Review Article Open Access
Bridging the Gap to Sustainable Salmon Farming: Overcoming the Gaping Problem
Karin Pittman1*, Grigory VM1 and Terry Brandebourg2
1Department of Biology, University of Bergen, High Technology Centre, N-5020 Bergen, Norway
2Department of Animal Sciences, Auburn University, Auburn, AL. 36849, USA
- *Corresponding Author:
- Karin Pittman
Department of Biology
University of Bergen
High Technology Centre
N-5020 Bergen, Norway
Tel: +47 55 58 44 72
E-mail: Karin.pittman@bio.uib.no
Received Date: February 23, 2013; Accepted Date: March 25, 2013; Published Date: March 27, 2013
Citation: Pittman K, Merkin GV, Brandebourg T (2013) Bridging the Gap to Sustainable Salmon Farming: Overcoming the Gaping Problem. J Fisheries Livest Prod 1:104. doi:10.4172/2332-2608.1000104
Copyright: © 2013 Pittman K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
As oily fish consumption has increased worldwide, farmed salmonid production has also dramatically increased. As such, farming and the satellite industries affiliated with the salmonid production chain form an increasingly important economic foundation for many communities in Norway and throughout Northern Europe. However, despite the successful growth of the European salmon industry, quality concerns pose significant challenges to the sustainability of farmed salmonid production. For instance, muscle gaping, the undesirable lace-like, irregular voids or gapes in the final product, can lead to the downgrade of up to 38% of salmon produced. These blemishes lead to consumer rejection of whole cuts at the fish counter while the resulting decrease in structural integrity of the meat also poses significant limitations to the further processing of value-added products. Because of such devastating losses, determining the underlying causes of gaping and developing better detection methods that allow evaluation of intervention strategies have become high research priorities for the industry and governmental agencies alike. Automated Image Analysis (IA) is one such technology that allows the objective measure of gaping on fish carcasses. Efforts to translate this technology to a platform that can be utilized efficiently in packing plants are progressing rapidly and producing promising results. The ability to objectively and rapidly detect graded differences in gaping of salmon products in commercial settings will allow the identification of critical points in the supply chain that impact upon product quality. Applying IA methods to identify these critical points and to assess the effectiveness of intervention strategies will ultimately allow salmon producers to bridge the quality gap that currently exists between the fish farm and the consumer.
Introduction
Global salmon farming produces about 1.4 million tonnes of fish annually [1] with an average price per kilo of about 4.6 Euros [2]. The positive health benefits associated with the consumption of oily fish and the high efficiency of converting protein to fish muscle are significant attributes of fish production that have driven the industry’s growth and increasing impact upon local communities. Countries located in Northern Europe, Canada and Chile comprises the primary producers of farmed salmon products while Europe, Japan and North America represent the industry’s primary markets.
Farmed salmon is marketed either as fresh whole fish, fillets and steaks or packaged as frozen or smoked products. As farm prices have tended to decrease, profitability has increasingly become dependent upon providing consistent, high-quality products and niche marketing including organic farming certification and fish welfare-related schemes. Furthermore, value-added processing like smoked fillets or smoked salmon slices place a premium on the flesh quality itself. Thus, intrinsic characteristics such as fillet yield, fillet coloration, chemical body composition, texture and loss of fillet integrity (gaping) have been given special attention to optimize the quality in fish products [3]. In general, both processers and consumers prefer firm fish fillets with even colouration and no gaping.
Muscle gaping in fillets is particularly problematic because gaping gives rise to lace-like slices and irregular shapes in the muscle that significantly detract from the desirability of the final product. Consequently, gaping can decrease salmon value by up to 38% during the secondary processing of the carcass. Thus gaping represents one of the most important quality issues facing the salmon industry [4]. In order to help the industry overcome this significant quality problem, better methods for identifying gaping in fish cuts need to be developed both to monitor the effects of intervention strategies upon the problem and to allow study of the underlying causes. The present review evaluates how gaping in salmonids is affected by parameters such as anatomical and histological features of the carcass, seasonal variation in growing conditions, current aquaculture practices, post-mortem changes that occur during the conversion of muscle to meat, and product storage procedures. Finally, current methods for gaping detection and their limitations and promise are briefly discussed.
Salmonid anatomy and physiology: going from muscle to meat
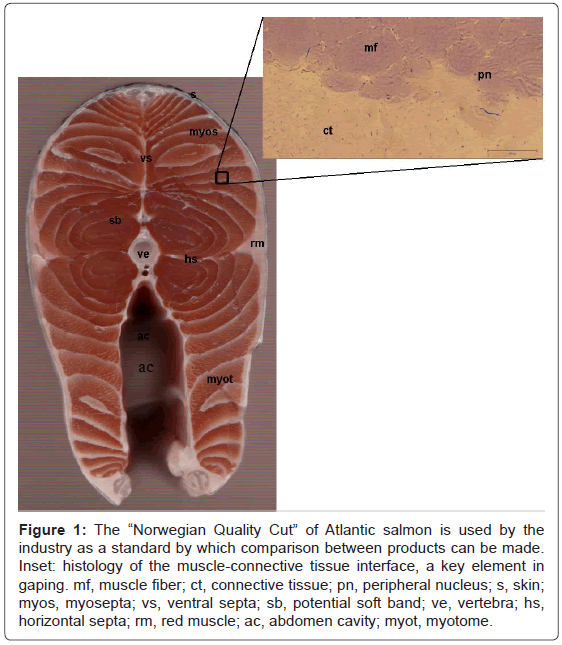
Anatomy: Salmon muscle structure is an important determinant of its textural characteristics including the structural cohesion of meat cuts [5]. For the purposes of meat quality, the fish carcass can primarily be thought of as a long axial muscle that facilitates swimming. Along this axial muscle, individual muscle cells are organized into “muscle blocks” or myotomes that are separated by sheets of connective tissue, called myocommata or myosepta consisting of collagenous connective tissue, adipocytes and non-adipose cells. Myosepta in turn function to anchor whole axial muscle to both the skeleton and the skin and are recognizable as repeating white bands separating the “salmon-colored” myotomes (Figures 1 and 2). Additionally, myotomes are further organized by horizontal and medial septa that function to separate the myotome into four sections comprising the left and right epaxial (dorsal) and hypaxial (ventral) muscles. Collagen fibers emerging from the myosepts ultimately give rise to the perimysium, the specialized connective tissue sheath that surrounds each muscle fiber [5].
Figure 1: The “Norwegian Quality Cut” of Atlantic salmon is used by the industry as a standard by which comparison between products can be made. Inset: histology of the muscle-connective tissue interface, a key element in gaping. mf, muscle fiber; ct, connective tissue; pn, peripheral nucleus; s, skin; myos, myosepta; vs, ventral septa; sb, potential soft band; ve, vertebra; hs, horizontal septa; rm, red muscle; ac, abdomen cavity; myot, myotome.
Fast-twitch or white muscle fibers comprise the predominant fiber type in the skeletal muscle of salmon (Figures 1 and 2). “White” muscle is poorly vascularized and its characteristically low myoglobin levels give rise to the pale color generally associated with fish muscle [6]. However, true “white” muscle in salmon generally takes on a reddish hue as the consequence of high levels of carotenoids in the typical salmon diet. True slow-twitch fibers or “red” muscle in salmon presents as thin lateral strips of darker muscle tissue between the “white” muscle and the skin (Figure 1). “Red” muscle in salmon is used for long and sustained swimming movement, whereas “white” muscle is involved in burst swimming activity.
On average, in salmon, white fibers have diameters between 50 and 100 μm while red muscle fibers range between 25 and 45 μm [6]. Tightly packed myofibrils, the organelles of contraction, contribute the greatest to myofiber volume, whereas sarcoplasmic constituents such as mitochondria, myoglobin and glycogen granules contribute relatively little to myofiber size. Anaerobic glycolysis is the main source of energy for white muscle, although aerobic breakdown of lipids in white muscle also takes place in salmonids [6].
Salmonids store carcass lipids both in adipocytes located within the myosepta and as intrafibrillar lipid droplets. The size and density of adipocytes varies according to anatomical location with adipocyte volume and number increasing closer to the belly [7]. Adipocytes contribute to carcass value because they represent the primary site for the storage of omega-3 fatty acids. The health benefits associated with consumption of this class of lipids has contributed to the increased demand for salmon meat that has occurred over the last several decades [7].
Growth and histological muscle features: Unlike in mammals, growth is continuous throughout the lifespan of fish. Such muscle growth in fish is accomplished both by the recruitment of new muscle fibers and by an increase in muscle fiber size [8]. Also in contrast to mammals, fish are poikilothermic. Fish thus experience depressed appetite and reduced rates of muscle fiber recruitment and hypertrophy in response to the shorter days and lower temperatures associated with the winter season [9]. Additionally, muscle fiber size and number and connective tissue remodelling within the developing muscle are also influenced by composition of the diet, feeding regimes, swimming speed, salmon strain and genotype [8,10,11].
Rigor mortis and post-mortem degradation of muscle tissue: Fish muscle undergoes conversion to edible meat through a series of post-mortem changes that must occur in a controlled manner for optimal meat quality. Immediately following harvest, fish muscle is soft (pre-rigor), but gradually carcass muscles stiffen (rigor) before finally undergoing a final post-rigor softening. These changes are necessary for the muscle to assume the texture most often associated with quality cuts at the fish counter. The timing of onset and the intensity and duration of rigor is heavily influenced by pre-harvest nutritional status [12], harvest-associated stress [12,13], and post-harvest storage conditions [14]. As post-mortem degradation of muscle protein occurs, detachments between individual myofibers become visible following one day of storage [15] while complete myofiber detachment from the myocommata can be observed by 5 days post-mortem [15].
The duration of rigor appears especially sensitive to stress responses in salmon and has been shown to last a mere 30 hours in stressed fish, up to 60 hours in fish exposed to stressors but calmed by anaesthesia [16], and to last between 72 and 120 hours in rested, unstressed fish [17,18]. Furthermore, intense muscular contraction in the whole fish during rigor can itself lead to gaping by damaging connective tissue and weakening the associations between myofibers and the myocommata, an outcome that is exacerbated by filleting the carcass. Finally, gaping after filleting is promoted with higher temperature as this weakens the connective tissue and tends to be associated with stronger contraction of the muscle [19].
Gaping: Typically a “quality” fillet is firm and devoid of gaping. However, gaping has been registered in farmed salmon [19], trout [19] both wild and farmed cod [20] as well as in other fish species [19]. Gapes in fish fillets have a characteristic appearance exemplified by grooves or splits creating voids in the muscle that reveal the membrane lining where collagen fibers transition to the perimysium between the myocommata and muscle fibers. As gaping becomes more pronounced, this membrane lining is lost resulting in detachment of the sarcolemma completely from the base of the muscle fiber [21]. This does not occur evenly over the entire fillet but is often concentrated near but not in the “soft stripe”, a medial area located near the vertebra where the connective tissue is less distinct. There is anecdotal evidence to suggest the co-occurrence of gaping and big fillet areas without clear separation between the myotome-myocommata (soft band) [22], an area which may be a growth region in the muscle [23]. Anecdotal evidence from the Norwegian fish farming industry in 2007 suggest a high co-occurrence of large gapes and a prominent jelly-like band, or soft stripe, in the salmon fillet [22]. An image analysis method to quantify areas of soft bands has been developed (Merkin et al., unpublished). However, gaping may occur anywhere in or on the periphery of the muscle tissue confounding attempts to correlate anatomy to the underlying cause of such structural issues.
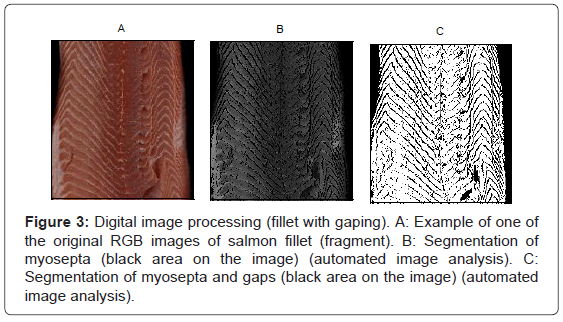
In more strict terms, gaping refers to the appearance of slits (gapes) (Figures 2 and 3) in the connective tissue between muscle segments or between individual myofibers in fish fillets. In whole salmon or fresh and smoked fillets, gaping appears as slits, whereas in smoked salmon slices, gaping appears as holes in the slices and notches in the slice borders [24]. There is a positive correlation between soft texture and the occurrence of gaping in salmonids, with firmer fillets having less gaping [25,26]. Gapes make the fillet difficult to skin and to slice, and badly affected fish can only be used in cheaper products like fish meal, fish oil or fish cakes [19,27]. Thus, the occurrence of gaping has a significant negative impact on profitability.
Figure 3: Digital image processing (fillet with gaping). A: Example of one of the original RGB images of salmon fillet (fragment). B: Segmentation of myosepta (black area on the image) (automated image analysis). C: Segmentation of myosepta and gaps (black area on the image) (automated image analysis).
Causes of gaping
The variation in gaping is partly explained by the variation in muscle fiber density which is inversely correlated with degree of gaping [8]. Since the rigor force of a muscle is proportional to the cross-sectional area of its muscle fibers, fiber density and the relative amount and distribution of connective tissue may be one of the determinants for the gaping phenomenon in post-mortem flesh [8]. Little or no gaping is observed in fish with a fiber density in excess of 95 fibers per square millimeter muscle [28]. Moreover Mørkøre et al. observed that raw fillets with low fiber cross-sectional area (<12.5 μm2 on average) had significantly firmer texture compared with fillets comprised of larger fibers [29]. This suggests that any selection program that influences muscle fiber dynamics may also unintentionally affect the degree of post-harvest gaping. Thus it is important to develop a better understanding of the association of muscle fiber size with gaping and meat quality as well as to develop markers for gaping that could potentially be incorporated into selection programs [30]. However, other factors extrinsic to muscle fiber development such as pre-slaughter growth patterns, slaughter season, stress, handling, and storage likely contribute to the incidence of gaping.
Seasonality influences fillet quality in poikilotherms and seasonal variation in salmon texture is well documented though the explanations for the causal mechanisms are manifold [31-35]. During rearing in sea-cages, Atlantic salmon are affected by predictable annual cycles in biotic and abiotic environmental factors, including photoperiod and temperature. Previous research has demonstrated that variation in water temperature affects feed intake, feed utilization and growth [31]. During a northern winter, salmon appetite normally declines and feeding drops off. Approximately 2 months after resumption of feeding, muscle carbohydrate stores improve. Anaerobic glycolysis of the increased carbohydrate pool may rapidly decrease postmortem pH in the muscle [36] and there is a strong negative correlation between the postmortem decline in pH and the increase in gaping during summer [19,27].
Periods of rapid growth in Rainbow trout (Onchorhynchus gairdneri) favour fiber hypertrophy, while periods of slow growth favour fiber recruitment [11]. During the winter season, the ratio of small diameter muscle fibers increases in Atlantic salmon [5]. Moreover, differential growth rates have been observed for the dorsal and lateral “white” epaxial muscle in trout, where the dorsal area had more small fibers and slower fiber enlargement [11]. Anecdotal information from fish farmers suggests that soft flesh is correlated with rapid growth rate. Hence post mortem muscle softness may be a “natural” result of muscle remodelling associated with periods of accelerated muscle growth [22]. While the underlying cellular events responsible for the apparent changes in structural integrity that appear associated with rapidly remodelled muscle are largely unknown, softer muscle expresses higher levels of gelatinase activity, ubiquitination, and protease activity, all changes that are consistent with the notion that muscle and connective tissue ultra structure is changed [22]. Therefore gaping may also be associated with phases in the growth cycle [4], although not all studies confirm the effect of fast growth on texture in salmon [37].
Regardless, degradation of the interface between muscle and connective tissue appears to be a key element in the development of gaping in whole muscle. It is likely that connective tissue remodelling elicited through changes in enzymatic activity and connective tissue fiber expression underlies the structural changes that result in suboptimal interaction of myofibers and the myocommata. It has been speculated that in the myocommata of farmed fish, the infiltration of lipid droplets can lead to a decrease in the strength of collagen thus stimulating gaping in fish loins after filleting [38]. Collagen influences the texture and function of muscles [39]. For instance, the structural integrity of the tissue itself is dictated by the degree of cross-linking between collagen and elastin fibers [40]. Li and coworkers [41] reported that there are at least 27 types of collagens with distinct domains and more than 20 proteins with collagen-like domains, although the majority can be classified as type I collagen in fish muscle. Species-specific differences in the prevalence of gaping are associated with seemingly small differences in muscle components. However differences in gaping between species appear to correlate best to inherent differences in collagen fiber dynamics. For instance, cod (Gadus morhua) are susceptible to gaping and generally have larger collagen fibers and less dense collagen networks in the myocommata than do wolfish (Anarhichas lupus) which are resistant to gaping [42]. In harvest-size Atlantic salmon, the non-reducible cross-links between elastin and collagen fibers represent only 1-3% of the total cross-linkages observed between connective tissue fibers [41]. Concerning potential mechanisms, each Hydroxylysyl Pyridinoline (PYD) crosslink connects three collagen molecules. Although an association between PYD and gaping has not been established, diet may influence PYD concentration and PYD-collagen cross-linkages, thus opening the possibility that flesh texture in farmed fish may be favorably altered through simply manipulating the fish diet [43].
The notion that manipulating feeding regimes and diet represent possible strategies to lessen the prevalence of gaping is supported by several observations. First, when starved for two months, Brown trout (Salmo trutta) had more connective tissue and thinner muscle fibers compared to their counterparts in the control group [44]. However the myosepta width was not affected by starvation and this resulted in an increased connective tissue–muscle tissue ratio, and hence in a higher muscle collagen content in the starved group [44]. Feeding a high-fat diet to Atlantic salmon resulted in carcasses containing more lipid than fish fed the medium-fat diet corresponding with 17% larger visible fat deposits on transversal carcass sections and 10% wider myosepta stripes on the fillets [45]. Similarly, feeding a restricted diet can lead to a decrease in gaping [46].
The potential impact of diet on gaping prevalence is especially relevant given the recent industry trend to utilize alternatives to fish meal as a protein source in salmon feeds. Vegetable-based alternatives currently in use have proven to adversely impact gut health and physiology although the overall effect of such diets upon the final protein composition of the fillet and therefore fillet quality remains to be fully investigated. One such study looked at the long term effects of feeding high-energy, low fish-meal feeds on growth and flesh characteristics, including gaping, using subjective scales and the authors found no significant difference between feed treatments [47]. Jessen et al. reared two groups of Rainbow trout on a more traditional diet of marine oils and proteins or a diet based exclusively on vegetable products, both of which had 42% protein and 26% fat. Analyzing the muscle using 2D proteomics, they found differences in 39 proteins including a number of proteases and proteins associated with protein and lipid metabolism and lipid transport. The authors concluded that while firmness was increased on the vegetable diet, proteins associated with flakiness and juiciness were also affected.
The application of microarray analysis to examine gene expression profiles of muscle within soft and firm Atlantic salmon [48] revealed a positive correlation between muscle firmness and genes that encode proteasome components, mitochondrial proteins, coordinate stress responses and regulate lipid metabolism. These results further suggested relationships between sugar metabolism and myofiber proteins and revealed several candidate marker genes although the function of these novel targets is currently unknown. Unfortunately, the Fish-Specific Whole Genome Duplication, an event that occurred in the evolution of bony fishes (teleosts) about 350 million years ago, complicates the interpretation of such data for fish [49]. Duplicated genes include those with basic functions like cell communication, regulation of physiological processes, the extracellular matrix and pattern binding, and there is also neo-functionalization or paralogs of other genes. While such genomic duplication forms the basis for the wide diversity exhibited by fishes, it makes the direct comparison to other vertebrate genes and interpretation of their functions more complex. The fishes have gene loss and enriched signaling as well as asymmetric duplication of developmental and behavioral genes compared to mammals [49]. Although the Atlantic salmon genome is being mapped [50], interpretation of molecular results will continue to be a challenge until the fish genome is better understood.
Slaughter welfare
Transport methods, the method of slaughter and post-harvest handling all affect flesh quality, much like traditional farm animal production. Pre-slaughter stress and activity in fish shorten pre-rigor time, increasing the possibility of damage to the flesh during processing [51]. An expected increase on rigor flow and mechanical gaping was indeed observed in rainbow trout in response to pre-harvest crowding and pumping [52], however there were no significant effects of these stressors on fillet gaping [52].
Nonetheless, increased pre-harvest muscle activity has been associated with external damage and carcass devaluation [51], earlier onset of rigor mortis [53], softer texture [54,55], and gaping [53]. Furthermore fish stunned at the cage site had significantly lower gaping scores than fish stunned after pumping or live chilling while no significant difference was detected between pumped and live chilled fish [56]. Empirical evidence suggests that the timing of onset of rigor is influenced by stress or muscular activity as post-mortem electrical muscle stimulation induced rigor at 2-4 h while stressed salmon entered rigor slightly later at 4-24 h and unstressed fish exhibited the latest onset at 12-36 h post-mortem [18]. Stressed fish had higher gaping scores and softer flesh than their counterparts, indicating that factors other than stress and rigor can activate the enzymatic responses necessary for softening flesh.
Although an increase in fillet gaping due to crowding stress has been documented in Atlantic salmon [18], these is not a consensus on this point [57]. Contradictory results could be explained by the difference in pre-harvest conditions between studies, e.g. different durations of crowding and pumping and the use of different harvesting equipment. These differences would be expected to be associated with differences in the magnitude of stress experienced by the fish across studies.
It is well established that rough handling of the fish carcass can result in physical damage that leads to severe gaping of the fillets [19,27]. Anecdotal information from the industry suggests that the necessary forcible straightening of fish carcasses that were in a bent position following the onset of rigor, causes further disruptions in muscle tissue [27].
Finally, texture and gaping in salmonids are further influenced by the methods of storage as thawed fillets gape more than fillets that are stored on ice [52,58,59]. Furthermore, time that a fillet is stored on ice is positively correlated with the incidence of gaping in salmon [33,60]. As such, even if fish farm husbandry and fish welfare are excellent during the lifetime of the salmon, peri-mortem and post-mortem practices can clearly impact product quality and thus the producer’s “bottom line”. Being able to determine which factors and what points in the supply chain impact product quality is a critical necessity.
Mapping gaping in the salmon industry
The estimation of gaping is typically done by visually grading fillets or fillet slices, either by counting gaps according to the “Andersen scale” from 0 to 5 [60], by evaluating the area covered by gaps [54], or by a sliding scale [61]. These subjective gaping analyses rely on expert opinion confounding the comparison of results across trials conducted by different specialists. Therefore, the use of several trained evaluators is recommended. In addition to the need for specialized personnel, current approaches for evaluating gaping are labour intensive and subjective in nature so the development of objective methods would represent a significant advance.
Nonetheless, subjective approaches have proven useful for detecting differences in the prevalence of gaping across the industry. In a large industrial trial (n=1953 salmon) carried out in Norway, different degrees of gaping in fillets was observed between processing plants [60]. While severe gaping was observed in less than 3% of fillets processed in one plant, a striking 22-25% of fillets exhibited multiple slits in the muscle at two other factories tested. In one of these factories, economic loss due to gaping was calculated to be 5-10% [60].
Importantly, some decrease in the degree of gaping has been achieved through the application of standardized protocols for harvest and storage. For example in one study conducted during 2010, market-size farmed salmon (n=1181) were harvested with 65% of fish in the study exhibiting no gaping in fillets while less than 5% of fish in the study were downgraded [62]. These results illustrate that a large variation in the incidence of gaping exists across the industry. Finding the underlying causes for such large variation in gaping across the supply chain is both a scientific and industrial challenge.
Given the important of monitoring the prevalence of gaping in harvested salmon and the limitations inherent in current subjective methods, considerable effort has been made to develop efficient, objective measures of gaping. Automated Image Analysis (IA) is one such modern and efficient method for monitoring quality in fish products [63]. The IA approach measures gaping by imaging the carcass and distinguishing between the visibly distinct bands of white myosepta (collagen) that form along thicker bands of the muscle fiber and the darker invaginations that appear corresponding to muscle gaping in salmon. IA permits objective segmentation of different parts of the fish carcass including white and red muscle, myosepts, dorsal fat deposit, and horizontal and vertical septum. This in turn facilitates analysis of the geometrical properties of these parts and their relative ratios and thus represents a very powerful tool to measure the degree of gaping on a carcass. While IA is now widely used for objective measures of quality traits in salmonids such as colour [64,65], fat content [66] and shape [65] there is no simple and efficient instrumental method for measuring gaping that could be applied in an automated fashion in a setting such as processing plants [25]. Given this significant limitation in objective approaches, there is great interest in developing automated IA protocols. In this regard, a semi-automatic method has been developed to analyze gaping in salmon fillets using a manually set threshold for each individual fillet image [67].
We have likewise further developed an automated IA method for gaping detection and quantification in smoked salmon slices [24]. The results obtained by our automatic image analysis strongly correlated with manual quantification of gaps (r=0.83, p-value <0.05). The automatic image analysis method can easily be extended to also include morphological parameters such as shape, red and white muscle area, and myocommata and myotome area. Our analysis demonstrated that gaps are strongly associated with “white” or fast-twitch muscle, and not “red” or slow muscle tissue. This observation suggests that decreases in the myotome-connective tissue ratio might make the fish carcass more susceptible to damages due to rigor tensions. Moreover, these results demonstrate the potential for industry-level quantification of fish slice/fillet characteristics associated with gaping. Importantly, this procedure holds promise as a tool to allow further investigation of the gaping phenomenon itself.
Conclusions
Gaping in fish muscle causes downgrading and restrictive use of the product resulting in lower value applications post-harvest. Gaping is somewhat seasonal, and is associated with peri-mortem and post-mortem factors such as temperature, muscle pH, stress, and crowding, among other factors. The association between the muscle fibers and the connective tissue, especially respective to collagen dynamics, is a key element in gaping. Growth rates, muscle fiber diameters, ratios between connective tissue and muscle have all been implicated as influencing factors. Attempts to deduce which genes are associated with gaping or muscle firmness have demonstrated a potential role for vegetable diets to influence these attributes. Interpretation of molecular data from fish is more complex than in other vertebrates due to the evolutionary Fish Specific Genome Duplication. Standardization of slaughter procedures and other methodologies to assess and quantify gaping on an industrial scale will permit identification of steps in the production chain that are critical for maintaining high quality, farm-fresh salmon all the way to the consumer.
References
- FAO (2010) Cultured Aquatic Species Information Programme.
- Fish Pool (2013) Price information.
- Rasmussen RS (2001) Quality of farmed salmonids with emphasis on proximate composition, yield and sensory characteristics. Aquaculture Research 32: 767-786.
- Michie (2001) Causes of downgrading in the salmon farming industry. Farmed Fish Quality. John Wiley & Sons, New Jersey, USA.
- Johnston AJ (2001) Implications of muscle growth patterns for the colour and texture of fish flesh. Farmed Fish Quality. Blackwell Science, Oxford, UK.
- Kiessling A, Ruohonen K, Bjørnevik M (2006) Muscle fiber growth and quality in fish. Arch. Tierz 49: 137-146.
- Zhou SY, Ackman RG, Morrison C (1995) Storage of Lipids in the Myosepta of Atlantic Salmon (Salmo Salar L.). Fish Physiol Biochem 14: 171-178.
- Johnston IA (1999) Muscle development and growth: potential implications for flesh quality in fish. Aquaculture 177: 99-115.
- Johnston IA, Manthri S, Alderson R, Smart A, Campbell P, et al. (2003) Freshwater environment affects growth rate and muscle fiber recruitment in seawater stages of Atlantic salmon (Salmo salar L.). J Exp Biol. 206: 1337-1351.
- Totland GK, Kryvi H, Jodestol KA, Christiansen EN, Tangeras A, et al. (1987) Growth and Composition of the Swimming Muscle of Adult Atlantic Salmon (Salmo salar L.) during Long-Term Sustained Swimming. Aquaculture 66: 299-313.
- Kiessling A, Kiessling KH, Storebakken T, Asgard T (1991) Changes in the Structure and Function of the Epaxial Muscle of Rainbow-Trout (Oncorhynchus-Mykiss) in Relation to Ration and Age: II. Activity of Key Enzymes in Energy-Metabolism. Aquaculture 93: 357-372.
- Morkore T, Mazo PI, Tahirovic V, Einen O (2008) Impact of starvation and handling stress on rigor development and quality of Atlantic salmon (Salmon salar L). Aquaculture 277: 231-238.
- Erikson U, Sigholt T, Rustad T, Einarsdottir IE, Jorgensen L (1999) Contribution of bleeding to total handling stress during slaughter of Atlantic salmon. Aquacult Int 7: 101-115.
- Stroud GD (1969) Rigor in Fish: The Effect on Quality. Torry Advisory Note No. 36.
- Taylor RG, Fjaera SO, Skjervold PO (2002) Salmon fillet texture is determined by myofiber-myofiber and myofiber-myocommata attachment. J Food Sci. 67: 2067-2071.
- Erikson U, Misimi E (2008) Atlantic salmon skin and fillet color changes effected by perimortem handling stress, rigor mortis, and ice storage. J Food Sci. 73: C50-C59.
- Skjervold PO, Fjaera SO, Ostby PB (1999) Rigor in Atlantic salmon as affected by crowding stress prior to chilling before slaughter. Aquaculture 175: 93-101.
- Roth B, Slinde E, Arildsen J (2006) Pre or post mortem muscle activity in Atlantic salmon (Salmo salar L.). The effect on rigor mortis and the physical properties of flesh. Aquaculture 257: 504-510.
- Lavety J (1984) Gaping in farmed salmon and trout. Torry Advisory Note No. 90.
- Love RM (1975) Variability in Atlantic Cod (Gadus morhua) from Northeast Atlantic - Review of 1Seasonal and Environmental Influences on Various Attributes of Flesh. Journal of the Fisheries Research Board of Canada 32: 2333-2342.
- Fletcher GC, Hallett IC, Jerrett AR, Holland AJ (1997) Changes in the fine structure of the myocommata-muscle fiber junction related to gaping in rested and exercised muscle from king salmon (Oncorhynchus tshawytscha). Food Science and Technology 30: 246-252.
- Martinez I, Wang PA, Slizyte R, Jorge A, Dahle SW, et al. (2011) Protein expression and enzymatic activities in normal and soft textured Atlantic salmon (Salmo salar) muscle. Food Chem 126: 140-148.
- Erikson U, Standal IB, Aursand IG, Veliyulin E and Aursand M (2012) Use of NMR in fish processing optimization: a review of recent progress. Magn Reson Chem 50: 471-480.
- Merkin GV, Stien LH, Pittman K, Nortvedt R (2012) Digital image analysis as a tool to quantify gaping and morphology in smoked salmon slices. Aquacult Eng.
- Mørkøre T, (2008), Tekstur i oppdrettslaks. Kunnskapsstatus og forhold som bidrar til fastere fillet, in Rapport/Report 32.
- Einen O, Thomassen MS (1998) Starvation prior to slaughter in Atlantic salmon (Salmo salar) II. White muscle composition and evaluation of freshness, texture and colour characteristics in raw and cooked fillets. Aquaculture 169: 37-53.
- Loye RM (1973) Gaping of Fillets. Torry Advisory Note No. 61: 3-6.
- Johnston IA, Manthri S, Alderson R, Campbell P, Mitchell D, et al. (2002) Effects of dietary protein level on muscle cellularity and flesh quality in Atlantic salmon with particular reference to gaping. Aquaculture 210: 259-283.
- Morkore T, Ruohonen K, Kiessling A (2009) Variation in Texture of Farmed Atlantic Salmon (Salmo Salar L.). Relevance of Muscle Fiber Cross-Sectional Area. J Texture Stud 40: 1-15.
- Vicira VLA, Norris A, Johnston IA (2007) Heritability of fiber number and size parameters and their genetic relationship to flesh quality traits in Atlantic salmon (Salmo salar L.). Aquaculture 272: S100-S109.
- Hevroy EM, Waagbo R, Torstensen BE, Takle H, Stubhaug I, et al. (2012) Ghrelin is involved in voluntary anorexia in Atlantic salmon raised at elevated sea temperatures. Gen Comp Endocrinol. 175: 118-134.
- Bjornevik M, Espe M, Beattie C, Nortvedt R, Kiessling A (2004) Temporal variation in muscle fiber area, gaping, texture, colour and collagen in triploid and diploid Atlantic salmon (Salmo salar L.). J Sci Food Agr 84: 530-540.
- Espe M, Ruohonen K, Bjornevik M, Froyland L, Nortvedt R, et al. (2004) Interactions between ice storage time, collagen composition, gaping and textural properties in fanned salmon muscle harvested at different times of the year. Aquaculture 240: 489-504.
- Morkore T, Rorvik KA (2001) Seasonal variations in growth, feed utilisation and product quality of farmed Atlantic salmon (Salmo salar L.) transferred to seawater as 0+smolts or 1+smolts. Aquaculture 199: 145-157.
- Roth B, Johansen SJS, Suontama J, Kiessling A, Leknes O, et al. (2005) Seasonal variation in flesh quality, comparison between large and small Atlantic salmon (Salmo salar L.) transferred into seawater as 0+ or 1+ smolts. Aquaculture 250: 830-840.
- Lavety J, Afolabi OA, Love RM (1988) The Connective Tissues of Fish .9. Gaping in Farmed Species. Int J Food Sci Tech 23: 23-30.
- Johnston IA, Bickerdike R, Li XJ, Dingwall A, Nickel D, et al. (2007) Fast growth was not associated with an increased incidence of soft flesh and gaping in two strains of Atlantic salmon (Salmo salar L.) grown under different environmental conditions. Aquaculture 265: 148-155.
- Torrissen O, Sigurgisladottir D, Slinde E (2001) Texture and technological properties of fish. Farmed Fish Quality, John Wiley & Sons, New Jersey, USA.
- Sikorski ZE, Scott DN, Buisson DH (1984) The Role of Collagen in the Quality and Processing of Fish. Crit Rev Food Sci Nutr. 20: 301-343.
- Silver FH, Freeman JW, Seehra GP (2003) Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 36: 1529-1553.
- Li XJ, Bickerdike R, Lindsay E, Campbell P, Nickell D, et al. (2005) Hydroxylysyl pyridinoline cross-link concentration affects the textural properties of fresh and smoked Atlantic salmon (Salmo salar L.) flesh. J Agric Food Chem. 53: 6844-6850.
- Ofstad R, Olsen RL, Taylor R, Hannesson KO (2006) Breakdown of intramuscular connective tissue in cod (Gadus morhua L.) and spotted wolffish (Anarhichas minor O.) related to gaping. Lwt - Food Science and Technology 39: 1143-1154.
- Johnston IA, Li XJ, Vieira VLA, Nickell D, Dingwall A, et al. (2006) Muscle and flesh quality traits in wild and fanned Atlantic salmon. Aquaculture 256: 323-336.
- Bugeon J, Lefevre F, Fauconneau B (2004) Correlated changes in skeletal muscle connective tissue and flesh texture during starvation and re-feeding in brown trout (Salmo trutta) reared in seawater. J Sci Food Agr 84: 1433-1441.
- Refstie S, Storebakken T, Baeverfjord G, Roem AJ (2001) Long-term protein and lipid growth of Atlantic salmon (Salmo salar) fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level. Aquaculture 193: 91-106.
- Johnsen CA, Hagen Ø, Solberg C, Björnsson BTH, Jönsson E, et al. (2013) Seasonal changes in muscle structure and flesh quality of 0+ and 1+ Atlantic salmon (Salmo salar L.): impact of feeding regime and possible roles of ghrelin. Aquacult Nutr 19: 15-34.
- Johnsen CA, Hagen Ø, Bendiksen EÅ (2011) Long-term effects of high-energy, low-fishmeal feeds on growth and flesh characteristics of Atlantic salmon (Salmo salar L.). Aquaculture 312: 109-116.
- Larsson T, Morkore T, Kolstad K, Ostbye TK, Afanasyev S, et al. (2012) Gene Expression Profiling of Soft and Firm Atlantic Salmon Fillet.
- Brunet FG, Crollius HR, Paris M, Aury JM, Gibert P, et al. (2006) Gene loss and evolutionary rates following whole-genome duplication in teleost fishes. Mol Biol Evol. 23: 1808-1816.
- Davidson WS, Koop BF, Jones SJM, Iturra P, Vidal R, et al. (2010) Sequencing the genome of the Atlantic salmon (Salmo salar). Genome Biol. 11: 403.
- Robb DHF (2001) The relationship between killing methods and quality. Farmed Fish Quality. Fishing News Books: Cornwall 220-233.
- Merkin GV, Roth B, Gjerstad C, Dahl-Paulsen E, Nortvedt R (2010) Effect of pre-slaughter procedures on stress responses and some quality parameters in sea-farmed rainbow trout (Oncorhynchus mykiss). Aquaculture 309: 231-235.
- Robb DHF, Kestin SC, Warriss PD (2000) Muscle activity at slaughter: I. Changes in flesh colour and gaping in rainbow trout. Aquaculture 182: 261-269.
- Kiessling A, Espe M, Ruohonen K, Morkore T (2004) Texture, gaping and colour of fresh and frozen Atlantic salmon flesh as affected by pre-slaughter iso-eugenol or CO2 anaesthesia. Aquaculture 236: 645-657.
- Roth B, Moeller D, Veland JO, Imsland A, Slinde E (2002) The effect of stunning methods on rigor mortis and texture properties of Atlantic salmon (Salmo salar). J Food Sci 67: 1462-1466.
- Roth B, Birkeland S, Oyarzun F (2009) Stunning, pre slaughter and filleting conditions of Atlantic salmon and subsequent effect on flesh quality on fresh and smoked fillets. Aquaculture 289: 350-356.
- Skjervold PO, Fjaera SO, Ostby PB, Einen O (2001) Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture 192: 265-280.
- Einen O, Guerin T, Fjaera SO, Skjervold PO (2002) Freezing of pre-rigor fillets of Atlantic salmon. Aquaculture 212: 129-140.
- Hall GM (1997) Fish Processing Technology (2nd edn.), Blackie Academic and Professional, London, UK.
- Andersen UB, Strømsnes AN, Steinsholt K and Thomassen MS (1994) Fillet gaping in farmed Atlantic salmon (Salmo salar). Norwegian J. Agric. Sci. 8: 165-179.
- Espe M, Kiessling A, Lunestad BT, Torrissen OJ, Rora AMB (2004) Quality of cold smoked salmon collected in one French hypermarket during a period of 1 year. Lebensmittel-Wissenschaft Und-Technologie-Food Science and Technology 37: 627-638.
- Acharya D, (2011), Fillet quality and yield of farmed atlantic salmon (Salmo salar L.): variation between families, gender differences and the importance of maturation. Norwegian University of Live Sciences, Norway.
- Gumus B, Balaban MO, Unlusayin M (2011) Machine Vision Applications to Aquatic Foods: A Review. Turkish Journal of Fisheries and Aquatic Sciences 11: 167-176.
- Quevedo RA, Aguilera JM, Pedreschi F (2010) Color of Salmon fillets by computer vision and sensory panel. Food and Bioprocess Technology 3: 637-643.
- Stien LH, Manne F, Ruohonene K, Kause A, Rungruangsak-Torrissen K, et al. (2006) Automated image analysis as a tool to quantify the colour and composition of rainbow trout (Oncorhynchus mykiss W.) cutlets. Aquaculture 261: 695-705.
- Stien LH, Kiessling A, Marine F (2007) Rapid estimation of fat content in salmon fillets by colour image analysis. Journal of Food Composition and Analysis 20: 73-79.
- Balaban MO, Sengor GFU, Soriano MG, Ruiz EG (2011) Quantification of gaping, bruising, and blood spots in salmon fillets using image analysis. J Food Sci 76: E291-E297.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 18019
- [From(publication date):
September-2013 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 13267
- PDF downloads : 4752