Brain Targeting Potential of Intravenous Carbamazepine SNEDDS
Received: 20-Sep-2017 / Accepted Date: 04-Oct-2017 / Published Date: 09-Oct-2017 DOI: 10.4172/2329-9053.1000137
Abstract
Purpose: The objective of this study was to evaluate the ability of nano-droplets of Self Nano Emulsifying Drug Delivery System to diffuse into the brain tissue.
Methods: The preconcentrate was prepared by dissolving oils, surfactants and cosolvents in 1:1 mixture of methanol and chloroform and flash evaporated at 50°C and was stored at room temperature until their use in subsequent studies. CSEDD with surfactant polysorbate-80 were radiolabel led with radioactive C18 triglyceride. The nanodroplets formed by CSEDD in 5% dextrose were subjected to evaluation in blood and brain.
Results: The intravenous pharmacokinetics of carbamazepine in rats of CSEDD formulation generated high initial serum levels (5.25 mcg/ml) at 0.25 h when compared to C-Sol (3.91 mcg/ml) at 0.5 h. It was found that oil to surfactant ratio had an impact on the physical characteristics of the nano-emulsion formed. The brain levels of CBZ from optimized CSEDD were significantly high at all-time points when compared to plain solution. The initial levels of CBZ from CSEDD was 8.023 mcg/ml thereafter the levels were consistently high till 8 h and the initial levels of CBZ from plain solution was 3.62 mcg/ml followed by a gradual decline till 4 h evidently showing that the clearance of CBZ from CSEDD was reduced. The brain targeting index of CSEDD and solution were 3 and 2 respectively. The brain enhancement factor value was found to be 22.29 at 15 min revealing a very rapid penetration of CBZ into brain.
Conclusions: This study proposes intravenous CSEDD as a new brain delivery system and highlights two requirements to design adequate delivery systems for long circulating properties of the carrier and appropriate surface characteristics to allow interactions with BBB endothelial cells.
Keywords: SNEDDS; Carbamazepine; Intravenous; Brain; Targeting
Introduction
The blood brain barrier (BBB), which acts as a natural guard to protect the brain from harmful substances in the blood stream while supplying the brain with the necessary nutrients for proper function, is the key challenge for delivering drugs to brain [1]. The BBB is a specialized system of capillary endothelial cells which are partially covered by pericytes and basement membrane is almost fully surrounded by the end feet of astrocytes preventing approximately 98% of the small molecules and nearly 100% of large molecules from being transported into the brain [2,3]. The BBB strictly limits drug transport into the brain by serving as a physical, metabolic and immunological barrier. To tackle this challenge, many kinds of active targeting strategies are adopted for developing effective drug delivery systems to the brain. The active targeting systems used are absorptive mediated transcytosis, transporter mediated transcytosis and receptor mediated endocytosis [4]. Generally, receptor mediated transcytosis is considered one of the most mature strategies for brain targeted drug delivery with the characteristics of high specificity, selectivity and affinity. Since many kinds of receptors are expressed on the capillary endothelium of the brain such as transferring receptor, the low density lipoprotein receptor, the insulin receptor and nicotinic acetylcholine receptors, targeting ligands including endogenous ligands have been exploited to facilitate receptor mediated BBB transport of drug delivery systems. Another common receptor, the low-density lipoprotein receptor related protein, has been reported to mediate transport of various ligands conjugated to nanocarriers across the BBB. Aprotinin is a LRP ligand and its BBB transport ability is evaluated using an in vitro model of the BBB and in situ brain perfusion. Its transcytosis across bovine brain capillary endothelial cell monolayers is found to be atleast 10 fold greater than that of holo transferrin [5]. Angiopep, derived from aprotinin with the Kunitz domains of human proteins exhibits even higher transcytosis capacity and parenchymal accumulation. It is reported that reported angiopep 2 modified cationic liposomes for the efficient co-delivery of a therapeutic gene with paclitaxel to the brain. After treatment with liposomes, the median survival time of mice is found to be significantly longer than that of other groups making it a promising drug delivery strategy against glioma [6]. In convulsive status epilepticus, intravenous administration of a benzodiazepine (BZD) such as diazepam or lorazepam is the preferred drug treatment. However, patients frequently have seizures refractory to this medication and parenteral phenytoin (PHT) or barbiturate treatment is required. However, this may depress respiration or induce cardiac irregularities. Furthermore, apart from PHT, all drugs currently used for control of status epilepticus depress cerebral function sufficiently to induce and maintain clouding of consciousness. Therefore, there is a need for a nonsedative antiepileptic drug for treatment of SE that fails to respond to bolus doses of a BZD. Carbamazepine (CBZ) is available as tablets and suspension for acute treatment of seizures. However certain formulations of CBZ, for intravenous administration, CBZ dissolved in glycofurol [7] and CBZ solubilized with 2-hydroxypropylb- cyclodextrin [8] are reported. Two lipid formulations of CBZ are reported, one is a parenteral formulation of CBZ developed using SolEmuls technology [9] in which the CBZ is solubilized in the interfacial layer of a lipid emulsion (lipofundin) by high pressure homogenization and the other is slow release lipospheres of CBZ prepared by melt dispersion technique [10]. The development of parenteral nanoemulsions through spontaneous emulsification and the testing of different oils and emulsifiers comprise another strategy which is currently the novel investigation [11]. Carbamazepine (CBZ) is a highly lipophilic compound and is very effective in the treatment of generalized tonic clonic and partial seizures. Since in seizure disorder focal point of treatment is brain, many methods to directly/ indirectly deliver drugs to brain have been approached [12]. Instead of direct delivery of anti-epileptic drugs (AEDs) to brain using some invasive techniques, it would be better to explore the possibility of AED targeting by non-invasive techniques. In such approaches, colloidal drug carriers such as Self Nano Emulsifying Drug Delivery System (SNEDDS) can be employed. The objective of the study was to assess the intravenous pharmacokinetics of CSEDD in rats and also to evaluate the brain targeting potential of such CBZ containing SEDD upon its intravenous bolus administration [13] under the hypothesis to make them mimic lipoprotein particles to enter BBB endothelium [14].
Materials and Methods
Preparation of preconcentrate
Preconcentrate was prepared by dissolving oleic acid, soya bean oil, Ascorbyl Palmitate, (Sigma, St.Louis, MO, USA); TPGS (Eastman chemicals UK); carbamazepine (Sun Pharmaceuticals); Ethanol, polysorbate 80, span80, PEG-200, benzyl Alcohol (Merck Ltd); Labrasol and MCT (Colorcon Laboratories, Goa) in 1:1 mixture of methanol and chloroform and flash evaporated at 50°C as described elsewhere (20). Preconcentrate was stored at room temperature until their use in subsequent studies. CSEDD with surfactant polysorbate 80 were radiolabelled with radioactive C18 triglyceride. The nanodroplets formed by CSEDD in 5% dextrose were subjected to evaluation in blood and for the transport of in different parts of the brain by their measurement of radioactivity.
Animal studies
This work was done in accordance with the Principles of Institutional Animal Ethical Committee of UCPSC, Kakatiya University. Mice (20-25 g) and rats (200-230 g) were obtained from Mahaveer Enterprises, Hyderabad. Animals were housed in plastic cages, were given food and water ad libitum and were maintained in temperature and humidity controlled rooms. Test formulation and control of CBZ were injected intravenously at a dose of 10 mg/kg in 8 groups of 3 rats (Intravenous Pharmacokinetics studies) and at a dose of 10 mg/kg in 8 groups of 3 mice (Brain distribution studies) and serum & brain concentrations were assessed at predetermined time intervals in which after administration with one group of animals used per time point. CBZ was determined by HPLC. Required volume of formulation containing CBZ (10 mg/kg) was injected as IV bolus via tail vein randomly into the rats and mice after 8 to 10 h of fasting. Rats were sacrificed at intervals of 0, 0.5, 1, 2, 4, 8, 12 and 24 h. Blood samples were also collected at above time points by direct heart puncture. Serum was separated from the blood by centrifuging at 6000 g and stored under frozen conditions until analysis by HPLC. Mice were sacrificed at intervals of 0, 0.25, 0.5, 1, 2, 4, 8 and 12 h. Brain tissue was excised, washed with saline, soaked on filter paper and stored at -40°C until analysis by HPLC. Blood samples were also collected at above time points by direct heart puncture. Serum was separated from the blood by centrifuging and stored under frozen conditions until analysis by HPLC.
HPLC Analysis
Carbamazepine was analyzed in serum and homogenized brain samples using HPLC. A HPLC waters agilent system equipped with LC-10 AT solvent delivery unit, SPD-10 AVP UV-Spectrophotometric detector, empower-2 software and Injector (Rheodyne) fitted with 20 ml capacity loop was used for the analysis. An octadecylsilane (C-18) reverse phase stainless steel analytical column (250 × 4.6 mm) with 5 μm particle size (Lichrospher 100) was employed for chromatographic separation. Mobile phase consists of methanol and phosphate buffer in the ratio of 8:2 with flow rate maintained at 1 ml/min. The drug concentration in the serum of mice and rat were separated from blood and used for preparing the standard graphs. To 0.2 ml of sample serum (mice and rat), 0.1 ml of diclofenac sodium as internal standard was added. To this 4 ml of dichloromethane is added and the mixture was vortexed for 2 min on cyclomixer, then centrifuged for 15 min at 3000 g. The organic layer was separated and dried under vacuum. The final residue was reconstituted with 200 μl of methanol, vortexed and 20 μl sample was injected into HPLC for analysis. The peak height ratios obtained at different concentrations of the drug were plotted against the concentration of the drug. The slope of this plot determined by least square regression analysis was used to calculate carbamazepine concentration in the unknown serum samples. Similarly, brain tissues were weighed and homogenized with 2 ml of saline using tissue homogenizer (Remi Motors, India) at 6000 g. To this whole brain homogenate, 0.1 ml of internal standard (diclofenac sodium), 0.1 ml methanol, 0.1 ml of 1 N Hcl and 4 ml of dichloromethane were added and mixed for 2 min on a cyclomixer. The mixture was centrifuged at 3000 g for 30 min. The organic layer was separated and dried under vacuum. The resultant residue was extracted with 2 ml of ethyl acetate and 1 ml of 0.1 N Hcl. The mixture was centrifuged at 2500 g for 15 min. The organic layer was separated and dried under vacuum. The resultant residue was reconstituted in 100 μl of methanol and 20 μl was injected on to the HPLC column.
Data analysis
Serum concentration vs time curves and brain concentration vs time curves were evaluated using Winonlin 3.3 Pharsight, Mountain View, CA, USA. Maximal serum and brain tissue concentrations (Cmax) and time to Cmax (Tmax) were obtained directly from the raw data. The terminal half-life t1/2 was calculated from the terminal slope. The area under curve from time zero to the last quantifiable plasma concentration (AUC) was calculated using the linear trapezoidal rule. The data of two formulations was subjected one way analysis of variance (ANOVA) to get the statistical significance. Significance of difference between formulations was calculated by student-Newman-Keuls with Instant Graph pad prism software. The difference was considered to be statistically significant at p<0.05.
Brain distribution studies
Brain targeting index and targeting enhancement factors: The brain targeting potential of CSEDD is assessed based on brain/serum drug concentration (B/S) ratio [15]. Brain to serum drug concentration ratio (B/S) is commonly employed as an index of targeting. If this ratio is more than one indicates drug targeting to that tissue. It is defined as the ratio between the area under the concentration-time curve (AUC) for the concentration of the drug itself at the targeted site and that at a systemic site:
BTI CSEDD=AUC brain/AUC serum, BTI solution=AUC brain/AUC serum
BTI gives an accurate measure on how effectively the active therapeutic agent is actually delivered to its intended site of action.
Brain enhancement factor (BEF): To assess the effectiveness of CSEDD compared with the drug itself, brain exposure enhancement factor (BEF) measures the change of the AUC of the active drug in the brain after administration of CSEDD, compared with administration of the drug alone:
BEF = AUC CSEDD in brain/AUC solution in brain
Targeting enhancement factor (TEF): Targeting enhancement factor (TEF) measures the relative improvement in the BTI produced by administration of CSEDD, compared with administration of the drug itself:
TEF = BTI CSEDD/BTI solution
TEF, as defined above, is the most rigorous measure that can be used to quantify the improvement in targeting produced by CSEDD. It compares not only concentrations, but also concentrations along a time period and it compares actual, active drug concentrations, both at target and systemic sites provided the targeting or delivery produces no toxicity of its own, the therapeutic effect is linearly related to AUC target and adverse effects are linearly related to AUC of blood.
Results
HPLC analysis
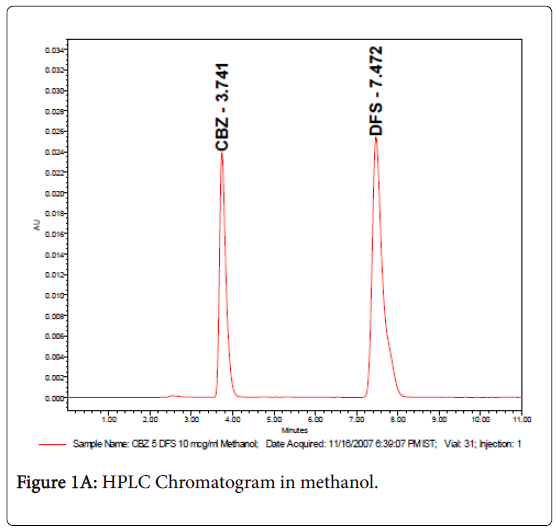
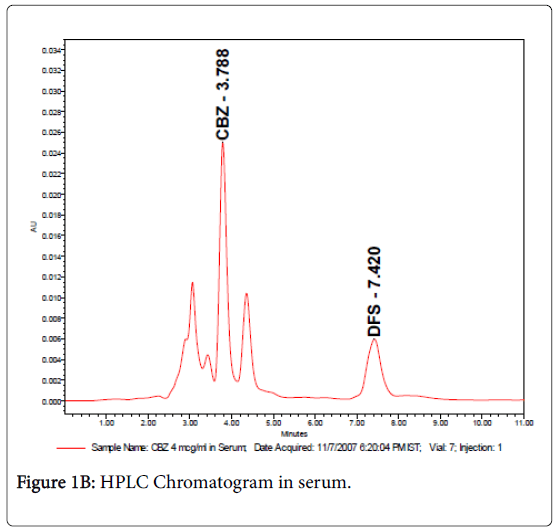
The retention times were 3.7 and 7.4 min for carbamazepine and internal standard (diclofenac sodium) respectively as shown in Figures 1A & 1B. Samples were detected using UV-Spectrophotometric detector at 276 nm and separation was at ambient temperature and the sensitivity was set at 0.005 AUFS. The method was validated for carbamazepine assay, according to ICH guidelines (ICH 2005) with respect to specificity, linearity (R2>0.99), precision (intra-day R.S.D<0.44 and inter-day R.S.D.<1.21%), and accuracy (recoveries between 99.4% and 102.1%).
Pharmacokinetic studies in rats
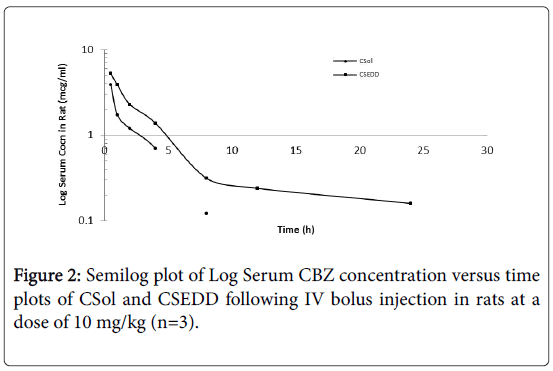
The intravenous pharmacokinetic studies were performed in rats. CSol and CSEDD were administered to rats through tail vein. The pharmacokinetic profiles of CSol and CSEDD were assessed based in rat serum. The serum concentrations of CBZ versus time profiles following intravenous bolus administration of CSol and CSEDD in rats are shown in Figure 2. The CSEDD formulation generated high initial serum levels (5.25 mcg/ml) at 0.25 h (P<0.01) when compared to C-Sol (3.91 mcg/ml) at 0.5 h (P<0.05). A rapid fall initially up to 1 h and then a gradual fall up to 8 h followed by fairly constant low levels from 8 h to 24 h were seen with CSEDD formulation. The serum levels of C-Sol showed an initial rapid fall at 1 h and then gradual fall up to 4 h and thereafter the levels were not detected at 8 h, whereas the CBZ levels from CSEDD formulation were detectable even at 24 h.
Pharmacokinetic parameters were calculated from data obtained in rat serum and their statistical significance was obtained using Anova. From Table 1, it is evident that a significant increase in AUC of CSEDD formulation (23.23 and 28.56 mcg.hr/ml) was observed when compared to C-Sol (17.64 and 19.67 mcg.hr/ml).
| Parameters (Units) | C-Sol (mcg/ml) Mean ± SD | C-SEDD (mcg/ml) Mean ± SD |
|---|---|---|
| Cmax | 3.91 ± 0.31 | 4.45 ± 0.35 |
| Tmax | 0.5 ± 0.06a | 0.25 ± 0.02c |
| AUC(0-12) | 7.628 ± 0.16 | 15.442 ± 0.05 |
| AUC(0-t) | 17.64 ± 2.81a | 23.23 ± 3.83 |
| AUC(0-∞) | 19.67 ± 4.27 | 28.56 ± 6.93d |
| Vd | 4.76 ± 1.03a | 7.95 ± 1.74 |
| CL | 0.51 ± 0.07 | 0.35 ± 0.02 |
| Vss | 9.34 ± 1.21 | 8.06 ± 2.15 |
| MRT | 18.37 ± 6.18 | 23.05 ± 1.92d |
| Ke | 0.107 ± 0.001d | 0.047 ± 0.001d |
| T1/2 | 6.491 ± 0.18a | 15.982 ± 2.17c |
Table 1: Mean pharmacokinetic parameters of CBZ following intravenous bolus administration of C-Sol and C-SEDD in rats at a dose of 10 mg/kg (n=3).
Similarly, 2.5 times increase in T1/2 (P<0.05) with CSEDD formulation was noticed than C-Sol and this is clearly reflected in reduced clearance and elimination (P<0.001). Among the other pharmacokinetic parameters a significant increase in Vd and MRT was seen with CSEDD formulation. In conclusion, a statistically significant increase was observed in all the pharmacokinetic parameters with CSEDD formulation.
Brain targeting potential studies in mice
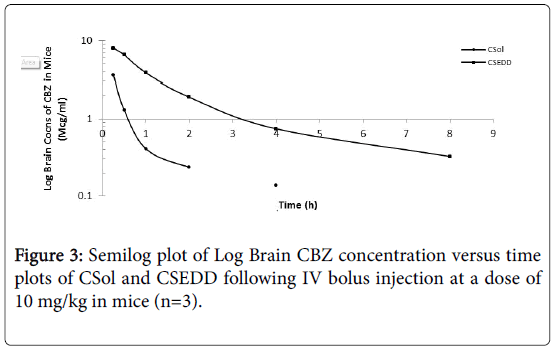
The brain targeting potential of CBZ solution and CSEDD was assessed from brain distribution studies in mice. The brain levels of CBZ due to CSEDD administration were significantly high at all-time points when compared to C-Sol (Figure 3). A slow decline of CBZ levels with time was observed for both the formulations. The initial levels of CBZ due to C-SEDD were 8.023 mcg/ml thereafter the levels were consistently high till 8 h and later to this time point levels were undetectable. The initial levels of CBZ due to C-Sol were 3.62 mcg/ml followed by a gradual decline till 4 h and thereafter the levels were undetectable.
The pharmacokinetic parameters for CBZ were calculated from brain distribution data obtained in mice and their statistical significance was obtained using Anova. The peak levels of CBZ (Cmax=9.15 mcg/ml) in brain were attained much rapidly (Tmax = 1.0 h) due to treatment with Test than control. This shows rapid and higher uptake of CBZ into brain. Further the levels of CBZ due to CSEDD retained in brain for a longer time because of slow elimination (T1/2=2.56 h, Ke=0.294/h) and clearance (CL=0.25L/h) from this tissue. As a result of this the brain availability of CBZ AUC(0-t) and AUC(0-∞)=3.66 and 40.6 mcg.hr/ml respectively) improved significantly over the C-Sol (Table 2).
| Parameters (Units) | C-Sol (mcg/ml) Mean ± SD | C-SEDDS (mcg/ml) Mean ± SD |
|---|---|---|
| Tmax | 2.5 ± 0.24 | 1.083 ± 0.01c |
| AUC(0-4) | 2.196 ± 0.21 | 10.825 ± 0.06 |
| AUC(0-t) | 15.16 ± 3.17a | 36.61 ± 2.9d |
| AUC(0-∞) | 15.81 ± 5.23d | 40.593 ± 8.92 |
| Vd | 0.79 ± 0.001d | 0.92 ± 0.08c |
| CL | 0.733 ± 0.13 | 0.251 ± 0.01 |
| Vss | 1.47 ± 0.93 | 1.06 ± 0.001 |
| MRT | 2.343 ± 3.91 | 4.238 ± 2.77a |
| Ke | 0.911 ± 1.34 | 0.294 ± 0.01c |
| T1/2 | 0.869 ± 0.03 | 2.565 ± 0.05a |
Table 2: Mean pharmacokinetic parameters of CBZ obtained from brain data due to CSol and SEDD administration at a dose of 10 mg/kg in mice (n=3).
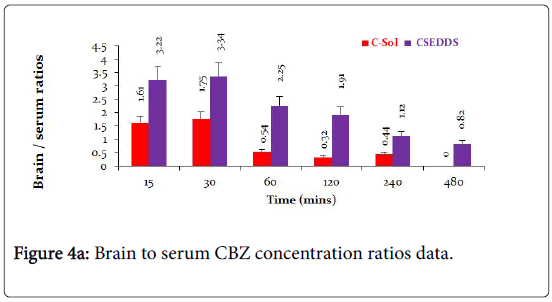
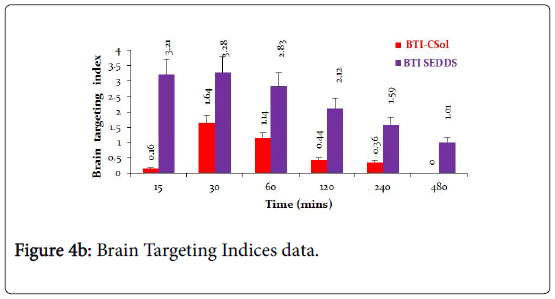
The brain to serum ratios (Figure 4A) due to treatment with C-Sol were either below or around 1 whereas brain to serum ratios for CSEDD treatment were higher than one at all-time points, excepting at 8th h. This clearly indicates that CSEDD has the potential for brain targeting of CBZ. The B/S ratios with CSEDD formulation were higher than one. The brain targeting index (Figure 4B) of CSEDD formulation was above 3 at 30 min and gradually declined from 1 to 8 h. These values were very high compared to brain targeting index values of CSol which were below 2 at all-time points.
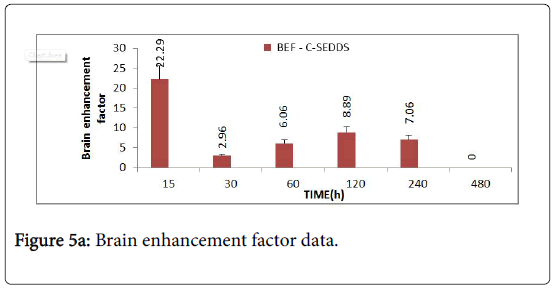
The brain enhancement factor (Figure 5A) of CSEDD to C-Sol was evaluated according to the previously mentioned equation. The BEF value was found to be 22.29 at 15 min revealing a very rapid penetration of CBZ into brain and thereafter a decrease followed by a sudden increase which maintained for 4 h.
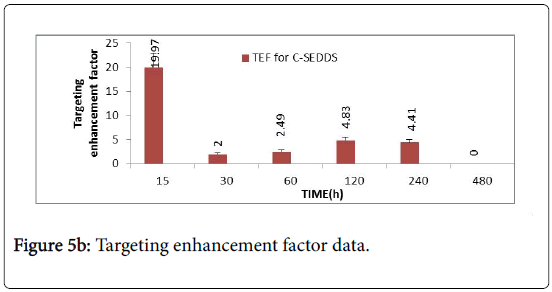
Based on the data it is evident that, very high TEF (Figure 5B) values were obtained at 15 min only. It was observed that the targeting enhancement factor at 0.25 h is significantly high followed by a rapid fall at 0.5 h and again a sudden increase at 2 h till 4 h.
Brain distribution studies
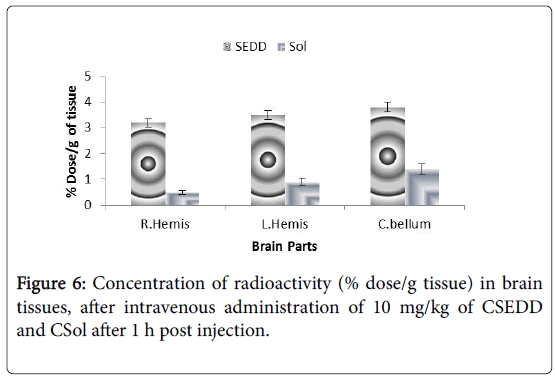
The brain concentrations of carbamazepine achieved with CSol and CSEDD were calculated as brain parenchyma concentrations and expressed as percentage of the injected dose/gram of tissue. The values of regional blood volumes used to correct brain radio activities were in accordance with those published previously [16,17]. Mice brains were divided into three regions, the two hemispheres and the cerebellum. In Figure 6 the concentrations of CBZ from CSEDD in mice brain after 1 h were observed to be the most effective carrier to improve CBZ brain concentrations. A three-fold increase of radioactivity was obtained in mice with CSEDD in comparison to CSol. The radioactivity measurement of CSEDD ranged between from 3.2 ± 0.1 to 3.7 ± 0.2% and 0.4 ± 0.2% to 1.2 ± 0.2% for plain solution in right hemisphere, left hemisphere and cerebellum respectively (Figure 6).
Discussion
The serum availability of CBZ due to intravenous administration of aqueous dispersion of SEDDS formulation in rats improved significantly compared to CBZ solution. The high mean residence time, less clearance and increased half-life clearly reveals that PEG 200 coat provides the required hydrophilicity to the globules thus reducing the opsonization process. The steady state volume of distribution was observed to be low. The high Cmax is a clear indication that both the size and hydrophilicity are preventing the opsonisation process which ultimately maintains increased serum levels of CBZ. The brain availability of CBZ due to intravenous administration of aqueous dispersion of SEDDS formulation in mice was significantly high compared to C-Sol. The brain transport in mice was enormously increased. The possible mechanisms that could be attributed are, the nanoglobules which were coated with polysorbate-80 might have been preferentially taken up by brain via BBB brain directed plasma proteins adsorption on such globules. This theory is supported by a study which reports that the coating of nanoparticles with polysorbate-80 leads to a specific alteration of the surface properties of the nanparticles. They further adsorb certain substances from the blood that, induce endocytic uptake from the blood stream by the endothelial cells of BBB [18]. The concentration of polysorbate-80 which we have used was 1% and this concentration was found to be a contributing factor for transport to brain. Our study is also supported by a report that states the translocation of chitosan nanoparticles across the BBB due to coating of such particles by polysorbate-80. Translocation of nanoparticles across the BBB was greatest with 1% w/w polysorbate-80 coating on chitosan nanoparticles. Interestingly, at a higher concentration of polysorbate-80, such as 2% w/w, the percent accumulation of radioactivity in the brain at 5 min was reduced [19]. The other possible mechanism for the higher brain levels of CBZ indicate that the CBZ containing oil droplets might have interacted with capillary endothelial cell surfaces in tissues via adhesion bonding probably involving ascorbyl palmitate moiety present on the surface of oil droplets. From such droplets ASP might desorb, enter the cell wall and consequently permeate cell walls. Similar mechanism was shown to be responsible for enhanced permeation of cancerostatic agents via BBB when these drugs were co-administered with 1-O-alkyl glycerols [20].
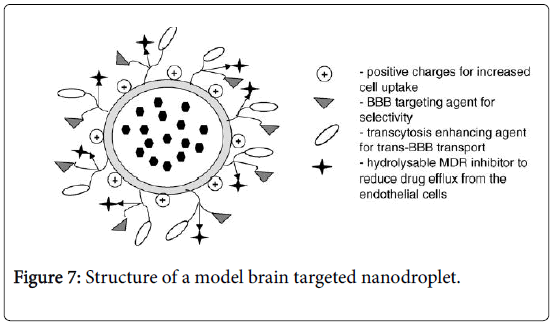
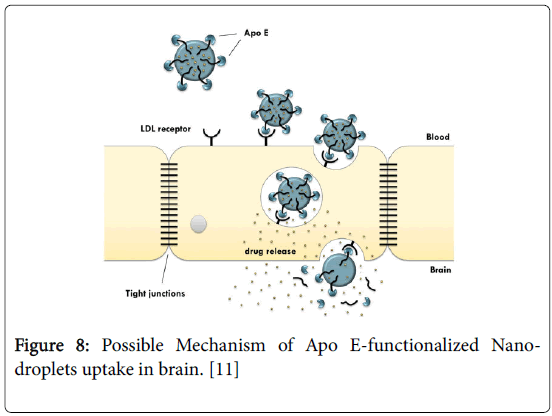
The structure of a model brain targeted nanodroplet is shown in Figure 7 to explain the role of ascorbyl palmitate and polysorbate-80 which enhance the targeting to brain. Since the degree of protein binding might not have been complete for CSEDD and the presence of PEG 200 could have given sufficient hydrophilicity on the surface, this could have contributed to variations in the brain uptake. The higher levels of CBZ due to CSEDD might be due to a relatively rapid distribution followed by slow clearance from the capillary bed of the brain. The droplets might have interacted with capillary endothelial cell surfaces more due to presence of the lipophilic moieties which could have helped in a better permeation of the formulation into the brain. From the therapeutic availability data it is clear that ASP allowed significant accumulation of CBZ into brain (2 times). A B/S ratio of 3 within 0.25 h and 2 times higher brain availability of CBZ is an important finding and it unveils the brain targeting potential of this system. Nanoparticles have previously been shown to enable the transport of a number of drugs across the blood-brain barrier that normally cannot cross this barrier after IV injection [21]. This bloodbrain barrier transport is achieved by coating the particles with polysorbate-80 and led to significant pharmacological and therapeutic effects in the brain [22] and postulated that the polysorbate-80 coating led to the adsorption of apolipoprotein E and possibly apolipoprotein B from the blood after the IV injection. Thus, the SNEDDS could mimic lipoprotein particles, which would then interact with the lipoprotein receptors located on the brain capillary endothelial cells. A subsequent endocytosis of the nanodroplets with drug would then occur. Apolipoprotein E plays an important role in the transport of lipoproteins to the brain [23]. Lipoproteins bind to and are internalized by the low-density lipoprotein receptor (LDL-R) (Figure 8) and the LDL-R-related protein [24]. The LDL-R is specifically up-regulated on the surface of the endothelium that forms the blood-brain barrier [25]. Apolipoprotein E-containing particles have been detected in human cerebrospinal fluid [26].
Figure 8: Possible Mechanism of Apo E-functionalized Nanodroplets uptake in brain. [11]
The present studies possibly presume the assumption that polysorbate-80 coated CSEDD could improve brain specific delivery due to the high affinity to adsorb plasma apolipoproteins on the surface, followed by low-density lipoprotein (LDL) receptor recognition and uptake by endothelial cells lining brain capillaries [27]. Moreover, the employment of apolipoprotein fractions containing only a binding sequence appears to be sufficient [28-31]. Due to the understanding of the uptake and transport mechanism of CSEDD into the brain a rational design of and the tailoring of very specific and effective carriers seems to be feasible. Concerning brain delivery, CSEDD were the most effective carrier to improve carbamazepine brain concentration. The concentrations of CBZ in CSEDD were, in most of the different brain structures like cerebellum, left & right hemispheres were significantly higher than those of plain solution. In fact, no toxic effect arising from the CSEDD has been noticed toward the permeability of the BBB. The results clearly demonstrated that the amount of CBZ found in brain was significantly higher with 1% polysorbate-80. It seems, therefore, that polysorbate-80 had a dramatic effect on BBB permeability.
Conclusion
It can be concluded that the serum levels of CSEDD in rat were high and declined gradually. The brain levels of CSol were far less when compared to CSEDD. A greater drug uptake in brain was seen with the CSEDD and brasin targeting could be achieved efficiently.
Acknowledgements
The authors are grateful to University College of Pharmaceutical Sciences, Kakatiya University. This work was supported by Natco Research Center (NRC), sanathnagar, Hyderabad and is sincerely acknowledged. We are grateful to the entire NDDS unit team of NRC for their assistance in the analytical measurements and their efforts are greatly appreciated. We are deeply indebted to Dr. B. Madhava Reddy, Professor& Principal, G.Pulla Reddy College of pharmacy, Hyderabad for permitting to conduct animal studies.
References
- Daneman R (2012) The blood–brain barrier in health and disease. Ann neurol72:648-672.
- De Boer AG, Gaillard PJ (2007) Drug targeting to the brain. Annu Rev PharmacolToxicol 47:323-355.
- Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, et al. (2013) The blood-brain barrier: anengineering perspective. Front neuroeng 6:7.
- Pardridge WM (2007) Drug targeting to the brain. Pharm res 24:1733-1744.
- Demeule M, Regina A, Che C, Poirier J, Nguyen T, et al. (2008) Identification and design of peptides as a new drug delivery system for the brain. J PharmacolExpTher 324:1064-1072.
- Sun X, Pang Z, Ye H, Qiu B, Guo L, et al. (2012) Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials 33:916-924
- Taubøll E, Lindström S, Klem W, Gjerstad L (1990) A new injectable carbamazepine solution—antiepileptic effects and pharmaceutical properties. Epilepsy research. 7(1):59-64.
- Loscher W, Hönack D (1997) Intravenous carbamazepine: comparison of different parenteral formulations in a mouse model of convulsive status epilepticus. Epilepsia 38:106-113.
- Akkar A, Müller RH (2003) Formulation of intravenous carbamazepine emulsions by SolEmuls® technology. Eur J Pharm Biopharm 55:305-312.
- Barakat NS, Yassin AE (2006) In vitro characterization of carbamazepine-loaded precifaclipospheres. Drug Delivery 13:95-104.
- Kelmann RG, Kuminek G, Teixeira HF, Koester LS (2008) Preliminary study on the development of nanoemulsions for carbamazepine intravenous delivery: An investigation of drug polymorphic transition. Drug DevIndPharm 34:53-58.
- Fisher RS, Ho J (2002) Potential new methods for antiepileptic drug delivery. CNS drugs 16:579-593.
- Juillerat-Jeanneret L (2008) The targeted delivery of cancer drugs across the blood–brain barrier: chemical modifications of drugs or drug-nanoparticles?. Drug Discov Today 13:1099-1106.
- Sporiš D, Baši? S, Serti? J, Mahovi?Lakuši? D, Babi? T (2017) Is Apolipoprotein E ε2 Associated with Delayed Onset of Non-Lesional Temporal Lobe Epilepsy? ActaClinica Croat 56:10-13.
- Brewster ME, Anderson WR, Webb AI, Pablo LM, Meinsma D, et al. (1997) Evaluation of a brain-targeting zidovudine chemical delivery system in dogs. Antimicrob Agents Chemother 41:122-128.
- Neves AR, Queiroz JF, Weksler B, Romero IA, Couraud PO, et al. (2015) Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: two new strategies of functionalization with apolipoprotein E. Nanotechnology 26:495103.
- Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, et al., (2006) Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J PharmacolExpTher 317:1246-1253.
- Kreuter J, Petrov VE, Kharkevich DA, Alyautdin RN (1997) Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood–brain barrier using surfactant-coated nanoparticles. J Controlled Release 49:81-87.
- Soni S, Babbar AK, Sharma RK, Banerjee T, Maitra A (2005) Pharmacoscintigraphic evaluation of polysorbate80-coated chitosan nanoparticles for brain targeting. Am J Drug Deliv 3:205-212.
- Erdlenbruch B, Jendrossek V, Eibl H, Lakomek M (2000) Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp Brain Res 135:417-422.
- Garcia-Garcia E, Andrieux K, Gil S, Couvreur P (2005) Colloidal carriers and blood–brain barrier (BBB) translocation: a way to deliver drugs to the brain? Int J Pharm 298:274-292.
- Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, et al. (2002) Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target 10:317-325.
- Packard CJ, Demant T, Stewart JP, Bedford D, Caslake MJ, et al. (2000) Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J Lipid Res 41:305-317.
- Dergunov AD (2004) Apolipoprotein E structure and substrate and receptor-binding activities of triglyceride-rich human plasma lipoproteins in normo-and hypertriglyceridemia. Biochemistry (Moscow) 69:720-737.
- Lucarelli M, Borrelli V, Fiori A, Cucina A, Granata F, et al. (2002) The expression of native and oxidized LDL receptors in brain microvessels is specifically enhanced by astrocytes?derived soluble factor (s). FEBS letters 522:19-23.
- Wagner S, Zensi A, Wien SL, Tschickardt SE, Maier W, et al. (2012) Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. PloS one 7:1-7.
- Hasadsri L, Kreuter J, Hattori H, Iwasaki T, George JM (2009) Functional protein delivery into neurons using polymeric nanoparticles. J BiolChem 284:6972-6981.
- Leupold E, Nikolenko H, Beyermann M, Dathe M (2008) Insight into the role of HSPG in the cellular uptake of apolipoprotein E-derived peptide micelles and liposomes. BBA-Biomembranes 1778:2781-2789.
- Leupold E, Nikolenko H, Dathe M (2009) Apolipoprotein E peptide-modified colloidal carriers: the design determines the mechanism of uptake in vascular endothelial cells. BBA-Biomembranes 1788:442-449.
- Sauer I, Dunay IR, Weisgraber K, Bienert M, Dathe M (2005) An apolipoprotein E-derived peptide mediates uptake of sterically stabilized liposomes into brain capillary endothelial cells. Biochemistry 44:2021-2029.
- Sauer I, Nikolenko H, Keller S, Ajaj KA, Bienert M, et al. (2006) Dipalmitoylation of a cellular uptake-mediating apolipoprotein E-derived peptide as a promising modification for stable anchorage in liposomal drug carriers. BBA-Biomembranes 1758:552-561.
Citation: Kumar GP, Rambhau D, Apte SS (2017) Brain Targeting Potential of Intravenous Carbamazepine SNEDDS. J Mol Pharm Org Process Res 5: 137. DOI: 10.4172/2329-9053.1000137
Copyright: © 2017 Kumar GP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5315
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 4405
- PDF downloads: 910