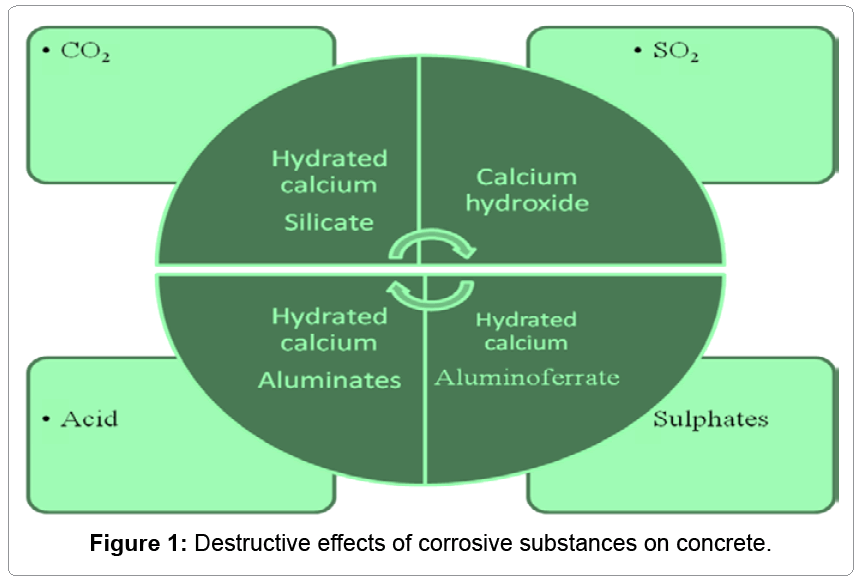
The set cement consists of hydrated calcium silicate, calcium hydroxide and reaction products originating from calcium aluminate (e.g. hydrated tetracalcium aluminate) and calcium aluminate ferrate. Hydrated calcium silicate, in particular, gives strength to concrete, while the calcium hydroxide is the reason for the alkaline nature of the set cement (pH > 12).
The products of hydration vary in their behavior against different aggressive media in Figure 1 shows the harmful effects upon concrete of these media.
Corrosion of set concrete
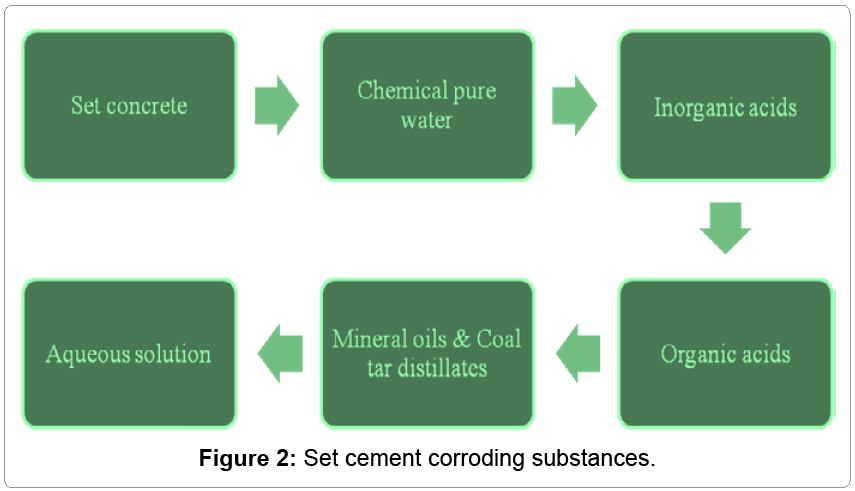
Set concrete is corroded by interaction of different types of corrosive substances like chemically pure water, inorganic acids, organic acids, alkalis, plant and animal oils and fats, mineral oils and coal tar distillates and aqueous solution and they are expressed in Figure 2. These hostile materials come in contact of set concrete to develop chemical reaction with it and deterioration of materials take place.
There are different types corrosive materials are described in Table 2 and their corroding action also observes. The results of Table 2 indicate that corrosion control of RCS is not an easy task. But FRP coatings give satisfactory outcome for RCS.
| Material | Occurance | Action |
|---|---|---|
| 1. Chemical pure water | water of condensation rainwater, molten snow soft spring water | Dissolves, leaches out active in practice |
| 2. Inorganic acids (HCl, H2SO4, H2SO3, HNO3 H3PO4, H2SiO4, H2CO3) | Chemical industry carbonic and sulphrous acids, natural waters | Dissolves, the stronger the acid the more intensely corrosive it is. Increasing activity is also found with falling pH value. H2SO4 Swelling effect Carbonic acid corrosion Depends upon free CO2. |
| 3. Organic acids ( acetic acid, lactic Acid, tannic acid, Formic acid, Oxalic acid) | During fermentation in dairies, canneries, fodder silos, dye works | Dissolves slowly can slow down setting process |
| Humic acid | In soils and impure Ballast | Can attack slowly, depending upon type Of humic acid |
| Oxalic acid | Dyeworks, chemical Factories | Nondestructive |
| Alkalis (sodium and Potassium Hydroxides) | In the chemical industry | Dissolves only when highly concentrated |
| Plants and animal oils and fats | food industry and food trade | Loosens the structure dissolves by reaction of the fatty acids with calcium salts to form soft calcium soaps. Turpentine has no destructive effect. |
| 4. Mineral oils and Coal tar distillates | Engineering sheds, filling stations, refineries | As these materials are non-acidic, they are Nondestructive. All low Viscosity oils penetrate Concrete and act as Lubricants between set Cement and ballast to lossen structure. Phenol and cresol slowly corrode concrete. |
| 5. Aqueous solution | ||
| Mg2+ | In natural and Industrial waters | Softens |
| NH4+ | Agricultural undertakings Artificial fertilizer factories | Dissolves |
| SO42- | In natural and industry waters | Swells |
| pH <6.5 | In natural and industry waters | Dissolves |
| CO,CO2and H2CO3 | In natural water | Dissolves |
| Na+, K+, Ca2+ Fe2+, Fe3+, Al3+, Si4+, NO3-, PO43- and SiO32- | In natural and industrial waters | Not Harmful |
Table 2: Action of Corrosive Materials on Set Concrete.
Acid corrosion
The corrosive action of acid depends upon their strength and their concentrations. The strong acids are hydrochloric acid, sulphuric acid and nitric acid. These acids dissolve all components of set cement to form salts of calcium, aluminum and iron and silica gel. Weak acids like carbonic acid many organic acids as humic acid and lactic acid which form water soluble salts with calcium compounds. Various types of damage observe after long periods of exposure.
Hydrogen sulphide is evolved as effluents during the decomposition of organic materials. It dissolves in water to give weak acid. It can be absorbed with humidity of the concrete and then oxidized to produce sulphrous and sulphric acid. In both cases an acidic attack is mainly involved.
SO2 gas is present in flue gases. It can be converted into sulphrous acid in presence of moisture and by oxidation into sulphuric acid. This is also mainly by acid.
Strong acids may be present in effluents, while acids are produced by dairies, fruit juice factories, breweries, paint and dyes, drugs, food processing and preservative industries and bog waters.
Carbonic acid dissolves limestone. Its ability to attack cannot be expressed by its pH value alone. It occurs in water. Even its small quantities create corrosive environment for concrete. Carbonic acid behaves like weak acids just like other weak acids and is capable of dissolving lime from concrete. Lime stone is partial soluble in water but its solubility is increased in presence of carbon dioxide. Addition of limestone does not prevent this from of corrosion.
CaCO3 + H2O + CO2 → Ca (HCO3)2
(Sparingly soluble) (Very soluble)
Salts corrosion with ion exchange properties
Magnesium chloride and ammonium chloride react with the set cement to from ammonium carbonate, ammonium oxalate, magnesium carbonate, magnesium sulphate and magnesium carbonate. Calcium hydroxide is present in the set cement which reacts with chloride to form water soluble compounds. Magnesium compounds may separate as hydroxide (a soft, gel-like mass) either on the outside or inside and can therefore cause swelling. Ammonia is released in the form of a gas.
Soft water corrosion
Soft water has less calcium and magnesium salts. For this reason water can dissolve relatively large quantities of these salts from concrete. Very soft water (less than 1.1 milliequivalent total hardness) can attack the surface, but dense concrete which has been made correctly is resistant even to very soft water.
Fats and oils corrosion
Organic compounds (plant and animal) fats and oils produce corrosive effect with concrete. All contain smaller or larger quantities of free fatty acids, which like other weak acids attack concrete. The fatty acids can react with calcium compounds contained in the set concrete with formation of calcium salts (soaps) of the fatty acids and glycerol. This decomposition of the fat (saponification) causes a softening of concrete. Mineral oils and fats provided they contain no acids or resins, do not attack concrete. If concrete is completely impregnated with fats and oils its hardness and strength of adhesion to the steel reinforcement are impaired. There is generally swelling action is possible. Sulphate solutions penetrate into concrete, chemical reactions take place between different parts of the set cement and the hydrates of calcium aluminate. These form voluminous new structures inside the concrete. The following equation gives:
3CaO.Al2O3 +3(CaSO4.2H2O) + 26H2O → 3CaO.Al2O3.3CaSO4.32H2O
Crystalline trisulphate is produced which is a very much larger volume than the solid starting materials because of the introduction of an appreciable quantity of water of crystallization. As the space it has to occupy is limited, it subjects its surrounding to pressure and therefore induces cracking.
This sulphate expansion can be avoided by using cements which contain little trisulphate. With very high sulphate concentration (1200 mg SO42-/litter) it is possible for gypsum to separate from calcium hydroxide solution in the hardened concrete. This also causes swelling. Mostly Sulphate occurs in ground and effluent waters.
Results and Discussions
Fiber reinforced polymers for RCS protection
Fiber reinforced polymers are not affected as severely as other building materials by various environmental conditions over the periods of time. The ageing process is not quickly influenced by the weather variations of ambient temperature, moisture in the air in the form of fog, rain, snow, hail and water vapour, air impurities, ultraviolet radiation, wind etc. Physical factors (e.g. mechanical stresses, static electricity) and chemical agents (e.g. salt solution, oxygen) have no influence. Other variable processes like chemical reactions as oxidation, displacement and double displacement reactions also can be minimized by the application of FRP. Fiber reinforced polymers check absorption of water, evaporation, swelling, dissolution, precipitation and all other factors that take part in these processes and stop breaking of reinforced concrete structures. The fiber reinforced polymers [4] provide chemical resistance, stability of surface structure, colour and embrittlement. The ageing process can be slowed down by addition of special additives. The additives should stabilize the molecular structure, filter out ultraviolet light and longer wave radiation and reduce oxidation tendencies, such materials are called stabilizers, light filters and antioxidants, respectively. The weather resistances of fiber reinforced plastics are very satisfactory and they can enhance the life of building materials. Good weather resistance and corrosion protection of reinforced concrete are shown by fiber reinforced polymers like polytetrafluorethylene (Teflon etc.) and phenolic and amino plastics, polyamides, polystyrene, polyethylene and polyvinylchloride [5]. The ageing reactants do not provide bad affect these reinforced polymers and concretes. Some important polymeric materials are mentioned in Table 3, they can protect reinforced concrete in acidic and alkaline medium.
| Polymers | Acids | Alkalis | ||
|---|---|---|---|---|
| weak | strong | weak | strong | |
| Polyvinly Chloride(PVC) | effective | effective | effective | effective |
| Polyethylene(PE) | effective | effective | effective | effective |
| Polypropylene(PP) | effective | effective | effective | effective |
| Polyisobutylene(PIB) | effective | effective | effective | effective |
| Polystyrene(PS) | effective | effective | effective | effective |
| Polymethyl Methacrylate (PMMA) | effective | effective | effective | effective |
| Phenolic resins | effective | noneffective | effective | noneffective |
| Melamine formaldehyde | effective | noneffective | effective | noneffective |
| Urea Formaldehyde | effective | noneffective | effective | noneffective |
| Polyester resins(UP) | effective | effective | effective | noneffective |
| Polyamides(PA) | noneffective | noneffective | effective | noneffective |
| Polyurethane(PUR) | effective | noneffective | effective | effective |
| Polytetra Fluoroethylene (PTFE) | effective | effective | effective | effective |
| Epoxy Resins(EP) | effective | effective | effective | effective |
| Polycarbonate(PC) | effective | effective | effective | effective |
Table 3: The Chemical resistance of acids and alkalis important fiber reinforced polymers.
Polymeric materials are written in Table 4, they can nullify the attack of organic solvent on reinforced concrete. These polymeric materials are coated on the surface of RC Structures and shave base building materials.
| Plastics | Solvents | ||
|---|---|---|---|
| alcohols | esters | ketones | |
| Polyvinly Chloride(PVC) | effective | noneffective | noneffective |
| Polyethylene(PE) | effective | effective | effective |
| Polypropylene(PP) | effective | effective | effective |
| Polyisobutylene(PIB) | effective | effective | effective |
| Polystyrene(PS) | effective | noneffective | effective |
| PolymethylMethacrylate (PMMA) | effective | noneffective | noneffective |
| Phenolic resins | effective | effective | effective |
| Melamine formaldehyde | effective | effective | effective |
| Urea Formaldehyde | effective | effective | effective |
| Polyester resins(UP) | effective | effective | effective |
| Polyamides(PA) | effective | effective | effective |
| Polyurethane(PUR) | noneffective | effective | effective |
| PolytetraFluoroethylene (PTFE) | effective | effective | effective |
| Epoxy Resins(EP) | effective | effective | effective |
| Polycarbonate(PC) | effective | noneffective | noneffective |
Table 4: The Chemical resistance of solvents important polymers.
Fiber reinforced polymers [6] are used for corrosion protection in RC Structures mentioned in Tables 5 and 6 describes the stability of plastic protective coatings against various aggressive media. Chlorinated rubber and epoxy resins with polyamide hardener are used for the protection of rebar steel. Polyethylenes are employed for winding around pipelines which are to be buried inside concrete [7]. Acid proof coating and claddings are made from polyvinylchloride. Polytetrafluorethylene shows particularly good resistance against aggressive media and can be used for example for the cladding of outer surface building in sea weather atmosphere and for coating sheet steel. Polyurethane lacquer is used for weather and chemical proof coating on aluminum [8]. Specific uses of fiber reinforced polymers for corrosion protection of building materials are commonly specified in connection with the appropriate building material. These are fiber reinforced polymers which are suitable for control corrosion of RC Structures [9] in given environment mentioned in Table 5.
| Fiber Reinforced Polymers | Benzene | Gasoline | Mixture of Fuels |
|---|---|---|---|
| Polyvinly Chloride(PVC) | noneffective | effective | effective |
| Polyethylene(PE) | noneffective | effective | noneffective |
| Polypropylene(PP) | effective | effective | effective |
| Polyisobutylene(PIB) | noneffective | effective | effective |
| Polystyrene(PS) | noneffective | effective | noneffective |
| PolymethylMethacrylate (PMMA) | effective | effective | effective |
| Phenolic resins | effective | effective | effective |
| Melamine formaldehyde | effective | effective | effective |
| Urea Formaldehyde | effective | effective | effective |
| Polyester resins(UP) | effective | effective | non effective |
| Polyamides(PA) | non effective | effective | non effective |
| Polyurethane(PUR) | effective | effective | effective |
| PolytetraFluoroethylene (PTFE) | effective | effective | effective |
| Epoxy Resins(EP) | effective | effective | effective |
| Polycarbonate(PC) | effective | effective | effective |
Table 5: The Chemical resistance of fuels important plastics.
Some minerals, animals, plants, oils and fats are corroding building materials which are mentioned in Table 6. In this environment RC Structures can be protected by application of given fiber reinforced polymers.
| Fiber Reinforced Polymers | Mineral | Animal and plant | Oils and fats |
|---|---|---|---|
| Polyvinly Chloride(PVC) | noneffective | effective | effective |
| Polyethylene(PE) | noneffective | effective | noneffective |
| Polypropylene(PP) | effective | effective | effective |
| Polyisobutylene(PIB) | noneffective | effective | effective |
| Polystyrene(PS) | noneffective | effective | noneffective |
| PolymethylMethacrylate (PMMA) | effective | effective | effective |
| Phenolic resins | effective | effective | effective |
| Melamine formaldehyde | effective | effective | effective |
| Urea Formaldehyde | effective | effective | effective |
| Polyesterresins(UP) | effective | effective | effective |
| Polyamides(PA) | noneffective | effective | effective |
| Polyurethane(PUR) | effective | effective | effective |
| PolytetraFluoroethylene (PTFE) | effective | effective | effective |
| Epoxy Resins(EP) | effective | effective | effective |
| Polycarbonate(PC) | effective | effective | effective |
Table 6: The Chemical resistance of fuels important fiber reinforced polymers.
Some reinforced elastomers are mentioned in Table 7 which controls the corrosion of RC Structures in mentioned environment.
| Elastomer | mineral oil | organic solvent | water, acids, alkalis |
|---|---|---|---|
| Natural rubber | noneffective | noneffective | effective |
| Chlorinated rubber | effective | noneffective | effective |
| Polysulphide rubber | effective | effective | effective |
| Silicone rubber | effective | effective | noneffective |
| Polyurethane rubber | effective | effective | noneffective |
Table 7: The chemical resistance of important elastomers.
Conclusion
Fiber reinforced polymers are coated on the surface of reinforced concrete structures, they do produce bonding between them. These protective materials minimize osmosis and diffusion process which are created by several types of hostile environment. Because of these two processes corrosive pollutants enter inside of concretes and activate corrosion reaction, causing disbonding among building materials. The FRP coatings work as fortification for RC Structures. But this type of coating do not give good results after long period because lot of porosities are present on the surface of FRP so osmosis and diffusion process of pollutants are not fully controlled. They can corrode FRP and RCS. Remedy of this problem is application of nanocoating on surface FRP that blocks osmosis and diffusion process.
Acknowledgement
I am thankful to UGC for providing me finical support for this work. I am thankful to Master Builder Company who gave me the idea for this work. I give my regards to Mr. PG Nair for his valuable suggestion.
References
- Carl CW (1968) The Reinforced-Concrete Sky Scrapers: The Ingalls Building in Cincinnati and its place in structural History. Technology and Culture 9: 1-33
- Steiger RW (1995) “History of concrete”.The Aberdeen Group.
- Morgan W (1995) Reinforced concrete the Elements of structure.
- Shaeffer RE (1992) History of Concrete Building Construction. Reinforced Concrete: Preliminary Design for Architects and Builders.McGraw-Hill.
- Collins P (1920-1981) Concrete: The Vision of a New Architecture. McGill-Queen’s University Press.
- Morsch E, Goodrich EP (1909) Concrete-steel Construction: (Der Eisenleltobaw). The Engineering News Publishing Company 204-205.
- Kim S, Surek JT, James R, Baker-Jarvis (2011) “Electromagnetic Metrology on concrete and corrosion”. Journal of research of the National Institute of standards and Technology 116:655-669.
- Nilson AH, Darwin D,Dolan W (2003) Design of concrete structures, The McGraw-Hill Education.
- Simescu F, Idrissi H (2008) “Effect of Zinc phosphate chemical conversion coating on corrosion behavior of Mild steel in alkaline medium: Protection of rebars in reinforced of concrete”. Sci Technol . Adv Mater 9.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 12159
- [From(publication date):
December-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11214
- PDF downloads : 945