Research Article Open Access
Building a Community - Academic Partnership to Enhance Hepatitis C Virus Screening
Irvin R1*, McAdams-Mahmoud A1, Hickman D2, Wilson J2, Fenwick W2, Chen I3, Irvin N4, Falade-Nwulia O1, Sulkowski M1, Chaisson R1, Thomas DL1 and Mehta SH51Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, MD, USA
2Sisters Together and Reaching, Incorporated, Baltimore, MD, USA
3Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
4Department of Emergency Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA
5Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
- *Corresponding Author:
- Risha Irvin
MD/MPH, Assistant Professor
Division of Infectious Diseases, Director
Generation Tomorrow, Center for AIDS Research
Johns Hopkins University, 725 N Wolfe Street
Room 218A, Baltimore, MD-21205, USA
Tel: +443-287-4843
Fax: +410-502-7029
E-mail: rirvin1@jhmi.edu
Received date: April 27, 2016; Accepted date: May 20, 2016; Published date: May 30, 2016
Citation: Irvin R, McAdams-Mahmoud A, Hickman D, Wilson J, Fenwick W, et al. (2016) Building a Community - Academic Partnership to Enhance Hepatitis C Virus Screening. J Community Med Health 6:431. doi:10.4172/2161-0711.1000431
Copyright: © 2016 Irvin R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Background: An estimated 3.5 million Americans are chronically infected with hepatitis C virus (HCV). However, the majority are unaware of their HCV diagnosis and few are treated. New models are required to diagnose and link HCV infected patients to HCV care. This paper describes an innovative partnership between Sisters Together and Reaching (STAR), Inc., a community organization, and Johns Hopkins University (JHU), an academic institution, for the identification of HCV cases.
Methods: STAR and JHU identified a mutual interest in increasing hepatitis C screening efforts and launched an HCV screening program which was designed to enhance STAR’s existing HIV efforts. STAR and JHU used the Bergen Model of Collaborative Functioning as theoretical framework for the partnership. We used descriptive statistics to characterize the study population and correlates of HCV antibody positivity were reported in univariable/ multivariable logistic regression.
Results: From July 2014 to June 2015, 325 rapid HCV antibody tests were performed in community settings with 49 (15%) positive HCV antibody tests. 33 of the 49 HCV antibody positive individuals answered questions about their HCV testing history and 42% reported a prior positive result but were not engaged in care and 58% reported that they were unaware of their HCV status. In multivariable analysis, factors that were significantly associated with screening HCV antibody positive were increasing age (AOR: 1.06, 95% CI 1.02-1.10), male sex (AOR: 5.56, 95% CI 1.92-14.29), and history of injection drug use (AOR: 39.3, 95% CI 15.20-101.49).
Conclusions: The community-academic partnership was successful in identifying individuals with hepatitis C infection through a synergistic collaboration. The program data suggests that community screening may improve the hepatitis C care continuum by identifying individuals unaware of their HCV status or aware of their HCV status but not engaged in care and linking them to care.
Keywords
Hepatitis C virus; Screening; Testing
Abbreviations
HCV: Hepatitis C Virus; STAR: Sisters Together and Reaching Inc; JHU: The Johns Hopkins University; CFAR: Center for AIDS Research
Introduction
It is estimated that 3.5 million Americans are chronically infected with hepatitis C virus (HCV) which is the leading cause of liver cancer and liver failure [1]. While HCV is now curable with highly effective all-oral regimens, the vast majority of Americans remain untreated because the HCV care continuum is often broken at the early stages.For example, approximately 50-80% of HCV infected individuals remain undiagnosed and fewer than 20% of those diagnosed have been linked to care [2-4]. Baltimore is an urban center that has the highest per capita prevalence of people who inject drugs (PWID) amongst individuals 15 to 64 years old in the United States, contributing to a large local HCV and HIV epidemic [5]. While there are limited surveillance data available for Baltimore, research studies suggest that the local Baltimore HCV epidemic is similar to national estimates [6,7].
The framework to engage individuals in HCV care is described by the HCV care continuum, analogous to the HIV care continuum. The traditional steps in the HCV care continuum include testing, linkage to and retention in care, treatment, and sustained virologic response (SVR) or “cure” [8,9].The US Action Plan for the Prevention, Care, and Treatment of Viral Hepatitis calls for improvements in the HCV care continuum in order to combat the epidemic of HCV by curing individuals and decreasing HCV transmission [9]. With the emergence of highly efficacious all-oral regimens for HCV, the possibility of curing many individuals is now real. However, to achieve subsequent steps in the care continuum, individuals must first be aware of their infection. Traditionally, most screening for HCV has been done within medical settings; however, many Americans are still unaware of their infection [4].Hence, there is a need to increase community awareness of HCV and seek alternative venues for HCV testing. It is with this knowledge that Sisters Together and Reaching (STAR), Inc., the Johns Hopkins University (JHU) Center for AIDS Research (CFAR), and the Division of Infectious Diseases set out to build a community-academic partnership to enhance HCV detection in the Baltimore community. The model for the HCV community-academic partnership builds off of a strong HIV infrastructure. This paper describes the elements used to build the community-academic partnership between STAR and JHU and the initial outcomes of the HCV testing program.
Methods
Setting
STAR is a federally recognized community and faith-based organization in East Baltimore City founded in 1991 to address the health and social support needs of persons living and affected by the HIV epidemic. The agency provides rapid HIV testing, spiritual support, direct services, and prevention education to the East Baltimore community in office settings, conferences, and on mobile testing units.
The JHU CFAR supports high-priority research on HIV and its coinfections and aims to develop a new generation of HIV/AIDS researchers and recruit under-represented minorities into the HIV/ AIDS field. One of the center’s primary aims is to enhance the university’s capacity to combat the HIV epidemic in Baltimore through training, outreach and community-based intervention studies. In 2013, CFAR and the Division of Infectious Diseases created Generation Tomorrow, a unique training and field experience program for undergraduate and graduate students and community health peers to gain hands-on experience in HIV and HCV counseling, testing, and educational outreach.
Scope of partnership through lens of community engagement in research framework
Since 2013, STAR has been one of Generation Tomorrow’s eight community-based partners and has hosted 13 students and peers for field experiences. STAR has performed community-based HIV outreach and testing for many years and decided upon an expansion into hepatitis C in 2014 through a community-academic partnership with JHU. Both partners share a mutual understanding of the other’s needs, capacities and goals, as stated in the partnership contract signed by both parties at the start of each academic year. Both organizations shared resources and funding throughout the partnership and maintained continuous communication to ensure the various community engagement projects kept moving.
Approach
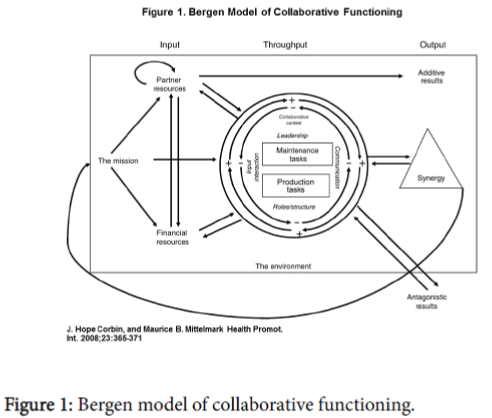
In 2014, leaders in STAR and Generation Tomorrow identified a mutual interest in increasing HCV testing efforts through community engagement. Leadership of both entities came together to start an HCV screening program using the Bergen Model of Collaborative Functioning as the theoretical framework (Figure 1) [10,11]. Both parties believed that combining partner resources, financial resources, and a unifying mission would lead to a more successful program. Both organizations worked closely to develop protocols for an HCV screening program that would be operated by STAR staff with additional funding and personnel support from Generation Tomorrow. This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
The HCV screening and linkage-to-care program was designed to enhance STAR’s existing HIV screening and outreach efforts. The aim of the program was simple: offer each person both an HIV and an HCV antibody test. Generation Tomorrow supplied STAR with interns to support outreach efforts and OraQuick rapid HCV antibody testing kits (Orasure Technologies, Inc., Bethlehem, PA) in which HCV antibody testing was performed on blood collected by finger stick. Individuals were asked subsequent demographic and behavioral questions, including questions about prior HCV testing and injection drug use (IDU). STAR provided invaluable expertise on successful community outreach approaches and recommendations for protocol amendments. All individuals screened for HCV were counseled on HCV transmission and substance use (including alcohol use).
In phase 1, individuals that tested rapid HCV antibody positive were given referrals for appropriate clinical follow up. Some individuals needed insurance while other individuals needed to see their primary care provider to get a referral to hepatitis C specialty care. Case management at STAR assisted individuals with signing up for insurance and with appropriate referrals. When possible (if patients had appropriate insurance and did not need a referral from primary care), patients were linked to the JHU Viral Hepatitis Center for follow-up care. HCV viral load testing was referred to partner sites/ specialty care. From July 2014 to June 2015, STAR facilitated hepatitis C testing efforts in 35 locations throughout Baltimore City including conferences, community block parties, intersections frequented by PWID, churches, shelters, markets, and other community gatherings.
Statistical analysis
Descriptive statistics were used to characterize the study population of individuals agreeing to be tested for hepatitis C with respect to demographic and behavioral characteristics. Differences by HCV status for various demographics were calculated using X2 analysis. Univariable logistic regression was used to assess association between demographic and behavioral characteristics and the outcome of interest, HCV antibody positivity. Correlates of HCV antibody positivity were reported in a univariable and multivariable logistic regression model. All analysis were performed using STATA version 13 (StataCorp, College Station, TX).
Results
This analysis included 325 individuals that were tested for antibodies to HCV through a community-academic partnership between STAR and Generation Tomorrow. Demographic and behavioral characteristics of these individuals are summarized in Table 1. The majority of the individuals were Black/African American (80%), male (62%), and insured (86%). The median age was 45 years (interquartile range 19 to 72), 20% had a history of IDU, and 3% had a positive HIV rapid test or were known to be HIV-positive. Of the 325 individuals tested for HCV antibodies, 49 (15%) were positive by rapid test.
| Characteristic | % Total sample N=325 |
% Testing HCV antibody negative N=276 (85%) |
% Testing HCV antibody positive N=49 (15%) |
P value |
|---|---|---|---|---|
| Age (median)* | 45 | 44 | 52 | <0.001 |
| Race Black/African American White American Indian/Alaskan Native Asian Other Missing |
260 (80%) 40 (12%) 3 (1%) 2 (1%) 8 (2%) 12 (4%) |
221 (80%) 32 (12%) 2 (1%) 2 (1%) 7 (3%) 12 (4%) |
39 (80%) 8 (16%) 1 (2%) 0 (0%) 1 (2%) 0 (0%) |
0.773 |
| Sex Female Male Missing |
120 (37%) 203 (62%) 2 (1%) |
112 (41%) 162 (59%) 2 (1%) |
8 (16%) 41 (84%) 0 (0%) |
<0.01 |
| Insured | 279 (86%) | 236 (86%) | 43 (88%) | 0.632 |
| IDU History (ever) | 65 (20%) | 28 (10%) | 37 (76%) | <0.001 |
| Screened HIV+ or Known HIV + | 11 (3%) | 6 (1%) | 5 (10%) | 0.410 |
| *Age (median): Missing data of 20 participants from total sample, 19 from participants testing HCV antibody negative, and 1 from participants testing HCV antibody positive | ||||
Table 1: Demographic and behavioral characteristics of STAR-JHU Community Outreach testing.
Of the 49 HCV rapid antibody positive individuals, 33 answered subsequent questions about prior HCV testing and 14 (42%) reported a prior positive result and were aware of their HCV status while 19 (58%) reported that they were unaware of their HCV positive status. Of those reporting a prior positive result, none were engaged in HCV specialty care or had been on HCV treatment. In multivariable analysis (Table 2), factors that were significantly associated with testing HCV antibody positive were increasing age (Adjusted odds ratio (AOR): 1.06, 95% CI 1.02-1.10), male sex (AOR: 5.56, 95% CI 1.92-14.29), and history of IDU (AOR: 39.3, 95% CI 15.20-101.49).
| Variable | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Age | 1.05 | 1.03 to 1.08 | 1.06 | 1.02 to 1.10 |
| Male (Ref: Female) | 3.45 | 1.59 to 7.69 | 5.56 | 1.92 to 14.29 |
| IDU History (Ref: No IDU History) | 27.31 | 12.78 to 58.36 | 39.3 | 15.20 to 101.49 |
| Screened HIV+ or Known HIV+ (Ref: HIV negative) | 1.73 | 0.46 to 6.54 | 0.87 | 0.13 to 5.82 |
| Black Race | 0.84 | 0.49 to 1.43 | 1.77 | 0.91 to 3.44 |
Table 2: Correlates of HCV antibody positivity: Results from univariable and multivariable logistic regression of factors associated with testing HCV antibody positive.
Of note, 47 (96%) of the 49 individuals testing HCV antibody positive, would have been identified through screening for the risk factor of IDU history ever and/or birth cohort screening (individuals born between 1945 and 1965), as recommended by the Centers for Disease Control and Prevention and the HCV Guidance Panel. While a higher percentage of individuals screening HCV antibody positive were also HIV-positive as compared to those who were HCV antibody negative (10% versus 1%), this difference was not statistically significant.
In qualitative assessments involving STAR, Generation Tomorrow, and JHU treatment staff, several barriers to HCV care were noted during the HCV testing and linkage process including limited knowledge in the community about hepatitis C and available treatment, the lack of insurance for hepatitis C (versus government sponsored programs such as the Ryan White Care Act for HIV), the requirement of many insurance plans for a referral from a primary care provider to access HCV specialty care, and restrictions placed on hepatitis C treatment based on liver fibrosis stage by both public and private insurance programs. Additionally, in qualitative staff discussions, staff noted that the combined efforts of the organizations led to a synergistic output where we created a hepatitis C screening program that would not have been possible by one entity alone.
Discussion
STAR and JHU have built a successful community-academic partnership to enhance HCV screening in the Baltimore community through integration of HCV screening into established HIV screening infrastructure. This community-academic partnership led to 325 individuals being tested for HCV in phase 1 of the program with 49 (15%) testing positive for HCV antibodies. This seropositivity rate is higher than the general population and suggests that community outreach screening focused on community events, drug treatment centers, intersections frequented by PWID, shelters, churches, and markets in Baltimore is a successful strategy to identify HCV amongst those infected [12]. Moreover, as documented in qualitative discussions, each partner firmly believes that our combined input (partner resources, financial resources, and unifying mission) along with a collaborative throughput with frequent communication and reassessment of protocols led to a synergistic output where we created something that was not otherwise possible by one entity alone using the Bergen Model of Collaborative Functioning (Figure 1) [10,11].
STAR brought years of community engagement, outreach testing, and program development to the table whereas JHU brought technical assistance, additional financial resources, and manpower through Generation Tomorrow. The program also succeeded by building on the existing HIV infrastructure and outreach testing instead of reinventing the process. By increasing awareness and detection of HCV, STAR and JHU are answering the call of the United States Action Plan for the Prevention, Care, and Treatment of Viral Hepatitis and are improving the HCV care continuum [9]. Of the individuals answering subsequent questions about their HCV status, 58% reported that they were unaware of their HCV antibody positive status. While 14 (42%) of the individuals testing positive for HCV antibodies subsequently reported a prior HCV positive result, none were engaged in HCV care or treatment. The fact that all of the HCV antibody positive individuals identified were either unaware of their status or aware of their HCV status but not engaged in care highlights the fact that the HCV care continuum is often broken at the early stages. All individuals testing HCV antibody positive were assisted by case management with signing up for insurance if needed and were referred to primary care or HCV specialty care in phase 1 of the program through partnership agreements.
Factors that were associated with testing HCV antibody positive were increasing age, male sex, and history of IDU. This is consistent with other data from Baltimore and from other cities in the United States [2,7,13]. It has been documented that many older individuals with HCV acquired it years or decades prior [14,15]. This is concerning due to the fact that as time progresses, HCV positive individuals are more likely to develop complications including cirrhosis and liver cancer [1]. These individuals, many in the birth cohort (born between 1945 and 1965), are recommended to be screened by current guidelines due to the high rate of IDU between the 1960s and 1980s [14]. Many in the birth cohort may have used injection drugs only once and may not consider themselves at risk so it is important that education about HCV also be a major community goal. STAR and JHU have incorporated community education at outreach events using creative games and seminars. Additionally, a major function of the counseling session for all is education around prevention, transmission, substance use (including alcohol) and treatment of HCV. As noted, 96% of the 49 individuals screening HCV antibody positive would have been identified through screening for the risk factor of IDU history ever and/or birth cohort screening and the program is considering whether a focused model of HCV screening is warranted for cost effectiveness. However, while birth information is easily obtained, the history of IDU typically only emerged during the counseling sessions while waiting on HCV antibody rapid results and it might be difficult to obtain this history on first approach of an individual on street outreach which would limit the value of this screening method.
The association of HCV infection with male sex is also notable. The community-academic partnership of STAR and JHU has had extensive outreach amongst men in the Baltimore community as it has been shown that men are less likely to engage in healthcare which limits the opportunity to be screened for HCV by a healthcare provider [16]. Therefore, the alternative of community screening may be important in identifying men that are not aware of their HCV antibody positive status. Community screening for HCV and other diseases may also offer a portal to engage men in healthcare by having a trusted community agency involved in the process.
A higher proportion of those testing HCV antibody positive were also HIV-positive compared with those testing HCV antibody negative. It is known that in the United States, about 80% of people with HIV who inject drugs also have HCV and overall 15-30% of HIV positive individuals are thought to be co-infected with HCV [17-22]. This is largely due to their shared mode of transmission of injection drug use. HIV/HCV co-infection is extremely important because HAART has drastically improved morbidity and mortality due to opportunistic infections amongst HIV/AIDS patients, however, HCV has emerged as a significant contributor to morbidity and mortality amongst HIV-positive individuals [20-22]. It will be important to identify HIV-positive individuals and also screen them for HCV to improve their health outcomes. The community-academic partnership is well positioned to do this as the HCV program is an expansion of the HIV framework and individuals are offered both HIV and HCV testing.
When interpreting these results, several methodological challenges exist. First, the sample of patients tested for HCV is a convenience sample in Baltimore and may not reflect the demographic make-up or HCV seroprevalence in other cities. This model is likely only effective at screening in cities where HCV seroprevalence is substantial or where targeted testing is feasible such as testing in areas of high injection drug use. Second, since the patients were not able to be followed across the care continuum, at this point, we do not have RNA testing data to confirm chronic HCV infection and cannot see the final impact on achieving SVR (“cure”). In phase 1 of the program, building HCV testing infrastructure was the focus of the community-academic partnership. Due to financial considerations and at times event logistics, HCV viral load testing was not offered in this phase but with additional funding for phase 2, HCV viral load testing will also be offered and recommended once an individual is found to be positive for HCV antibodies. This confirmatory step being performed at the same time will decrease the chance that individuals fail to get follow up HCV viral load testing and will also allow the program to follow up with anyone that might have cleared HCV. Additionally in phase 1, individuals could not be tracked across the care continuum but for phase 2, we are actively working with HCV treatment facilities to track patients across the care continuum with their permission. This is complicated by the fact that many individuals have to engage in primary care and get a referral from their primary care clinician before they can be seen by a specialist to treat HCV. However, currently in Baltimore, a project funded by the Centers for Disease Control and Prevention and led by the Maryland Department of Health and Mental Hygiene and the Johns Hopkins Division of Infectious Diseases is training primary care clinicians to treat HCV which will help alleviate the barrier of workforce shortage and allow more individuals to benefit from the advances in HCV treatment. Additionally, the JHU Viral Hepatitis Center has dedicated additional resources to this partnership including additional case managers and peer navigators to assist in linkage to care and treatment adherence. It should also be noted that current restrictions on HCV treatment based on liver fibrosis stage by both private and public insurance prevent all patients with chronic HCV from being treated and STAR and JHU are committed to additional advocacy around this topic to ensure treatment for all.
HCV has made a large imprint on Baltimore which is reflective of the high prevalence of IDU in the city. While HCV is the leading cause of liver cancer and liver failure, it is now curable with the emergence of highly active all-oral regimens. However, individuals must be aware of their diagnosis and unfortunately many individuals are not aware of their HCV status which prevents them from benefiting from the advances in HCV treatment. In order to find individuals that are HCV positive, STAR and JHU established a community-academic partnership built on a shared mission and mutual respect. The community-academic partnership between STAR and JHU has been a success and remains an active partnership due to extensive preprogram planning, the establishment of theoretical framework (the Bergen Model of Collaborative Functioning) and continuous communication and program evaluation [10,11]. Each partner brought expertise to the collaboration under a unified mission—to improve the health of Baltimore through enhancing HCV screening—which led to a synergistic collaboration. This model of collaboration may be the key to finding community cases of HCV amongst individuals not screened in traditional healthcare settings or engaged in care.
Acknowledgements
The authors would like to thank the study participants; Sisters Together and Reaching, Inc. leadership and staff; The Johns Hopkins University Center for AIDS Research leadership and staff; The Johns Hopkins Division of Infectious Diseases and the Viral Hepatitis Center; Generation Tomorrow students and peers.
Additional Sources of Funding
Support for this project was provided by the Johns Hopkins Center for AIDS Research and Division of Infectious Diseases and the Gilead Foundation. Additional support was provided to the primary author by the Johns Hopkins University Center for AIDS Research, National Institute of Allergy and Infectious Diseases (1P30AI094189) and the UCLA HIV/AIDS, Substance Abuse, and Trauma Training Program (1R25DA035692-01).
References
- CDC (2015) National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention.
- Thomas DL, Cannon RO, Shapiro CN, Hook EW, Alter MJ, et al. (1994) Hepatitis C, hepatitis B, and human immunodeficiency virus infections among non-intravenous drug-using patients attending clinics for sexually transmitted diseases. J Infect Dis 169:990-995.
- Mehta SH, Astemborski J, Kirk GD, Strathdee SA, Nelson KE, et al. (2011) Changes in blood-borne infection risk among injection drug users J Infect Dis 203: 587-594.
- Denniston MM, Klevens RM, McQuillan GM, Jiles RB (2012) Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology 55: 1652-1661.
- Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, et al. (2008) Estimating the prevalence of injection drug users in the U.S and in large U.S metropolitan areas from 1992 to 2002 J Urban Health 85: 323-351.
- Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, et al. (2006) Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS 20: 2361-2369.
- Falade-Nwulia O, Irvin R, McAdams MA, Mehta SH, Niculescu A, et al. (2016) Senior Center-Based Hepatitis C Screening in Baltimore. Open forum infectious diseases 3: ofv217.
- Yehia BR, Schranz AJ, Umscheid CA, Lo Re (2014) The treatment cascade for chronic hepatitis C virus infection in the United States: A systematic review and meta-analysis. PLoS ONE 9.
- Combating the silent Epidemic of Viral Hepatitis (2015) The US Action Plan for the Prevention, Care, and Treatment of Viral Hepatitis.
- Corbin JH, Mittelmark MB (2008) Partnership lessons from the Global Programme for Health Promotion Effectiveness: a case study. Health PromotInt23: 365-371.
- Corbin JH, Fernandez ME, Mullen PD (2015) Evaluation of a community-academic partnership: lessons from Latinos in a network for cancer control. Health PromotPract16: 345-353.
- Ditah I, Ditah F, Devaki P, Ewelukwa O, Ditah C, et al. (2014) The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. Jhepatol 60: 691-698.
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, et al. (2008) Limited uptake of hepatitis C treatment among injection drug users. J Community Health 33: 126-133.
- Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, et al. (2012) Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR 61: 1-18.
- Smith BD, Yartel AK, Brown KA, Krauskopf K, Massoud OI, et al. (2014) Effectiveness of hepatitis C virus (HCV) testing for persons born during 1945-1965-summary results from three randomized controlled trials. Hepatology 60:295A.
- Sandman DS, Elisabeth S, Christina A (2000) Out of Touch: American Men and the Health Care System. Commonwealth Fund Men’s and Women’s Health Survey Findings.
- CDC (2015) HIV/AIDS and Viral Hepatitis.
- Sulkowski MS, Thomas DL (2003) Hepatitis C in the HIV-Infected Person. Ann Intern Med 138: 197-207.
- Sherman KE, Rouster SD, Chung RT, Rajicic N (2002) Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials GroupClin Infect Dis 34: 831-837.
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England j med338: 853-860.
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, et al. (2001) Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 32: 492-497.
- Monga HK, Rodriguez BMC, Breaux K, Khattak K, Troisi CL, et al. (2001) Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis 33: 240-247.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 10821
- [From(publication date):
June-2016 - Apr 06, 2025] - Breakdown by view type
- HTML page views : 9992
- PDF downloads : 829