Broad Humoral Immunity Generated in Mice by a Formulation Composed by Two Antigens from Delta Variant of SARS-CoV-2
Received: 27-Oct-2022 / Manuscript No. JIDT-22-76240 / Editor assigned: 31-Oct-2022 / PreQC No. JIDT-22-76240 (PQ) / Reviewed: 14-Nov-2022 / QC No. JIDT-22-76240 / Revised: 10-Feb-2023 / Manuscript No. JIDT-22-76240 (R) / Published Date: 17-Feb-2023
Abstract
Study background: Due to the rapid development of new variants of SARS-CoV-2 virus, as well as the real threat of new Coronavirus zoonosis events, the development of a preventive vaccine with a broader scope of functionality is highly desirable. Previously, we reported the functionality of a nasal formulation based on the preparation of the nucleocapsid protein with the ODN-39M and combined with RBD, both antigens from Delta variant of SARS-CoV-2. This combination induces a cross reactive immunity in mucosal and systemic compartments until sarbecovirus level. In the present study, we explored the magnitude of the immunity generated in Balb/C mice by the same formulation, but adding alum as adjuvant, so as to enhance the humoral immunity against the two antigens.
Methods: Animals were immunized with three doses of the bivalent formulation, administered by subcutaneous route. The humoral immunity was tested by ELISA and by a surrogate of viral neutralization test. The cell mediated immunity was also explored.
Results: High levels of antibodies against both antigens (N and RBD) were obtained upon immunization. Additionally, the anti-RBD Abs with neutralizing capacity reacted against the three SARS-CoV-2 variants of RBD assayed, including omicron. At the same time, the Abs also recognized the nucleocapsid proteins from: SARSCoV- 1 and SARS-CoV-2 delta and omicron.
Conclusion: Taken together, these results make the bivalent formulation tested, an attractive component of a pan Corona vaccine able to broaden the scope of humoral immunity against both antigens. This will be particularly important in the reinforcement of immunity from previously vaccinated and/or infected populations.
Keywords: SARS-CoV-2; Bivalent vaccine; Subcutaneous; Cross reactivity; Delta variant; Neutralizing Abs
Introduction
SARS-CoV-2 virus has resulted in several variants and sub variants which have provoked different waves of infections across the globe [1]. On the other side, SARS-CoV-2 infection is the third zoonosis related to human lethal coronaviruses after SARS-CoV in 2002 and MERS-CoV in 2012 [2]. Altogether it highlights the importance to develop a new generation of vaccines with broader scope of protection [3,4]. Generally, the current approved vaccines are based on the spike protein which is a variable antigen among Coronavirus with a limited functionality against the emerging new variants [5].
The Nucleocapsid (N) is a conserved protein among Coronaviruses which has emerged as an attractive antigen to compose a novel generation of multivalent vaccines [6]. The study published by Matchett demonstrated that N protein, presented in the Ad5 platform, protected mice against SARS-CoV-2 challenge [7]. Dangi proved that addition of the N protein in a spike vaccine formulation, improved distal protection in mouse brain [8]. In turn, the N protein is already contained in the registered COVID-19 vaccine in India called RelCovax®. The vaccine is composed by the mixture of RBD and N protein with alum and CpG ODN-1018 as adjuvants and was able to induce, in mice, humoral and cell mediated immunity against both antigens [9].
Our group has also been involved on the development of bivalent formulations based on the same antigens. Nevertheless, there are two differences compared to RelCovax®: our antigens are based on delta variant and the CpG ODN used in our preparations has a phosphodiester backbone, different to the all phosphorothioate ODNs used so far in humans, which have been associated to some safety issues due to this chemical modification. In our previous study, the combination of recombinant N protein with the ODN-39M, favored the formation of aggregate conformations. The resulting preparation, administered by intranasal route, induced anti-N Cell Mediated Immunity (CMI) in spleen and humoral immunity in both, sera and lungs. Importantly, the overall immunity generated was cross reactive against N protein from SARS-CoV-2 omicron variant and SARSCoV- 1. Furthermore, the nasal bivalent formulation, based on N +ODN-39M preparation and combined with RBD delta protein, enhanced the local and systemic immune response against RBD with a modulation toward a Th1-like pattern [10].
In the present study we explored the same delta based bivalent formulation, now administered by subcutaneous route and adding alum as adjuvant to systemically potentiate the induction of neutralizing Abs. As a result, high levels of antibodies against both antigens were obtained. Additionally, the anti-RBD Abs with neutralizing capacity reacted against the three SARS-CoV-2 variants of RBD assayed: Ancestral, delta and omicron. At the same time, the Abs also recognized the three nucleocapsid proteins tested (SARSCoV-2 delta and omicron and SARS-CoV-1).
Materials and Methods
Recombinant proteins, peptide and ODN-39M
The recombinant antigens were purchased from Sino biological Inc. (China). N proteins from: SARS-CoV-2, delta (40588-V07E29) and omicron (40588-V07E34) variants and SARS-CoV-1 (40143-V08B). RBD proteins from SARS-CoV-2: Delta (40592-V08H90), ancestral (40592-VNAH) and omicron (40592-V08H123) variants and ACE-2-His (10108-H08B).
The peptide N351-365 from SARS-CoV-2 (ILLNKHIDAYKTFPP) was synthesized with ≥ 97% purity by Zhejiang peptides biotech (China).
The ODN-39M, a 39 mer, whole phosphodiester backbone ODN (5’-ATC GAC TCT CGA GCG TTC TCG GGG GAC GAT CGT CGG GGG-3’), was synthesized by Sangon biotech (China).
In vitro aggregation procedure of N protein with ODN-39M
The N protein from SARS-CoV-2, delta variant, was subjected to in vitro aggregation, as previously described with few modifications. Briefly, in a 100 μL of reaction, 40 μg of N protein were mixed with 60 μg of ODN-39 M in 10 mMTris, 6 mM EDTA (pH 6.9) buffer [11]. The mixture was incubated for 30 min at 30°C in water bath and after was stored at 4°C for 4 hours. Finally, the preparation was centrifuged at 14,000 x g for 10 min. The supernatant was collected and tested for protein concentration.
Source of human sera
Human sera from COVID-19 convalescent (N=10) and negative (N=10) individuals were collected at the eighth and ninth people’s hospital of Dongguan city (Guangdong province, China). The study protocol was approved by the institutional ethics committee from both hospitals and was carried out in accordance with the principles of Helsinki declaration. Informed written consent was obtained from each participant before enrolment in the study. Blood samples were collected between April-May 2022 during an outbreak of omicron variant in Guangdong, China. SARS-CoV-2 patients confirmed by real time polymerase chain reaction and individuals with no history of SARS-CoV-2 infection neither vaccination voluntarily donor the blood. The serum was obtained by centrifugation, inactivated by incubation at 56°C for 30 min in water bath and stored at 20°C until use.
Antigenic characterization using human sera positive to SARS-CoV-2
Ninety six well high binding polystyrene plates (Costar, USA) were coated with 3 μg/mL of N protein delta variant, aggregated or not with ODN-39M, in sodium carbonate sodium bicarbonate buffer and incubated overnight at 4°C. Unspecific binding of the antibodies was avoided by blocking with 5% skimmed milk (Oxoid, USA) 1 h at 37°C. After 5 times washing with Phosphate Buffered Saline with 0.05% Tween 20 (PBST), 100 μL of appropriately diluted serum sample in 2% milk PBST were added and incubated for 2 hour at 37°C. After washing 5 times with PBST, bound antibodies were detected with the secondary antibodies conjugated with horseradish peroxidase of goat anti-human IgG (1:20000). After incubation for 1 hour at 37°C and five PBST washes, 100 μL of OPD substrate solution were added to each well and the mixture was incubated for 10 minutes at room temperature. The reaction was stopped by adding 0.2 N sulfuric acid to the mixture and the Optical Density (OD) at 492 nm was measured. The data is represented as OD measures.
Immunization experiments
Adult (6 to 8 weeks old) females Balb/c mice (inbred, H-2d) were housed at Beijing vital river laboratory animal technology Co, Ltd. The standard of laboratory animal room complied with the national standard of the people's republic of China GB14925-2010. All the experimental protocols were approved by the institutional animal care and use committee.
Groups of six to eight animals each were immunized with three doses, administered on days 0, 7 and 21 by subcutaneous (sc) route. Formulations were prepared with aluminum hydroxide (Alum; Alhydrogel, Invitrogen), as adjuvant, at a final concentration of 1.4 mg/ ml. A dose of 10 μg, of each protein per mouse, was evaluated. All the immunogen were dissolved in sterile PBS. The immunogen was administered in a final volume 100 μL in each experiment, a placebo immunized group was included as control.
Animals were randomly assigned to four groups. Group 1 receiving N (Delta)+ODN-39M (NO), group 2 was immunized with RBD (Delta), group 3 was immunized with NO+ RBD and group 4 received PBS; all adjuvated with alum as previously described. Blood extraction was carried out at 12 and 18 days after the third dose. Spleen removal was carried out at day 18 after third immunization.
Assessment of humoral immune response by ELISA
The antibody response in sera was monitored by ELISA. Briefly, anti-IgG anti-IgG1 and anti-IgG2a subclasses were carried out as previously described [12]. Briefly, 96 well high binding plates were coated with 3 mg/mL of N protein (Delta/omicron variants or SARS-CoV-1) or 2 mg/mL of RBD protein (Delta/omicron/ancestral variants or SARS-CoV-1) and blocked with 2% skimmed milk solution. Sera samples were evaluated in duplicates using different dilution starting from 1/50. Specific horseradish peroxidase conjugates and OPD (Sigma, USA)/hydrogen peroxide substrate solution were used. After 10 min of incubation in the dark, the reaction was stopped using 2 N sulfuric acid and the OD was read at 492 nm in a multi plate reader. The arbitrary units of titers were calculated by plotting the OD values obtained for each sample in a standard curve (a hyper immune sera of known titer). The positivity cut off was established as 2 times the average of OD obtained for a pre immune sera pool.
Surrogate virus neutralization test
For detecting functional antibodies generated by the evaluated formulations, a surrogate Virus Neutralization Test (sVNT) was used that allows testing the capacity of mice sera to inhibit the interaction of RBD with ACE-2 protein. To evaluate the inhibitory activity against the Ancestral strain, mice sera were assayed using the SARS-CoV-2 inhibitor screening ELISA kit (KIT001, Sino Biological, China), according to the supplier instructions. In this assay, antibodies present in sera compete with ACE-2 for binding to RBD (Ancestral variant) immobilized on the plate. Bound ACE-2 is detected via the polyhistidine tag using an anti-polyhistidine Mab conjugated to HRP. In this experimental set up, the signal decreases as the inhibitory capacity of the serum increases.
For detecting inhibitory antibodies against RBD Delta variant, a house sVNT was developed following the same design but using RBD from Delta variant as coating protein. Samples, positive and negative controls were diluted 1:25, followed by two serial dilutions 1/3 or 1/5 with PBS 0.3% BSA, 0.05% tween 20. The dilutions were combined with equal volume of ACE-2-His in a dilution plate. Fifty microliters of each mixture of ACE-2 and serum dilution were added to separate RBD coated wells of a 96 well plate (Thermo scientific 442404) and incubated at 25°C for 1 h. Bound ACE-2 was detected with the conjugate anti-His tag-HRP and developed using OPD/H2O2 as substrate. Absorbance was read at 492 nm on an ELISA micro plate reader.
In both assays, binding inhibition was calculated as (1-OD value of sample/OD value of negative control) × 100%. Inhibitory titer was defined as the highest dilution in which each animal serum inhibits 20% of the binding of ACE-2 without any competitor, calculated by linear regression using graph pad prism version 5.00.
Assessment of cellular immune response by IFN-γ ELISPOT
IFN-γ NFIELISPOT assay was performed using a Mouse IFN-γ ELISPOT antibody pair. Splenocytes were isolated in RPMI culture medium. Samples (three per group) were processed individualized, with the exception of the control group (Placebo) which was processed as pooled samples of three randomly selected mice. Duplicates cultures (5 × 105 and 1 × 105 splenocytes per well) were settled at 37°C for 48 h, at 5% CO2, in a 96 well round bottom plate with 10 μg/mL of: N351-365 peptide, N protein (Delta) or RBD protein (Delta), Concanavalin A (Con A) or medium. After, the whole content of this plate was transferred to an ELISPOT pre coated plate and incubated at 37°C for 16-20 h, at 5% CO2. The incubation conditions for conjugated antibodies and following steps were done as recommended by the manufacturers. A stereoscopic microscope coupled to a digital camera was used for spots count.
Statistical analysis
For statistical analyses the graph pad prism version 5.00 statistical software was used. Antibody titers were transformed to log10 for a normal distribution. For the non sero converting sera, an arbitrary titer of 1:50 was assigned for statistical processing. The one way ANOVA test followed by a Tukey's post-test was used as parametric tests for multiple group comparisons. In case of non arametric multiple comparisons, the Kruskal Wallis test and Dunns posttests was employed. A standard P value consideration was as follows, ns, p>0.05*, p<0.05**, p<0.01***, p<0.001.
Results
Antigenic characterization of recombinant antigens RBD and N preparations with COVID-19 convalescent sera
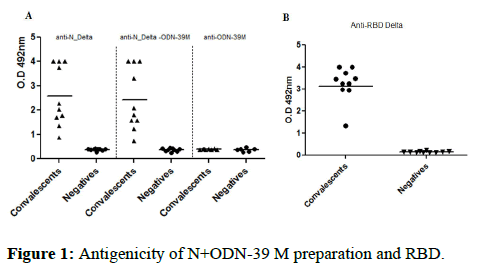
The recognition of N protein from delta strain, without treatment and after aggregation process with the ODN-39M, were tested using human sera from COVID-19 convalescent donors and negative control samples, both obtained during an outbreak of omicron variant, at Guangdong, China. As shown in Figure 1A, the two protein preparations were equally recognized by the human sera from convalescent donors. It indicates that ODN-39M did not significantly affect the protein conformation which is properly recognized by Abs generated upon natural infection. Similarly, the RBD delta protein, without treatment, was also recognized by the sera of SARS-CoV-2 convalescents (Figure 1).
Figure 1: Antigenicity of N+ODN-39 M preparation and RBD.
Note: Recognition by human sera: A) N protein alone (left panel), aggregated with ODN (center panel) and ODN (right panel), in this assay all the sera samples were evaluated at 1:2000 dilution, B) RBD, in this assay the sera were evaluated at 1:200 dilution. The data is presented as OD492 nm values from each individual serum; horizontal bar represents the mean of the group in each case. (▲) convalescents and (●) negative sera.
Humoral immunity generated by subcutaneously administered bivalent preparations
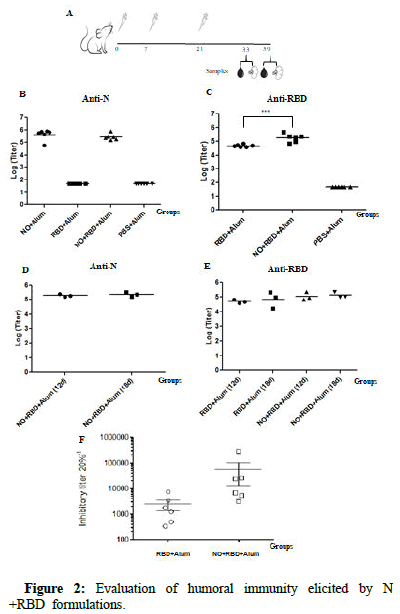
Mice experiment was conducted to explore the immunogenicity of the bivalent formulation N+ODN-39M+RBD+Alum, administered by subcutaneous route. Twelve days after three doses, the two groups receiving the N protein induced high levels of anti-N IgG antibodies in sera without significant differences among them (Figure 2). In turn, both groups immunized with RBD (NO+RBD+Alum and RBD +Alum) elicited high levels of anti-RBD IgG titers, though titers induced by the bivalent formulation were higher than those induced by RBD alone (p<0.001). However, such a difference was not detected when Abs were measured at day 18. Similar behaviour was observed for the anti-N Ab response generated by the bivalent group in the two evaluated time points.
In order to test the functionality of Abs elicited against RBD, a sVNT was employed using the RBD protein from SARS-CoV-2 delta variant. As shown, in both groups (NO+RBD+Alum and RBD+Alum) all animals elicited antibodies with inhibitory activity. The Geometric Mean Titer (GMT) of the group receiving the bivalent formulation was higher than that detected for the group receiving RBD+Alum. Nevertheless, no significant differences were detected between them (p>0.05).
Figure 2: Evaluation of humoral immunity elicited by N +RBD formulations.
Note: A) Diagram of BALB/c mouse immunization. Four groups of 6-8 week old mice were immunized subcutaneously with three doses at 0, 7 and 21 days, of each formulation: G1: N+ODN-39M (NO) + Alum, G2: RBD+Alum, G3: NO+RBD+Alum, G4: PBS+Alum. IgG antibody response in sera was evaluated by ELISA, at 12 and 18 days after the last immunization, against N Delta, B) and D) respectively and against RBD Delta C) and E) respectively. Data are represented individually as the log10 of titers and horizontal bars represent Geometric Mean of the Titers (GMT). F) Inhibitory titers of sera from RBD immunized mice were evaluated by sVNT 12 days after the third dose. Horizontal bars represent median value and error bars represents the standard error of the mean.
Assessment of the IgG subclass and cell mediated immunity
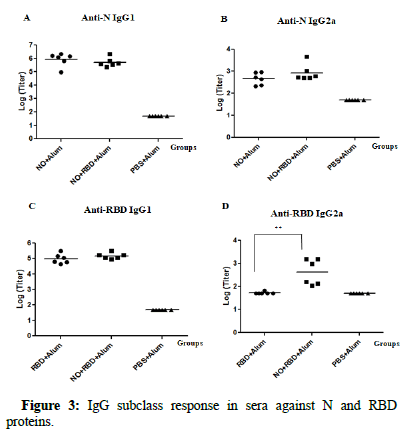
In order to obtain evidences of the pattern of the immunity induced, the IgG1 and IgG2a subclass against the two antigens: N and RBD, were also determined (Figure 3). For N protein, both groups receiving N protein (NO+Alum and NO+RBD+Alum) induced similar and high titers (105-106) of anti-N IgG1 Abs. In accordance, the anti N-IgG2a levels were also similar for both groups, though the magnitude of the titers was lower (103-104) compared to that from IgG1. For RBD protein, again, there were no significant differences between the IgG1 Abs titers between groups receiving the RBD protein (NO+RBD +Alum and RBD+Alum). On the contrary, for IgG2a Abs, only the group receiving the bivalent formulation (NO+RBD+Alum) exhibited certain level of response.
Figure 3: IgG subclass response in sera against N and RBD proteins.
Note: Twelve days after the third immunization the IgG subclass responses were evaluated by IgG1 ELISA against N and RBD A) and C) respectively and by IgG2a ELISA against N and RBD B) and D) respectively. Data are expressed as the log10 of the titers (n=6). Horizontal bars represent geometric mean in each case.
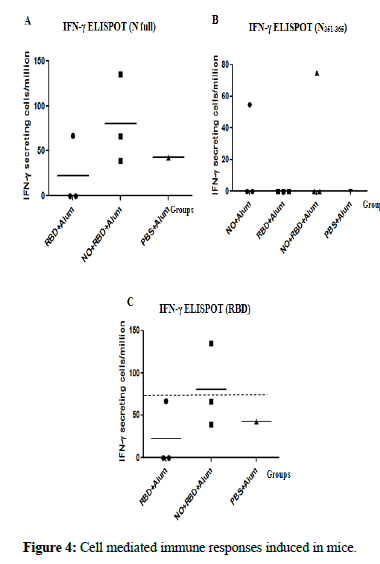
Eighteen days after the last immunization, the frequency of IFN-g secreting spleen cells was evaluated against the conserved peptide N351-365 and the whole N and RBD proteins from delta variant. When the splenocytes were stimulated with the N protein, 2 out 3 animals from the (NO+RBD+Alum) group showed a positive response, whereas 3 out 3 animals were positive in the group receiving NO+Alum (Figure 4). In turn, for the N351-365 peptide, both groups behaved similar, with only 1 animal out of 3 showing a positive response. Regarding the stimulation with RBD protein, only the group receiving the bivalent formulation exhibited positive response in one of the three animals tested.
Figure 4: Cell mediated immune responses induced in mice.
Note: Eighteen days after the third immunization the frequency of IFN-γ secreting cells were measured by ELISPOT in spleen after in vitro stimulation with A) N protein Delta, B) N351-365 peptide and C) RBD Delta. The response of each individual mice (n=3) is represented, the horizontal bars correspond to the mean for each group.
Evaluation of cross reactive humoral immune response elicited by the formulation N+ODN-39M+RBD+Alum
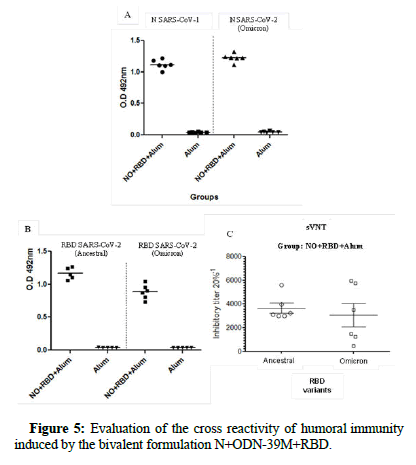
Based on the positive humoral immune response elicited by the bivalent formulation against the homologous antigens, this group was selected to test the Abs generated against heterologous antigens such as: N protein from SARS-CoV-2 omicron variant and from SARS-CoV- 1 and RBD from SARS-CoV-2 ancestral and omicron variants.
Results from IgG ELISAs, against each antigen are shown in Figure 5. A wide cross-reactive profile was found since high levels of IgG titers against each antigen were detected. In all cases, the 100% of animals were positive against N and RBD antigens at the same time.
Finally, to test the scope of the generated neutralizing Abs, the sVNT was employed using the RBD protein from ancestral and omicron variants. Similar to the response obtained against the homologous RBD, all animals induced inhibitory titers against the RBD ancestral variant (GMT=3800) and omicron variant (GMT=3000).
Figure 5: Evaluation of the cross reactivity of humoral immunity induced by the bivalent formulation N+ODN-39M+RBD.
Note: Sera from groups NO+RBD+Alum and Placebo were evaluated at 1:100 dilution by A) IgG ELISA against N proteins from SARS-CoV-2 omicron variant (right panel) and SARS-CoV-1 (left panel), B) IgG ELISA against RBD Ancestral and Delta variants. Graphics show O.D 492 nm values, horizontal bar represents mean for each group and condition. C) sVNT in Group NO+RBD+Alum, against ancestral (left panel) and omicron (right panel) RBD variants. Inhibitory titer was defined as the highest dilution in which each serum inhibits more than 20%. Horizontal bar represents median and error bars represent the standard error of the mean.
Discussion
Given the fact that SARS-CoV-2 delta variant infection produces high viral load in infected individuals and consequently, more severity of the disease, our group selected this variant to be considered as a background of a future vaccine. Due to its rapid expansion, as well as the efficacy of the prototype original vaccines against the severe disease, there is limited information about the immunogenicity of vaccines comprising antigens from this variant.
The two delta antigens (N and RBD) contained in the vaccine formulations evaluated in the present study were properly recognized by human sera from convalescent donors of the heterologous omicron variant SARS-CoV-2 wave in Guangdong province, China, during April-May 2022. In turn, the addition of ODN-39M to the N protein, did not significantly affect such recognition, indicating that the interaction between both molecules (N and ODN-39M), after the aggregation process, did not induce detectable changes in the antigenicity of the protein.
The preparation N+ODN-39M, administered by subcutaneous route, both as monovalent and bivalent formulations (combined with RBD as inductor of neutralizing Abs) induced similar and higher anti-N Abs titers. In accordance, high levels of anti-RBD and homologous neutralizing Abs were obtained for the bivalent group. This fact indicates that both antigens from delta variant are highly immunogenic by parenteral route and suggest that no antigen competition took place in the mixture, at least for the induction of a proper humoral immune response. Particularly, for the bivalent formulation, the total IgG and IgG2a anti-RBD Abs, measured at day 12 after the last immunization, were higher than those generated in the RBD monovalent group. Nevertheless, this superiority was not statistically reflected on the neutralizing Ab levels. In addition, titers of anti-RBD Abs measured 18 days after the last dose were similar in both groups therefore, the enhancer capacity of the N+ODN-39M over the humoral immunity generated against RBD should be further studied. Similar results have been obtained by other authors. Phatarphekar reported the adjuvant effect of the dual adjuvant (Alum+ODN) on RBD at day 14 after the last dose; however, at day 28, this effect was not observed [13]. Concerning the possible immune enhancing effect of the N protein alone on RBD in this formulation, in our previous report using a similar preparation (w/o Alum) by intranasal route, it was demonstrated that N protein alone did not contribute to the RBD immunity. This in accordance with the results obtained with another bivalent formulation of N+RBD where the anti-RBD response was similar in the monovalent group (RBD alone) compared to the bivalent one [14]. Based on the aforementioned elements, for our bivalent formulation, further experiments are required to define the exact contribution of the N and ODN-39M in the immunity obtained against RBD.
In the present work the cell mediated immunity was also explored. Positive response, measured by the in vitro stimulation with the full N protein, was obtained for the monovalent and bivalent formulations containing the nucleocapsid antigen. On the contrary, in the same groups, only 1 animal out of 3 was positive upon stimulation with the N351-365 peptide and the RBD. Taken together we consider that CMI induced by the bivalent formation was weak and no clear adjuvant effect over RBD cellular immune response was obtained for the N +ODN-39M preparation.
In general, the immunogenicity results obtained for the present parenteral bivalent formulation are different compared to those previously reported by our group, for the nasal bivalent formulation N +ODN-39M+RBD. In the previous study, the adjuvant effect of the N +ODN-39M preparation on the RBD immunogenicity was clearly obtained under a Th1 modulation. The differences obtained in the two studies could be explained by the presence of alum in the parenteral bivalent formulation and/or the intrinsic effect of the inoculation route. Alum is a typical Th2 adjuvant, which was used in the present study at 1.4 mg/mL therefore, it can affect the modulation toward a Th1 and consequently, the CMI response induced against the two antigens. Additional studies are planned to determine the real effect of the alum amount in the parenteral formulation and to define the main cause of such differences.
The level of cross reactivity of the humoral immune response against the two antigens was also overcome in the present work. The Abs anti-N also recognized the N protein from SARS-CoV-2 Omicron variant and SARS-CoV-1. Antibodies against N protein have been recently focused as contributor of protection in mice. Dangi demonstrated, upon passive transfer of anti N-Abs generated by a vaccine candidate, that anti N Abs were able to decrease the viral load upon SARS-CoV-2 challenge in a relevant animal model [15]. Accordingly, an interesting study in humans also suggested that anti-N Abs could have a protective role against severity of the disease [16]. Authors found that the clinical benefit of COVID-19 convalescent plasma is related to a shift toward reduced inflammatory spike responses and enhanced nucleocapsid humoral responses. One of the possible mechanisms of protection of Abs induced by N proteins was described using LCMV as a model system. Authors demonstrated that TRIM21 uses anti-N antibodies to target N for cytosolic degradation and generate Cytotoxic T cells (CTLs) against N peptide. These CTLs rapidly eliminate N-peptide displaying cells and drive efficient viral clearance [17]. Taken together, if the bivalent formulation assessed in the present work is able to induce anti-N Abs, even against SARS-CoV-1, a broader protection capacity might be expected.
On the other hand, the neutralizing Abs is the main correlate of protection against viral infections and particularly for SARS-CoV-2 [18,19]. The Abs against RBD generated by the bivalent formulation N+ODN+39M+RBD+Alum, had inhibitory capacity with the three SARS-CoV-2 RBD variants assayed: Ancestral, delta and omicron. These results are very interesting, since to our acknowledge, this is one of the few works describing the neutralizing capacity against ancestral and omicron variants for Abs induced by RBD from delta variant.
In the present work, the sVNT was selected as a regular test for evaluating the functionality of the Abs induced by RBD based on two main reasons. First, it is well recognized that almost all antibodies with potent viral neutralizing activity bind to RBD and many of them, block interactions with the human ACE-2 [20]. One illustrative example is the phase I-II clinical trial reported for Abdala vaccine composed by the recombinant RBD protein with alum as adjuvant [21]. In the study it was clearly demonstrated a positive correlation between the neutralizing Abs measured by the sVNT system and the authentic viral assay. Second, this test has emerged as an economic and simple tool that can be indeed used to estimate the major portion of neutralizing Abs without BSL-2 and BSL-3 requirements. In our study, we even used one commercial kit provided by Sino biological (Kit 001).
Conclusion
It is also important to highlight that the magnitude of the neutralizing Abs obtained in the present study against the RBD variants was higher than those reported by the nasal bivalent formulation, supporting the alum effect and the route of administration on the humoral immune response enhancing. This fact opens the possibility to explore, in the future, strategies of combination of administration routes. The nasal formulation can provide cross CMI and humoral immunity including the nasal compartment whereas, the subcutaneous administration of the present bivalent formulation would enhance the systemic humoral immunity, mainly the intra variant neutralizing response. Further mice experiments will be conducted to define the suitability of this combined approach as well as for determining the real contribution of the ODN-39M in the parenteral formulation.
Ethics Statement
The animal study was approved by the institutional animal care and use committee at Beijing vital river laboratory animal technology Co. Ltd. The standard of laboratory animal room complied with the national standard of the people's republic of China GB14925-2010. The human sera from COVID-19 convalescent and negative individuals were collected at the eighth and ninth people’s hospital of Dongguan city (Guangdong Province, China). The study protocol was approved by the institutional ethics committee from both hospitals and was carried out in accordance with the principles of Helsinki declaration.
Acknowledgments
This work was supported by Most "National key R and D program of China (2021YFE0192200)", “PNCT CITMA, Cuba”, "Hunan provincial base for scientific and technological innovation cooperation (2019CB1012)", "The science and technology innovation program of Hunan province, (2020RC5035)", "Hunan provincial innovative construction program (2020WK2031)".
Authors Contributions
Yadira Lobaina: Conceptualization, investigation, formal analysis, writing-original Draft. rong Chen: Investigation. Edith Suzarte: Conceptualization, formal analysis. Panchao Ai: Investigation, resources. Vivian Huerta: Investigation, formal analysis. Changyuan Tan: Investigation, resources. Liz Alvarez-Lajonchere: Formal analysis. Yang Liling: Investigation. Alexis Musacchio: Investigation. Ricardo Silva: supervision. Gerardo Guillén: supervision. Jiang Zaixue: Supervision. Ke Yang: Project administration. Yasser Perera: Conceptualization, supervision, Formal analysis. Project administration. Lisset Hermida: Conceptualization, supervision, funding acquisition, formal analysis, writing review and editing.
References
- Thakur V, Bhola S, Thakur P, Patel SKS, Kulshrestha S, et al. (2022) Waves and variants of SARS-CoV-2: Understanding the causes and effect of the COVID-19 catastrophe. Infection 50: 309-325. [Crossref] [Google Scholar] [PubMed]
- Swerdlow DL, Finelli L (2020) Preparation for possible sustained transmission of 2019 novel Coronavirus: Lessons from previous epidemics. JAMA 323: 1129-1130. [Crossref] [Google Scholar] [PubMed]
- Rubin R (2021) The search for a single vaccine against Coronaviruses yet to come. JAMA 326: 118-120. [Crossref] [Google Scholar] [PubMed]
- Morens DM, Taubenberger JK, Fauci AS (2022) Universal Coronavirus vaccines: An urgent need. N Engl J Med 386: 297-299. [Crossref] [Google Scholar] [PubMed]
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, et al. (2022) COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 386: 1532-1546. [Crossref] [Google Scholar] [PubMed]
- Dutta NK, Mazumdar K, Gordy JT (2020) The nucleocapsid protein of SARS-CoV-2: A target for vaccine development. J Virol 94: 647-620. [Crossref] [Google Scholar] [PubMed]
- Matchett WE, Joag V, Stolley JM, Shepherd FK, Quarnstrom CF, et al. (2021) Cutting edge: Nucleocapsid vaccine elicits spike independent SARS-CoV-2 protective immunity. J Immunol 207: 376-379. [Crossref] [Google Scholar] [PubMed]
- Dangi T, Class J, Palacio N, Richner JM, Penaloza MacMaster P (2021) Combining spike and nucleocapsid based vaccines improves distal control of SARS-CoV-2. Cell Rep 36: 109664. [Crossref] [Google Scholar] [PubMed]
- Phatarphekar A, Vidyadhar Reddy GEC, Gokhale A, Karanam G, Kuchroo P, et al. (2022) RelCoVax®, a two antigen subunit protein vaccine candidate against SARS-CoV-2 induces strong immune responses in mice. Vaccine 40: 4522-4530. [Crossref] [Google Scholar]
- Lobaina Y, Chen R, Suzarte E, Ai P, Huerta V, et al. (2022) The nucleocapsid protein of SARS-CoV-2, combined with ODN-39M, is a potential component for an intranasal bivalent pancorona vaccine. bioRxiv 6:1-34 [Crossref]
- Gil L, Marcos E, Izquierdo A, Lazo L, Valdes I, et al. (2015) The protein DIIIC‐2, aggregated with a specific oligodeoxynucleotide and adjuvanted in alum, protects mice and monkeys against DENV‐2. Immunol Cell Biol 93: 57-66. [Crossref] [Google Scholar] [PubMed]
- Lobaina Y, Trujillo H, Garcia D, Gambe A, Chacon Y, et al. (2010) The effect of the parenteral route of administration on the immune response to simultaneous nasal and parenteral immunizations using a new HBV therapeutic vaccine candidate. Viral Immunol 23: 521-529. [Crossref] [Google Scholar] [PubMed]
- Hong SH, Oh H, Park YW, Kwak HW, Oh EY, et al. (2021) Immunization with RBD-P2 and N protects against SARS-CoV-2 in nonhuman primates. Sci Adv 7: 7156. [Crossref] [Google Scholar] [PubMed]
- Dangi T, Sanchez S, Park M, Class J, Richner et al. (2022) Nucleocapsid specific humoral responses improve the control of SARS-CoV-2. bioRxiv. [Crossref] [Google Scholar]
- Herman JD, Wuang C, Burke JS, Zur Y, Compere H, et al. (2022) A role for nucleocapsid specific antibody function in COVID-19 convalescent plasma therapy. Medrxiv. [Crossref] [Google Scholar]
- Caddy SL, Vaysburd M, Papa G, Wing M, O’Connell K, et al. (2021) Viral nucleoprotein antibodies activate TRIM21 and induce T cell immunity. EMBO J 40: e106228. [Crossref] [Google Scholar] [PubMed]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, et al. (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27: 1205-1211. [Crossref] [Google Scholar] [PubMed]
- Zhu DY, Gorman MJ, Yuan D, Yu J, Mercado NB, et al. (2022) Defining the determinants of protection against SARS-CoV-2 infection and viral control in a dose down Ad26.CoV2.S vaccine study in nonhuman primates. PLOS Biol 20: e3001609. [Crossref] [Google Scholar] [PubMed]
- Kleanthous H, Silverman JM, Makar KW, Yoon IK, Jackson N, et al. (2021) Scientific rationale for developing potent RBD based vaccines targeting COVID-19. NPJ Vaccines 6: 1-10. [Crossref] [Google Scholar] [PubMed]
- Hernandez-Bernal F, Ricardo-Cobas MC, Martin-Bauta Y, Navarro-Rodríguez Z, Piñera-Martínez M, et al. (2022) Safety, tolerability and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised, double blind, placebo controlled, phase 1-2 clinical trial (ABDALA Study). E Clinical Medicine 46: 101383. [Crossref] [Google Scholar] [PubMed]
- Tan CW, Chia WN, Qin X, Liu P, Chen MI-C, et al. (2020) A SARS-CoV-2 surrogate virus neutralization test based on antibody mediated blockage of ACE2 spike protein protein interaction. Nat Biotechnol 38: 1073-1078. [Crossref] [Google Scholar] [PubMed]
Citation: Lobaina Y, Chen R, Suzarte E, Ai P, Huerta V, et al. (2023) Broad Humoral Immunity Generated in Mice by a Formulation Composed by Two Antigens from Delta Variant of SARS-Cov-2. J Infect Dis Ther 11:534
Copyright: © 2023 Lobaina Y, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2191
- [From(publication date): 0-2023 - Nov 09, 2025]
- Breakdown by view type
- HTML page views: 1806
- PDF downloads: 385