BTG1 Low Expression in Pancreatic Ductal Adenocarcinoma is Associated with a Poorer Prognosis
Received: 24-Mar-2017 / Accepted Date: 31-Mar-2017 / Published Date: 08-Apr-2017 DOI: 10.4172/2476-2024.1000126
Abstract
Objective: BTG1 is a member of the TOB/BTG protein family, which is a transducer of ErbB-2 and TOB2. BTG1 is known to inhibit tumor genesis, but the role of it in pancreatic ductal adenocarcinoma is still unknown. The purpose of this study is to investigate the expression of BTG1 protein in pancreatic ductal adenocarcinoma (PDAC) and to determine its prognostic significance.
Methods: Immunohistochemistry is used to determine the protein expression level of BTG1 gene in 79 surgically resected pancreatic ductal adenocarcinoma. Association of BTG1 expression with all the patients’ clinicopathologic parameters was analyzed using statistical software SPSS22.0. The correlations between BTG1 expression and clinicopathological features were evaluated by Pearson’s chi-square (χ2) test, Fisher’s exact test, and Spearman’s rank. Univariate and multivariate Cox regression analyses were used to identify correlations between the immunohistochemical data for BTG1 expression and the clinicopathologic characteristics in pancreatic ductal adenocarcinoma. Kaplan-Meier analysis was used to demonstrate the correlation between overall survival and the expression of BTG1.
Results: BTG1 positive expression was observed in 27.8% (22/79) of the PDAC tissues, which was significantly lower than the 58.2% (46/79) of corresponding normal adjacent non-cancerous tissues by immunohistochemical staining (P<0.001). Through the stratified analysis, we found that significant difference of BTG1 expression in PNI (P=0.002), T stage (P=0.000), N stage (P=0.018) and TNM stage (P=0.000). Univariate and multivariate Cox analysis revealed that BTG1 expression status was an independent prognostic factor in pancreatic ductal adenocarcinoma (P=0.027). Moreover, overall survival was better in PDAC cases with positive than negative BTG1 expression (P=0.027).
Conclusion: This study demonstrated for the first time that lower expression of BTG1 might be involved in the progression of pancreatic ductal adenocarcinoma, suggesting that BTG1 might be a novel prognostic marker and target for therapy.
Keywords: Pancreatic ductal adenocarcinoma; BTG1; Prognosis; Immunohistochemistry; Marker; Overall survival; Target
7338Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers. In spite of its incidence rate, it is the tenth in the western countries; PDAC is still the fourth leading cause of cancer-related death in the western countries [1]. Totally, the 5-year overall survival (OS) rate of PDAC is about 5% around the world, which indicates its prognosis is very poor [1]. Although radical surgical resection of the primary tumor is the only potentially curative option in patients with PDAC, only 15~20% of PDAC patients are suitable for surgery because most PDAC patients are difficult to be detected at an early stage and they often were diagnosed only after regional invasion or distant metastasis [2]. Even among patients whose diseases are surgically respectable, at least 80% of these cases will subsequently develop local recurrences or distant metastases within 2 years of surgery [3]. PDAC is almost resistant to all available radiotherapies and chemotherapies, so we aim to search a new molecular target which will contribute to early diagnosis and prognostic judgment of PDAC.
BTG1, which named B cell translocation gene 1, is a member of the TOB/BTG protein family, who contains six proteins (BTG1, BTG2/ TIS21/PC3, BTG3, BTG4/PC3B, Transducer of ErbB-2 and TOB2). All members of this family can inhibit cell proliferation, inhibit cell cycle progression and stimulate cellular differentiation in different kinds of cells [4]. Human BTG1, which is localized on chromosome 12q22, is initially discovered in a case of Bcell chronic lymphocytic leukemia and recognized as a translocation partner of the cMyc gene, so named B cell translocation gene 1 [5,6]. BTG1 expression varies with the progression of cell cycles, it is highest in the G0/G1 phases and decreased when cells progress through G1 [4]. Previous research also showed that BTG1 overexpression can reduce vascular endothelial growth factor expression in tumors and contribute to anti-sense Bcl-2 induced cytotoxic effects in apoptotic cells [7,8].
Previous studies have showed that BTG1 is down regulated in multiple cancers,such as breast cancer [9], NSCLC [10], gastric cancer [11], ovarian cancer [12], liver carcinoma [13]. These results consistently showed that BTG1 overexpression decreases proliferation, migration and invasion, which suggests that BTG1 overexpression may contribute to a better prognosis.
Above all, BTG1 may be a candidate for tumor suppressor genes. However, the relation between BTG1 and clinicopathological features and prognostic factors in PDAC have not been reported. Therefore, we performed immunohistochemistry on 79 PDAC tissues, and then examined the association of BTG1 expression level and patient overall survival and clinicopathological features in PDAC.
Materials And Methods
Patients and tissue samples
We collected 79 formalin-fixed paraffin-embedded tissue samples of patients with pathologically diagnosed pancreatic ductal adenocarcinoma who had undergone surgical resection at Fuzhou General Hospital of Nanjing Military Command between January 2005 and December 2014. We excluded patients receiving any preoperative anticancer therapies. We included only patients who had survived at least 60 days after surgery to exclude any bias related to perioperative mortality. The clinicopathologic features of each patient was carefully collected from the clinical database, including sex, age, tumor size, tumor site, histological differentiation, PNI, surgical margin, T stage, N stage and TNM stage. We got the overall survival data via one by one telephone follow-up. Follow-up data were updated on September 21, 2015. Median follow-up time was 10.0 months (range: 1.0 month to 114 months). Moreover, pathologic tumor staging was performed according to the American Joint Committee on Cancer (AJCC) TNM classification, 7th edition [14].
Our study was approved by the ethical committee of Fuzhou General Hospital of Nanjing Military Command and each patient signed an informed consent form before enrollment into the study to obtain the use of clinical specimens.
Immunohistochemistry
To detect the expression of BTG1 in PDAC, we used formalin-fixed and paraffin-embedded tumor tissue cut into 4 μm thick sections. The immunohistochemical analysis was performed using Elivision method. After cutting into 4 μm thick sections of paraffin-embedded tissue were incubated at 37°C overnight. Put simply, tissue sections were deparaffinized in xylene and rehydrated in graded alcohol and use citrate buffer (PH 6.0) in a pressure cooker for 2 min to retrieve antigen. Endogenous peroxidase blocking was performed at room temperature for 10 min with 3 % hydrogen peroxide, and then washed three times with 0.01 mM PBS. Then, the sections were incubated with goat anti-human BTG1 polyclonal antibody (1:100 dilutions, no. sc-18540, Santa Cruz Biotechnology, Inc) in a wet box at room temperature for 1 h. After twice 0.01 mM PBS washing, the secondary antibody (PV polymer HRP anti-goat IgG, ZSGB-BIO) was put into the wet box for incubation for 30 min and then washed three times with 0.01 mM PBS, followed by DAB staining for 5 min. The hematoxylin stain can make DAB staining visualized, the last step is dehydration with the coverslip sealed. PBS as the primary antibody was used to be a negative control.
The immunohistochemical scoring was performed independently by two pathologists without knowing patients’ information. Five representative fields of each section were randomly selected to be counted under microscope. Cytoplasm labeling with the BTG1 antibody was regarded as positive cell. The BTG1 expression level was defined by the staining index (SI) which was calculated based on the percentage of positive tumor cells multiplied by the staining intensity. The percentage of positive tumor cells was graded as follows: 0=1-5%; 1=5–25%; 2=25–50%; 3 ≥ 50%. The intensity of staining was classified as: 0=no staining; 1=weak staining (light yellow); 2=intermediate staining (brown); and 3=strong staining (yellowish-brown). The total points of BTG1 expression was the product of the BTG1 positivity and staining intensity, graded as 0 for negative, + to +++ for positive(+=1– 2; ++=3–5; +++=6–9) [9,11].
Statistical analysis
All data were analyzed using SPSS22.0 software and p<0.05 was considered statistically significant. The relation between various variables and BTG1 expression in cancer tissue was analyzed by Chi-square test. Comparison of BTG1 expression status with overall survival in different groups of PDAC patients was performed using a log-rank correlation test and Cox regression test. Clinicopathologic variables with P<0.05 on univariate analysis were selected in the multivariate Cox regression analysis. OS distributions were estimated using the Kaplan–Meier method. The overall survival was defined as the time from the date of confirmed diagnosis to death or the date of last telephone follow-up.
Results
BTG1 protein expression in PDAC tissues and adjacent noncancerous tissues
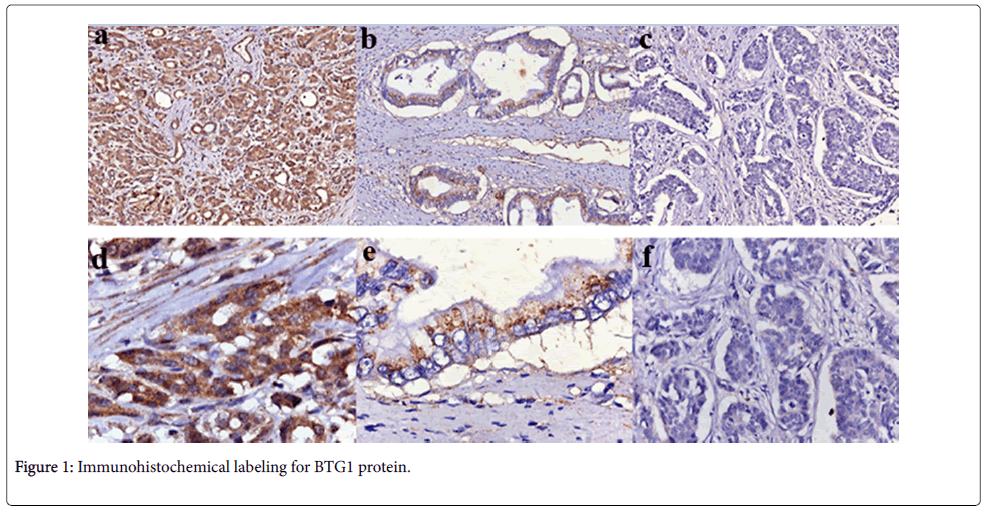
BTG1 protein was positively detected in the cytoplasm of adjacent non-cancerous pancreas tissues, the staining was strong. But in most of the PDAC tissues, cells staining were weak or negative, especially in the poor-differentiated PDAC which is shown in Figure 1. On the basis of the immunohistochemical scoring, we found significantly lower rates of BTG1 positive expression in PDAC tissues (27.8%) compared with corresponding normal adjacent non-cancerous tissues (58.2%) (P<0.001, Table 1).
| Groups | n | BTG1 expression Positive Negative |
PR(%) | P | |
|---|---|---|---|---|---|
| Normal tissue | 79 | 46 | 33 | 58.2 | <0.001 |
| PDAC tissue | 79 | 22 | 57 | 27.8 | |
| *PR: Positive Rate | |||||
Table 1: BTG1 expression in pancreatic cancer tissue and in normal tissue.
The relationship between BTG1 expression and clinicopathological parameters of PDAC
We analyzed the BTG1 expression difference according to various variables using Chi-square test, including sex, age, tumor site, tumor size, histological differentiation, PNI, surgical margin, T stage, N stage, TNM stage. Our results showed that BTG1 expression was statistically correlated with PNI (P=0.002), T stage (P=0.000), N stage (P=0.018) and TNM stage (P=0.000) (Table 2). However, no significant correlations were found with sex, age, tumor site, tumor size, histological differentiation and surgical margin (Table 2).
| Variables | n | BTG1 expression in cancer tissues positive (%) negative(%) |
P* | |
|---|---|---|---|---|
| Sex | P=0.290 | |||
| Male | 54 | 17(31.5%) | 37(68.5%) | |
| Female | 25 | 5(20.0%) | 20(80.0%) | |
| Age | P=0.247 | |||
| <57 | 37 | 8(21.6 %) | 29(78.4%) | |
| ≥57 | 42 | 14(33.3%) | 28(66.7%) | |
| Tumor site | P=0.943 | |||
| Head | 57 | 16(28.1%) | 41(71.9%) | |
| Non-head | 22 | 6(27.3%) | 16(72.7%) | |
| Tumor size | P=0.183 | |||
| ≤2cm | 52 | 17(32.7%) | 35(67.3%) | |
| >2cm | 27 | 5(18.5%) | 22(81.5%) | |
| Histological differentiation | P=0.738 | |||
| Poor | 30 | 9(30%) | 21(70.0%) | |
| Well-Moderate | 49 | 13(26.5%) | 36(74.5%) | |
| PNI | P=0.002 | |||
| Present | 44 | 6(13.6%) | 38(86.4%) | |
| Absent | 35 | 16(45.7%) | 19(54.3%) | |
| Surgical margin | P=0.126 | |||
| R0 | 76 | 20(26.3%) | 56(73.7%) | |
| R1 | 3 | 2(66.7%) | 1(33.3%) | |
| T stage | P=0.000 | |||
| T1-2 | 32 | 17(53.1%) | 15(46.9%) | |
| T3-4 | 47 | 5(10.6%) | 42(89.4%) | |
| N stage | P=0.018 | |||
| N0 | 37 | 15(40.5%) | 22(59.5%) | |
| N1 | 42 | 7(16.7%) | 35(83.3%) | |
| TNM Stage | P=0.000 | |||
| I-II | 30 | 19(63.3%) | 11(36.7%) | |
| III-IV | 49 | 3(6.1%) | 46(93.9%) | |
| *Chi-square test; Age: Mean age; PNI: Peri-Neural Invasion |
||||
Table 2: Summary of relation between BTG1 expression and clinicopathological parameters.
Relationship between BTG1 expression and prognosis in PDAC patients
We collected survival data of all the patients via telephone followup, there were only 6 patients still alive and 73 patients were dead at the end of our follow-up. According to different BTG1 expression level in 79 PDAC tissues, patients were divided into two groups: including 22 BTG1 positive expression patients and 57 BTG1 negative expression patients. We performed univariate and multivariate analyses to identify the relative risk of prognostic factors for the 79 PDAC cases (Table 3). Univariate analysis showed that there are six significant prognostic factors correlated with poor prognosis, including histological differentiation (poor differentiation), T stage (T3-4), N stage (N1), peri-neural invasion present, III-IV stage, BTG1 negative expression (Table 3). The Cox multivariate analysis indicate that histological differentiation (hazard ratio 2.342, 95% confidence interval 1.417-3.873, P=0.001) and BTG1 expression status (2.331, 95% confidence interval 1.1044.924, P=0.027) were all independent prognostic factors for poor overall survival in patients with PDAC (P<0.05, Table 3).
| Variables | OS(Univariate) Median±SE 95%CI |
P* | HR | OS(Multivariat) 95%CI |
P# | |
|---|---|---|---|---|---|---|
| Sex | 0.725 | |||||
| Male | 12.75±1.93 | 8.96-16.55 | ||||
| Female | 11.88±2.69 | 6.59-17.17 | ||||
| Age | 0.944 | |||||
| <57 | 12.56±2.33 | 7.99-17.11 | ||||
| ≥57 | 11.73±1.79 | 8.22-15.25 | ||||
| Histological differentiation | 0.003 | |||||
| Poor | 7.40±1.10 | 5.26-9.54 | 2.342 | 1.417-3.873 | 0.001 | |
| Well-Moderate | 14.84±2.18 | 10.58-19.11 | 1 | |||
| Tumor size | 0.081 | |||||
| ≤2cm | 14.59±2.30 | 10.07-19.12 | ||||
| >2cm | 8.40±1.01 | 6.43-10.28 | ||||
| T stage | 0.001 | |||||
| T1-2 | 18.15±3.34 | 11.60-24.70 | 0.824 | 0.381-1.783 | 0.623 | |
| T3-4 | 8.28±0.78 | 6.76-9.80 | 1 | |||
| N stage | 0.014 | |||||
| N0 | 16.67±2.94 | 10.91-22.44 | 0.735 | 0.408-1.324 | 0.305 | |
| N1 | 8.42±0.91 | 8.27-14.4 | 1 | |||
| PNI | 0.008 | |||||
| Present | 8.43±0.85 | 6.75-10.11 | 1.305 | 0.777-2.191 | 0.315 | |
| Absent | 16.24±2.86 | 10.63-21.84 | 1 | |||
| Surgical margin | 0.703 | |||||
| R0 | 12.44±1.64 | 9.23-15.65 | ||||
| R1 | 12.33±3.93 | 4.63-20.03 | ||||
| TNM Stage | 0.001 | |||||
| I-II | 18.9±3.52 | 11.99-25.80 | 1 | |||
| III-IV | 8.22±0.75 | 6.75-9.70 | 1.009 | 0.400-2.547 | 0.984 | |
| BTG1 expression | 0.000 | |||||
| BTG1(+) | 22.36±4.50 | 13.54-31.19 | 1 | |||
| BTG1(-) | 8.52±0.79 | 6.96-10.09 | 2.331 | 1.104-4.924 | 0.027 | |
| *log-rank test; #Cox regression test |
||||||
Table 3: Overall survival in patients with pancreatic cancer according to multiple variables on univariate and multivariate analysis.
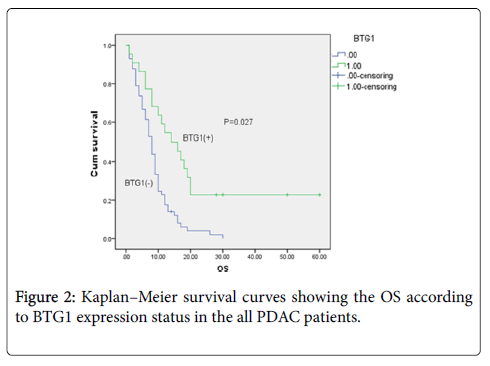
Survival analysis was performed in all the patients on the basis of their overall survival and corresponding BTG1 expression status. Our results showed that the median survival time in the negative BTG1 expression group (8.000 ± 0.749 months, n=57) was significantly shorter than that in the positive BTG1 expression group (14.000 ± 3.518months, n=22) (P=0.027, Table 4).
| BTG1 Expression | Median Survival Time(Months) | SE | P* Value |
|---|---|---|---|
| Positive (n=22) | 14.000 | 3.518 | P=0.027 |
| Negative (n=57) | 8.000 | 0.749 | |
| *log-rank test | |||
Table 4: BTG1 expression and median survival time in PDAC patients.
Patients with negative BTG1 expression in PDAC samples had a significantly lower overall survival than those with positive BTG1 expression (P=0.027, Figure 2). These results suggested that patients with negative BTG1 expression have a worse prognosis than those with positive BTG1 expression.
Discussion
Despite the steady increase in survival for different kinds of cancer in the last decades, the 5-year survival rate of pancreatic cancer is still less than 5% [1].This is mainly because only 15~20% of patients have the chance to receive surgical resection at the time of diagnosis and it’s almost resistant to all available chemotherapy and radiation therapies [15]. For pancreatic cancer, the prediction value of some prognostic indicators that had been known, such as tumor size, histological differentiation, lymph node metastases, may not be obvious [16,17]. The most common pathological type of pancreatic cancer is pancreatic ductal adenocarcinoma (over 90%), which originates from the exocrine cells [18]. It is in need of finding a new way to early detect and treat the PDAC. Furthermore, for patients with PDAC, they are deeply concerned about the prognosis of the disease. TNM-staging is known as the preferred prognostic indicator. However, different cancer patients’ prognosis differ considerably even when they are at the same TNM stage. Therefore, it is important for us to find a significant marker, which will contribute to predict tumor patients’ private outcomes.
BTG1 expression is barely detectable in brain and muscle tissues which are fully differentiated, but it will be detectable in cells which can still respond to various signals, especially in G0/G1 transition [19,20]. The expression of BTG1 is highest in the G0/G1 phases of the cell cycle and is decreased when cells progress through G1 [4], so BTG1 overexpression can inhibit cell proliferation and cell cycle progression, increase cell apoptosis, reduce vascular endothelial growth factor expression in tumors [19].
According to previous studies, we can draw some useful conclusions about BTG1. BTG1 overexpression was considered as a marker for favorable prognosis in breast, non-small cell lung cancer, gastric cancer, ovarian carcinoma, hepatocellular carcinoma, nasopharyngeal cancer, esophageal squamous cancer and thyroid cancer [9-13,20]. Zheng HC et al. indicated that BTG1 expression is associated with the worse prognosis in gastric cancer patients by inhibiting proliferation, enhancing autophagy and apoptosis [11]. Zheng HC et al. also suggested that lower BTG1 expression might promote gastric tumorigenesis partly due to its promoter methylation [11]. Scheijen B et al. also indicated that BTG1 is a tumor suppressor gene in leukemia, when deleted, strongly enhances the IKZF1-deleted B-cell precursor acute lymphoblastic leukemia relapse risk [21]. Our results are consistent with recent researches that BTG1 may be a tumor suppressor gene.
Our study is the first instance which searches BTG1 expression in PDAC tissues using immunohistochemistry. The results showed that BTG1 expression levels were significantly lower in PDAC tissues than in normal adjacent non-cancerous tissues (P<0.001, Table 1). We found that BTG1 expression level was correlated with PNI (P=0.002), T stage (P=0.000), N stage (P=0.018) and TNM stage (P=0.000). According to univariate and multivariate analysis, lower BTG1 expression (P=0.027) and poor differention (P=0.001) may predict decreased overall survival. We drew a conclusion that tumoral BTG1 loss may contribute to the development and progression of PDAC.
Our study revealed that the overall survival is significantly higher in positive BTG1 expression groups than negative BTG1 expression groups. Consequently, combining BTG1 expression status with clinical stage may help clinicians to decide treatment plans and to predict prognosis.
Conclusion
This study demonstrated for the first time that lower expression of BTG1 was correlated with poor survival in pancreatic ductal adenocarcinoma patients, which suggests that BTG1 expression may serve as an available prognostic biomarker and a novel molecular in the future. However, there are still some defects in our study, more large population-based studies and long-term follow-up are still needed to support our findings. A potential signal mechanism about down-regulated of BTG1 in pancreatic ductal adenocarcinoma is still needed in the future, and we will conduct in our further study.
Conflict of interest
Not declared.
Funding
This work was funded by grants from the National Natural Science Foundation of China (No.81502360) and Natural Science Foundation of Fujian Province (No.2016J01576 and No.2016J01586 and No. 2017J05121).
References
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics. CA Cancer J Clin 65: 5-29.
- Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, et al. (2013) Recent progress in pancreatic cancer. CA Cancer J Clin 63: 318-348.
- Stathis A, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev ClinOncol 7: 163-172.
- Winkler GS (2010) The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol 222: 66-72.
- Rimokh R, Rouault JP, Wahbi K, Gadoux M, Lafage M, et al. (1991) A chromosome 12 coding region is juxtaposed to the MYC protooncogene locus in a t(8;12)(q24;q22) translocation in a case of B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer 3: 24-36.
- Rouault JP, Rimokh R, Tessa C, Paranhos G, Ffrench M, et al. (1992) BTG1, a member of a new family of antiproliferative genes. EMBO J 11: 1663-1670.
- Corjay MH, Kearney MA, Munzer DA, Diamond SM, Stoltenborg JK, et al. (1998) Antiproliferative gene BTG1 is highly expressed in apoptotic cells in macrophage-rich areas of advanced lesions in Watanabe heritable hyperlipidemic rabbit and human. Lab Invest 78: 847-858.
- Nahta R, Yuan LX, Fiterman DJ, Zhang L, Symmans WF, et al. (2006) B cell translocation gene 1 contributes to antisense Bcl-2-mediated apoptosis in breast cancer cells. Mol Cancer Ther 5: 1593-1601.
- Sheng SH, Zhao CM, Sun GG (2014) BTG1 expression correlates with the pathogenesis and progression of breast carcinomas. Tumour Biol 35: 3317-3326.
- Sun GG, Lu YF, Cheng YJ, Hu WN (2014) The expression of BTG1 is downregulated in NSCLC and possibly associated with tumor metastasis. Tumour Biol 35: 2949-2957.
- Zheng HC, Li J, Shen DF, Yang XF, Zhao S, et al. (2015) BTG1 expression correlates with pathogenesis, aggressive behaviors and prognosis of gastric cancer: a potential target for gene therapy. Oncotarget 6: 19685-19705.
- Urzúa U, Roby KF, Gangi LM, Cherry JM, Powell JI, et al. (2006) Transcriptomic analysis of an in vitro murine model of ovarian carcinoma: functional similarity to the human disease and identification of prospective tumoral markers and targets. J Cell Physiol 206: 594-602.
- Sun GG, Lu YF, Cheng YJ, Yang CR, Liu Q, et al. (2014) Expression of BTG1 in hepatocellular carcinoma and its correlation with cell cycles, cell apoptosis, and cell metastasis. Tumour Biol 35: 11771-11779.
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17: 1471-1474.
- Garrido-Laguna I, Hidalgo M (2015) Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 12: 319-334.
- Tempero MA, Arnoletti JP, Behrman S, Ben-Josef E, Benson AB, et al. (2010) Pancreatic adenocarcinoma. J Natl Compr Canc Netw 8: 972-1017.
- Zhang LJ, Wang KB, Liu LS, Chen LZ, Peng BG, et al. (2014) Overexpression of GOLPH3 is associated with poor prognosis and clinical progression in pancreatic ductal adenocarcinoma. BMC Cancer 14: 571.
- Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, et al. (2015) Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 15: 8-18.
- Rouault JP, Rimokh R, Tessa C, Paranhos G, Ffrench M, et al. (1992) BTG1, a member of a new family of antiproliferative genes. EMBO J 11: 1663-1670.
- Pramesh CS, Mistry RC, Jambhekar NA, Laskar SG (2006) Does the TNM staging system for esophageal cancer need revision. J Am Coll Surg 202: 855-856.
- Scheijen B, Boer JM, Marke R, Tijchon E, van IngenSchenau D, et al. (2017) Tumor suppressors BTG1 and IKZF1 cooperate during mouse leukemia development and increase relapse risk in B-cell precursor acute lymphoblastic leukemia patients. Haematologica 102: 541-551.
Citation: Huang Y, Zheng J, Tan T, Song L, Huang S, et al. (2017) BTG1 Low Expression in Pancreatic Ductal Adenocarcinoma is Associated with a Poorer Prognosis. Diagn Pathol Open 2: 126. DOI: 10.4172/2476-2024.1000126
Copyright: © 2017 Huang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4020
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 3130
- PDF downloads: 890