Commentary Open Access
Breaking the Vicious Circle of Dementia and Hypoglycemia in Type 2 Diabetes: New Niches and New Opportunities
YJ Sheen1 and Wayne HH Sheu2-5*1Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Hospital, Ministry of Health and Welfare, No. 199 Section 1, Sanmin Road, Taichung 403, Taiwan
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Veterans General Hospital, No. 1650, Section 4, Taiwan Boulevard, Taichung 40705, Taiwan
3School of Medicine, National Defense Medical Center, Taipei, Taiwan
4School of Medicine, National Yang-Ming University, Taipei, Taiwan
5Institute of Medical Technology, National Chung-Hsing University, Taichung, Taiwan
- *Corresponding Author:
- Wayne HH Sheu
Division of Endocrinology and Metabolism
Department of Internal Medicine
Taichung Veterans General Hospital
No. 1650 Sect. 4, Taiwan Boulevard
Taichung 40705, Taiwan
Tel: +886-4-2374-1300
E-mail: whhsheu@vghtc.gov.tw
Received Date: September 21, 2016; Accepted Date: September 27, 2016; Published Date: October 04, 2016
Citation: Sheen YJ, Sheu WHH (2016) Breaking the Vicious Circle of Dementia and Hypoglycemia in Type 2 Diabetes: New Niches and New Opportunities. J Alzheimers Dis Parkinsonism 6:266. doi: 10.4172/2161-0460.1000266
Copyright: © 2016 Sheen YJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abbreviations
TZD: Thiazolidinedione, DPP4I: Dipeptidyl Peptidase-4 Inhibitors, GLP-1R: Glucagon-like Peptide 1 Receptor Agonist, SGLT2i: Sodium Glucose Co-transporter 2 Inhibitors, SMBG: Self-Monitoring of Blood Glucose
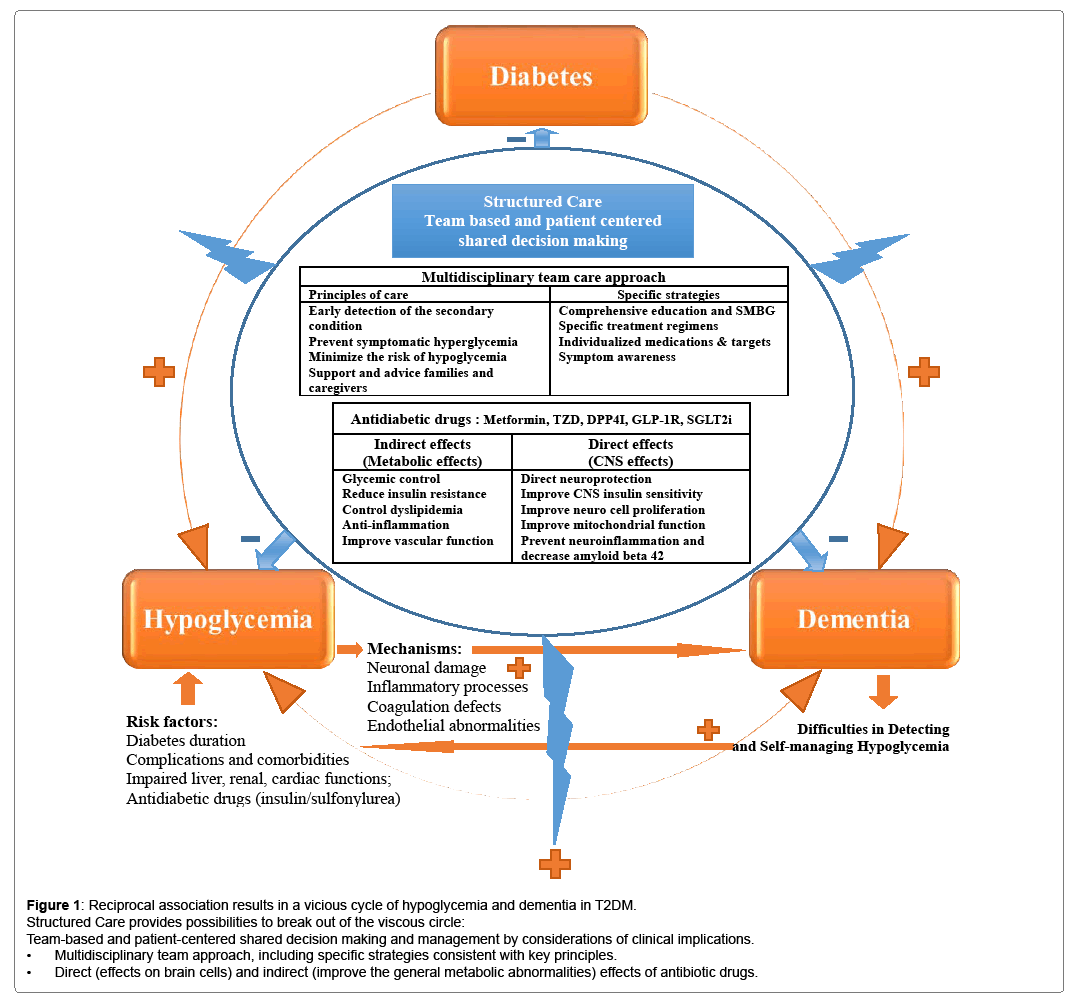
Dementia is a major cause of disability in elderly populations. As the prevalence of dementia still continues to increase globally, the World Health Organization has recognized dementia as a top public health priority [1]. Poor glycemic control is well known to lead to both macrovascular and microvascular complications. Recent studies have also confirmed that diabetes subjects may develop central nervous system disorders, presenting as cognitive impairment and dementia progression [2,3]. Although the causes of dementia in patients with type 2 diabetes (T2DM) are considered to be multifactorial [4,5], researchers proposed that the association between poor glycemic control (including high hemoglobin A1C values and glucose variability) and impaired cognitive function is independent of other metabolic abnormalities [6,7]. Moreover, hypoglycemia, a common consequence of diabetes management that is known to be associated with severe morbidity and life-threatening conditions, has been currently suggested as an independent risk factor to the development of dementia [8-11]. The results of several cross-sectional and longitudinal epidemiological studies have shown not only that hypoglycemic events were associated with an increased risk of dementia [5,9,12] but also that their associations seem to be dose dependent [9], i.e., the risk of dementia increased with severity and the number of hypoglycemic episodes. So far, a series of potential pathophysiological hypotheses has been proposed, including but not limited to, post-hypoglycemic neuronal damage, inflammatory processes, coagulation defects, endothelial abnormalities and synaptic dysfunction of hippocampal neurons during hypoglycemic episodes [3,9,13]. In consideration of the rapid aging population, we believe that hypoglycemia and dementia in diabetes should receive greater attention. In this article, we review the updated information between hypoglycemia and cognitive dysfunction, and provide relevant strategies specifically to reduce the potential burdens associated with hypoglycemia-related dementia in patients with diabetes [10].
Hypoglycemia events may be more common than we know. In the RECAP-DM study conducted in 5 Asian countries, we found that symptoms of hypoglycemia, obtained by asking history 6 months previously, were 35.8% of patients with type 2 diabetes treated with oral antihyperglycemic agents [14]. Among these patients, symptoms were severe in 11.6% and very severe in 8.2% of patients experiencing hypoglycemia [14]. Through continuous glucose monitoring (CGM), we also identified several midnight asymptomatic hypoglycemia in a group of patients with diabetes who underwent intensification of glucose managements by adding or increasing the doses of oral anti-diabetic agents [15]. In fact, repeated episodes of asymptomatic hypoglycemia could also be detrimental [15-17].
Hypoglycemia can lead to the development of dementia and an increased risk of subsequent cognitive dysfunction; alternatively, dementia in patients with diabetes can also increase the risk of hypoglycemia [5,12]. Both hypoglycemia and dementia are clinically underestimated and can seriously affect the quality of life and reduce the life expectancy of patients with diabetes [5,14,18-21]. These issues are more complicated in consideration of a bidirectional relationship, and the reciprocal causation results in a vicious cycle [5,10,12,21]. A recent meta-analysis of 12 cohorts (total number of patients enrolled: 1,439,818; mean age: 75 years) reported a significantly increased risk of dementia in patients with hypoglycemic episodes (pooled odds ratio: 1.68; 95% confidence interval: 1.45–1.95) and a significantly higher risk of hypoglycemia in patients with dementia (pooled odds ratio: 1.61; 95% confidence interval: 1.25–2.06) [12]. Other potential risk factors of hypoglycemia-associated dementia were genetic and demographic factors; diabetes duration; disease-related complications and comorbidities; impaired liver, renal and cardiac functions; and the choice of anti-diabetic drugs (especially insulin or sulfonylurea) [5,10,22,23].
Several pharmacological and non-pharmacological strategies may provide new possibilities to break out this viscous circle (Figure 1). For example, development and implementation of structured care by team-based approach offered specific strategies to tailor individual needs and therapeutic glycemic goals. In addition, new anti-diabetic medications seem to have favorable effects on neuroprotection through several direct (effects on brain cells) and indirect (improve the general metabolic abnormalities) mechanisms. Details are described in the following paragraphs.
Diabetic patients with dementia may have difficulties in acknowledging symptoms of hypoglycemia and learning how to avoid hypoglycemia. Therefore, professional education and adopted team especially with geriatric training appear particularly relevant [5,24]. In 2013, the International Diabetes Foundation launched a “Global Guideline for Managing Older People with Type 2 Diabetes” to respond to the rapidly aging population [25]. According to this guideline, specific recommendations of care programs (including personalized medications choice and targets) are required for each of the functional categories. They also address the special considerations (including hypoglycemia, frailty, and dementia) of elderly patients and their physical, cognitive, and social needs [25]. Recommendations of the American Diabetes Association 2016 emphasized that care systems should support structured educations (self-management and evidencebased decision support), community involvement (community policies for identifying and developing resources to support healthy lifestyles, and moving from a reactive to a proactive care delivery system), patient registries (using registries that could provide population-based and patient-specific support to the health-care system) and shared decision making by team members (including patients, families, caregivers, physicians, and social supporting systems) to meet individualized goals and patient needs [26]. The National Institute for Health and Care Excellence recommendations also suggest that a consultant-led multidisciplinary team may be involved in primary, secondary, and community care to ensure that structured education programs are evidence-based, suit the needs of the patient and are available locally, and that diabetic patients and their family members or caregivers have the opportunity to contribute to the design and provision of local programs [27]. Taken together, team care approach and multidisciplinary management are mandatory and considered to be effective for the prevention of hypoglycemia episodes and early detection of cognitive impairment among diabetic patients.
A number of new anti-diabetic drugs that confer low risk of hypoglycemia have been introduced in the past decade and have changed significantly the medication prescribing practices. This change is consistent with the clinical guidelines recommended in favor of medications with lower risk of hypoglycemia [25-31]. In fact, a recent Canadian population-based study found that the trends of the uses of anti-diabetic medications being prescribed from 2002 until 2013 changed greatly [31]. They identified a greater number of prescriptions for metformin and new medications, including dipeptidyl peptidase-4 (DPP4) inhibitors; a decreased percentage of using glyburide; and an increased use of combinations of medications, even in newly treated patients [31]. These findings are consistent with those of other nationwide population-based cohort study [32]. Furthermore, the overall percentage of hypoglycemia declined during the study period, the uptake of medications with low hypoglycemia risk and decline in the use of glyburide may have contributed to this trend [31].
Combination therapy with certain drugs by patient-centered decision making at submaximal doses that provide better postprandial glycemic control and lower risk of hypoglycemia may help to promote effective managements and provide possibilities to avoid longterm complications of diabetes, including cognitive dysfunction and dementia [30]. Besides, recent studies reported that some antidiabetic medications may promote neuronal survival (Figure 1) [33,34]. Metformin, a widely accepted first-line agent for patients with type 2 diabetes, could reduce tissue inflammation and oxidative stress, normalize the reduction of cell proliferation and neuroblast differentiation in animal models, activate AMP-activated protein kinase (AMPK)-dependent pathways, which may act against amyloid-betainduced mitochondrial dysfunction and help reduce neuronal injury [33,35]. A population-based study showed that long-term treatment with metformin may reduce the risk of cognitive decline in both cross-sectional (odds ratio: 0.30; 95% confidence interval: 0.11–0.80) and longitudinal analyses (odds ratio: 0.27; 95% confidence interval: 0.12–0.60) among patients with diabetes [36]. The insulin sensitizer thiazolidinedione (TZD) is an agonist of the peroxisome proliferatoractivated receptor gamma that may reduce inflammation, modulate cell-signaling pathways, and improve endothelial functions and ischemic brain injuries. However, only some studies indicate that TZD treatment is associated with a reduction in dementia risk [33,34,37]. The glucagon-like peptide 1 (GLP-1) receptor agonist, a kind of incretin mimetics, may help regulate insulin secretion, overcome an increasingly recognized GLP-1-resistant state, and improve peripheral and central insulin signaling. The agents can cross the blood-brain barrier (BBB); thus, they might have a therapeutic effect for minimizing neuronal cell loss and even possibly rescuing cognitive decline in Alzheimer’s disease and other forms of dementia [33,38,39]. Growing evidence suggests that GLP-1 receptor agonists provide a neuroprotective effect in reducing tissue damage after cerebral ischemia, increase proliferation of neuro stem cells, and improve memory performance in animal models, but clinical trials of these agents in Alzheimer’s disease are still underway [33,38,39]. DPP4 inhibitors inhibit the degradation of incretins and may offer neuroprotective effects by increasing GLP-1 levels and decreasing amyloid beta 42, total tau, and phosphorylated tau levels [33,40]. The neuroprotective effects of DPP4 inhibitors have been evaluated in cell cultures and animal models, but not in human clinical trials [41]. Novel classes of anti-diabetic drugs such as dapagliflozin and canagliflozin might act as potent dual inhibitors of sodium glucose cotransporter 2 (SGLT2) and acetylcholinesterase. The results are expected to lead to the development of a therapy for Alzheimer’s disease and diabetesassociated neurological disorders [42,43].
Dementia is an important but much overlooked complication of diabetes, which has physical, psychological, and socioeconomic effects on diabetes patients, their families, and the society [1]. Hypoglycemic episodes can lead to the development of dementia in diabetic patients, while diabetic patients with dementia may face difficulties in detecting and self-managing hypoglycemia. Anti-diabetic agents with lower risk of hypoglycemia might provide favorable effects on the central nervous system. It is hope that multidisciplinary team approach aim to provide the possibilities and new therapeutic opportunities to break out of the vicious circle of dementia and hypoglycemia in patients with T2DM.
References
- Wortmann M (2012) Dementia: A global health priority - highlights from an ADI and World Health Organization report. Alzheimer's Research &Therapy 4: 40.
- Weng SC, Shu KH, Tang YJ, Sheu WH, Tarng DC, et al. (2012) Progression of cognitive dysfunction in elderly chronic kidney disease patients in a veteran's institution in central Taiwan: A 3year longitudinal study. Intern Med 51: 29-35.
- Scheen AJ (2010) Central nervous system: A conductor orchestrating metabolic regulations harmed by both hyperglycaemia and hypoglycaemia. Diabetes &Metabolism 36 Suppl 3: S31-38.
- Kawamura T, Umemura T, Hotta N (2012) Cognitive impairment in diabetic patients: Can diabetic control prevent cognitive decline? Journal of Diabetes Investigation 3: 413-423.
- Abdelhafiz AH, McNicholas E, Sinclair AJ (2016) Hypoglycemia, frailty and dementia in older people with diabetes: Reciprocal relations and clinical implications. Journal of diabetes and its complications S1056-8727: 30329-30334.
- Tournoy J, Lee DM, Pendleton N, O'Neill TW, O'Connor DB, et al. (2010) Association of cognitive performance with the metabolic syndrome and with glycaemia in middle-aged and older European men: The European male ageing study. Diabetes Metab Res Rev 26: 668-676.
- Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, et al. (2009) Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: The action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 32: 221-226.
- Morales J, Schneider D (2014) Hypoglycemia. Am J Med 127: S17-24.
- Lin CH, Sheu WH (2013) Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7year follow-up study. J Intern Med 273: 102-110.
- Sheen YJ, Sheu WH (2016) Association between hypoglycemia and dementia in patients with type 2 diabetes. Diabetes Research and Clinical Practice 116: 279-287.
- Desouza CV, Bolli GB, Fonseca V (2010) Hypoglycemia, diabetes and cardiovascular events. Diabetes Care 33: 1389-1394.
- Mattishent K, Loke YK (2016) Bi-directional interaction between hypoglycaemia and cognitive impairment in elderly patients treated with glucose-lowering agents: A systematic review and meta-analysis. Diabetes, Obesity &Metabolism 18: 135-141.
- Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, et al. (1997) Specific changes in human brain after hypoglycemic injury. Stroke 28: 584-587.
- Chan SP, Ji LN, Nitiyanant W, Baik SH, Sheu WH (2010) Hypoglycemic symptoms in patients with type 2 diabetes in Asia-Pacific-real-life effectiveness and care patterns of diabetes management: The RECAP-DM study. Diabetes Research and Clinical Practice 89: e30-32.
- Wang JS, Lee IT, Lee WJ, Lin SD, Su SL, et al. (2016) Glycemic excursions are positively associated with changes in duration of asymptomatic hypoglycemia after treatment intensification in patients with type 2 diabetes. Diabetes Research and Clinical Practice 113: 108-115.
- Dave KR, Tamariz J, Desai KM, Brand FJ, Liu A, et al. (2011) Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke 42: 1404-1411.
- Moore H, Craft TK, Grimaldi LM, Babic B, Brunelli SA, et al. (2010) Moderate recurrent hypoglycemia during early development leads to persistent changes in affective behavior in the rat. Brain BehavImmun 24: 839-849.
- McGuire LC, Ford ES, Ajani UA (2006) The impact of cognitive functioning on mortality and the development of functional disability in older adults with diabetes: the second longitudinal study on aging. BMC Geriatr 6: 8.
- Bordier L, Doucet J, Boudet J, Bauduceau B (2014) Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes &Metabolism 40: 331-337.
- Wang CY, Lin CL, Huang TS, Chien MN, Hsieh SH, et al. (2012) Inertia on hypoglycemia: Highlight from a Taiwan subgroup analysis of real-life effectiveness and care patterns of diabetes management (RECAP-DM) study. Diabetes Research and Clinical Practice 98: 61-67.
- Sheu WH, Ji LN, Nitiyanant W, Baik SH, Yin D, et al. (2012) Hypoglycemia is associated with increased worry and lower quality of life among patients with type 2 diabetes treated with oral antihyperglycemic agents in the Asia-Pacific region. Diabetes Research and Clinical Practice 96: 141-148.
- Yaffe K, Falvey CM, Hamilton N, Harris TB, Simonsick EM, et al. (2013) Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Internal Medicine 173: 1300-1306.
- Lin YY, Hsu CW, Sheu WH, Chu SJ, Wu CP, et al. (2010) Risk factors for recurrent hypoglycemia in hospitalized diabetic patients admitted for severe hypoglycemia. YonseiMedical Journal 51: 367-374.
- Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH (2015) Hypoglycemia in type 2 diabetes--More common than you think: A continuous glucose monitoring study. Journal of Diabetes Science and Technology 9: 999-1005.
- http://www.idf.org/guidelines-older-people-type-2-diabetes
- http://care.diabetesjournals.org/content/39/Supplement_1
- https://www.nice.org.uk/guidance/ng28
- Sinclair AJ, Hillson R, Bayer AJ (2014) Diabetes and dementia in older people: A Best Clinical Practice Statement by a multidisciplinary National Expert Working Group. Diabetic Medicine: A Journal of the British Diabetic Association 31: 1024-1031.
- Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, et al. (2016) Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm--2016 executive summary. Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 22: 84-113.
- Lavernia F, Adkins SE, Shubrook JH (2015) Use of oral combination therapy for type 2 diabetes in primary care: Meeting individualized patient goals. Postgraduate Medicine 127: 808-817.
- Clemens KK, Shariff S, Liu K, Hramiak I, Mahon JL, et al. (2015) Trends in antihyperglycemic medication prescriptions and hypoglycemia in older adults: 2002-2013. PloSOne 10: e0137596.
- Ko SH, Kim DJ, Park JH, Park CY, Jung CH, et al. (2016) Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002-2013: Nationwide population-based cohort study. Medicine 95: e4018.
- Patrone C, Eriksson O, Lindholm D (2014) Diabetes drugs and neurological disorders: New views and therapeutic possibilities. The Lancet Diabetes &Endocrinology 2: 256-262.
- Chen Y, Zhang J, Zhang B, Gong CX (2016) Targeting Insulin Signaling for the Treatment of Alzheimer's Disease. Current topics in medicinal chemistry 16: 485-492.
- Chiang MC, Cheng YC, Chen SJ, Yen CH, Huang RN (2016) Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against Amyloid-beta-induced mitochondrial dysfunction. Experimental Cell Research 347: 322-331.
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, et al. (2014) Long-term metformin usage and cognitive function among older adults with diabetes. Journal of Alzheimer's Disease41: 61-68.
- Ye F, Luo YJ, Xiao J, Yu NW, Yi G (2016) Impact of Insulin Sensitizers on the Incidence of Dementia: A Meta-Analysis. Dementia and geriatric cognitive disorders 41: 251-260.
- Athauda D, Foltynie T (2016) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: Mechanisms of action. Drug Discovery Today 21: 802-818.
- Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, et al. (2013) Crosstalk between diabetes and brain: Glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. BiochimicaetBiophysicaActa1832: 527-541.
- Kosaraju J, Madhunapantula SV, Chinni S, Khatwal RB, Dubala A, et al. (2014) Dipeptidyl peptidase-4 inhibition by Pterocarpusmarsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer's disease. Behavioural Brain Research 267: 55-65.
- Matteucci E, Giampietro O (2015) Mechanisms of neurodegeration in type 2 diabetes and the neuroprotective potential of dipeptidyl peptidase 4 inhibitors. Current Medicinal Chemistry 22: 1573-1581.
- Shaikh S, Rizvi SM, Shakil S, Riyaz S, Biswas D, et al. (2016) Forxiga (dapagliflozin): Plausible role in the treatment of diabetes-associated neurological disorders. Biotechnology and applied biochemistry 63: 145-150.
- Rizvi SM, Shakil S, Biswas D, Shaikh S, Bagga P, et al. (2014) Invokana (Canagliflozin) as a dual inhibitor of acetylcholinesterase and sodium glucose co-transporter 2: advancement in Alzheimer's disease- diabetes type 2 linkage via an enzoinformatics study. CNS &Neurological Disorders Drug Tar gets 13: 447-451.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 12648
- [From(publication date):
October-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11711
- PDF downloads : 937