Research Article Open Access
Blockade of Lactate Transport in the Insular Cortex Impairs Reconsolidation, but not Retrieval, of Morphine-associated Memo ry and Prevents Subsequent Reinstatement
Yan Nie1, He Ren2, Jia-Rui Gu3, Chong-Ran Sun4, Yang Hui3, Yong-Bin Jing3 and En-You Li1*
1Department of Anesthesiology, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, PR China
2Radiology Department, Chongqin Hospital of Traditional Chinese Medicine, Chongqin City, PR China
3Harbin Medical University, Harbin, Heilongjiang Province, PR China
4Department of Neurosurgery, 2nd Affiliated Hospital of Zhejiang University Medical College, PR China
- Corresponding Author:
- Enyou Li
Department of Anesthesiology, The First Affiliated Hospital of Harbin Medical University
Harbin, Heilongjiang Province, PR China
Tel: 086-15996914018
E-mail: doctorPRC@hotmail.com
Received date: February 16, 2016; Accepted date: March 31, 2016; Published date: April 07, 2016
Citation: Nie Y, Ren H, Gu JR, Sun CR, Hui Y, et al. (2016) Blockade of Lactate Transport in the Insular Cortex Impairs Reconsolidation, but not Retrieval, of Morphine-associated Memory and Prevents Subsequent Reinstatement. J Alzheimers Dis Parkinsonism 6:227. doi:10.4172/2161-0460.1000227
Copyright: © 2016 Nie Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Drug-associated memories are critical for addictive behaviors, as these memories can trigger drug seeking and relapse by contextual cues. The transfer of lactate from astrocytes to neurons plays an important role in reward memory. Recently, studies have indicated that the insular cortex has a vital role in addictive procedure, which can be induced by contextual cues using both rat and human memory models. However, the neural locus in which the role of astrocyte–neuron lactate transport in long-term conditioning is required for reward memories is unclear. In investigating the involvement of insular astrocyte–neuron lactate transport in the processing of reward memory, using the conditioned place preference (CPP), we show that the local blockage of astrocyte–neuron lactate transport via the infusion of an inhibitor of glycogen phosphorylase (DAB) into the insular cortex impairs CPP expression of reconsolidation, but not extinction. Co-administering L-lactate and DAB confirmed that lactate could restore DABinduced memory deficit. The expression of c-fos in the insula cortex, the product of an immediate early gene, is also inhibited following memory reactivation. We found that the administration of DAB in the insula prior to reactivating the memory could inhibit the reconsolidation of reward memory, which could be reversed by the co-administration of DAB and L-lactate, and decrease the number of c-fos-positive cells. However, these treatments have no contribution to the extinction procedure, thereby indicating that the inhibitory contribution is reactivation dependent. Our results demonstrate that insular astrocyte–neuron lactate transport has a role in the processing of drug memory and that the blockage of insular astrocyte–neuron lactate transport could inhibit the reconsolidation of reward memory. This offers a novel therapeutic target to reduce the long-lasting conditioned responses to drug abuse.
Keywords
Reward memory; Drug reward; Reconsolidation; Conditioned place preference; Insular cortex; Astrocyte–neuron lactate transport
Introduction
Drug addiction is a type of brain disease that is characterized by behavioral features and is thought to be a form of distorted memory [1]. During the usage of the addicted substance, both reward effects and environmental cues are entered into reward memory after consolidation, thereby resulting in the context-cue stimulus being able to induce the drug craving behavior [2]. The consolidated memories are stable and difficult to disturb [1]. However, other studies have indicated that the retrieval of memory traces could induce an additional labile phase after reactivation/ retrieval [3,4]. This phase was termed as the reconsolidation of memory, which has been thought of as an important component of long-term memory processing [5]. In 2000, Nader et al. challenged consolidation during the Pavlovian association of a tone (CS) with a shock (US), thereby showing that a CS-alone reminder that is presented long after the consolidation phase was completely re-engaged during the temporary susceptibility phase of memory access [6]. In memory formation, protein synthesis is needed, thereby suggesting that disturbances in protein synthesis may prevent memory formation [7]. Previous studies have considered that the stabilization of a new memory occurs through consolidation, which requires gene expression. Importantly, memories can again become transiently labile and sensitive to protein synthesis inhibitors if memories are reactivated after consolidation [8,9]. Many studies have indicated that disturbing the reconsolidation of different memories, including reward memory and fear-conditioned memory, could destroy consolidated memories [10-12]. Therefore, disturbing the reconsolidation of reward memory to inhibit drug-craving behavior has become a novel target for curing the addiction.
During abstinence, drug-related cues induce drug-seeking behaviors by the activation of reward memory. The insular cortex is a key cortical region for activation, which is regulated by the interoception procedure for reward memory [13]. Viscerosensory and nociceptive nonspecific thalamic inputs were transmitted into the insular cortex, which stores emotional and affective state information in the parahippocampal-hippocampal system [14]. Reward effects of morphine and environmental cues were integrated to become reward memories. Therefore, disturbing the insular cortex may contribute to the reconsolidation of reward memory.
Previous studies have indicated that metabolic coupling between astrocytes and neurons is considered a key mechanism in response to neuronal activity [15]. Further studies have shown that interference with lactate transfer from astrocytes to neurons could affect this memory procedure. The astrocyte network could be interconnected through gap junctions to form many synapses, thereby permitting a continuous supply of energy substrates [16]. Astrocytic storage of glycogen has been used as a supplemental energy bank for neurons when more energy is needed. Therefore, metabolic coupling between astrocytes and neurons is considered to be glycogenolysis dependent [17]. Inhibiting the releasing lactate transfer from astrocytes into neurons in the hippocampus impairs memory formation by astrocytic (MCT4) and neuronal (MCT2) lactate transporters [18,19]. Therefore, growing evidence suggests that lactate may directly play an important role in memory.
We hypothesized that the reconsolidation of memories of contextual cues that were previously paired with morphine is mediated by lactate transport in the insular cortex. To address this issue, the glycogen phosphorylation inhibitor 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) was used to disturb glycol genolysis in astrocytes and neurons in an effort to affect memory reconsolidation. Furthermore L-lactate with DAB was co-administered to confirm that lactate could restore the DAB-induced memory deficit by activating the MCTs. The conditioned place preference (CPP) that was induced by morphine was used to examine memory in rats. The expression of c-fos, the product of an immediate early gene related to memory reconsolidation was used as a marker at the cellar level following a brief reward memory reactivation [20].
Materials and Methods
Animals
Male Sprague Dawley rats (weighing 220–240 g upon experiment) were obtained from the Laboratory Animal Center, Kunming Medical University. They were housed four per cage in a temperature- (23 ± 2°C) and humidity-controlled environment with ad libitum access to food and water. They were fed on a normal cycle (12 h/12 h, lights off at 8:00 A.M.). All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals. The experimental procedures were approved by the Biomedical Ethics Committee for animal use and protection of Kunming Medical University.
Drugs
Morphine sulfate was dissolved in sterile saline at a concentration of 10 mg/ml and administered at a dose of 10 mg/kg ((Shenyang first pharmaceutical factory). DAB (No. 1542, obtained from Sigma Aldrich, Shanghai) was dissolved in sterile saline. DAB was injected using a 10- μl Hamilton syringe at a rate of 200 nl min-1 over 2 min. After infusion, the injectors were kept in place for an additional 2 min.
CPP
Precondition (days 1-3): As described in previous works, CPP of rats was trained using an unbiased procedure [21]. During the precondition phase, the rats were individually habituated in the neural chamber with the doors freely removed for 15 min. Animals with a strong preference for any chamber (i.e., >540 s spent in each chamber) were removed from the experiments.
Condition (days 4-11): Then, animals underwent conditioned training once per day for 8 consecutive days. During conditioning, animals received morphine (10 mg/kg, intra peritoneal injection, i.p.) or an equal volume of vehicle (i.p.) immediately before being placed in the drug-paired or non-drug-paired chamber for 30 min on alternating days and were then returned to their home cages.
Post-conditioning test (day 12): After conditioning training, the time spent in the drug-paired compartment was recorded (day 12). Expression of CPP was conducted 24 h after the last training session.
Reconsolidation and extinction (days 13 and 14): For reconsolidation of reward memory in the CPP model, animals were re-exposed to the drug-paired or non-drug-paired chamber for 30 min without any injections, which was considered to be a memory retrieval trial (day 13), before performing the reconsolidation test (day 14). During extinction, animals were exposed to the CPP chamber (US) in the absence of any administration after the post-conditioning test (day 12). Finally, on day 14, CPP expression was examined. CPP scores were analyzed by comparing the time spent between the precondition and cross CPP tests. All locomotors could be recorded using an infrared-detecting instrument.
Insular injection
Rats were anesthetized with 100 mg/kg of ketamine (i.p., No. 6740881, Shenyang first pharmaceutical factory). Sterile stainless-steel guide cannulae (26G, 4.5 mm) were bilaterally implanted into the insular field, which were fixed to the skull with screws and dental acrylic using a stereotaxic apparatus before being sealed with an occlude. The injection cannulae (33 G) protruded 1 mm from the tip of the guide cannulae. These operations were based upon the coordinates of the rats’ brain atlas (insular cortex: bregma 0.72, midline ± 5.2 mm; depth 6.8 mm) [22]. The location was verified by Nissl-stained sections. Rats will be trained for CPP after the operation. DAB was dissolved in sterile saline. A 10-μl Hamilton syringe was coupled with an injection cannula to inject DAB or sterile saline at a rate of 200 nl min- 1 over 2 min. Controls received the same volume of vehicle for insular microinjections. All microinjections were performed in the DAB group using an automatic pump. The injection needle was located for 5 min and then slowly removed, with the occluders being reinserted.
Brain tissue processing for immunocytochemistry
Immediately after the behavioral test, animals were anesthetized with pentobarbital (500 mg/kg, i.p.; Shanghai Medical Pharm) and perfused transcardially with 4% paraformaldehyde (4%PFA) in 0.01 M sodium phosphate buffer, pH 7.4 (0.01 M PBS). Brains were post-fixed for 4 hours in 4% PFA and then stored at 4°C. Serial coronal sections (30 μm, one slice per five slices) were cut with a freezing microtome (Leica). After being washed in 0.01 M PBS ((3×5 min, pH 7.4), free floating brain sections were incubated for 5 min in PBS containing 1% H2O2 and rinsed again in PBS (3×5 min). Then, the sections were incubated with rabbit anti-c-fos polyclonal antibody (1:200, dilution in BSA, cat numbers: sc-52, Santa Cruz Biotechnology) overnight at 4°C. The sections were washed with 0.01 M PBS (3×5 min) and incubated at room temperature for 60 min in biotinylated goat anti-rabbit IgG (1:200, cat numbers: sc-45101, Santa Cruz Biotechnology). They were then washed (3×5 min) with PBS and subsequently incubated for 2 h in avidin–biotin complex (1:500, dilution in PBS-X, Vector Laboratories). Sections were rinsed (3×5 min) in PBS and then stained with 3,3’-diaminobenzidine (DAB, Sigma-Aldrich). The reaction was stopped by washing in PBS. Finally, the sections were dried using gradient alcohol solutions and exposed to xylene for delipidation.
Experimental Design
Experiment 1: Retrieval-dependent effects of intra-insula DAB on the reconsolidation of CPP
To determine the effect of DAB on the retrieval of reward memory, morphine (10 mg/kg, i.p.) or sterile saline (0.5 ml per injection, i.p.) was used to train CPP in rats after preconditioning. Then, CPP expression was tested (day 12). Animals from every group were randomly divided into two parts after retrieval (day 13) and were bilaterally administered with DAB (0,3,30 pmol/0.5 μl per side) in the insular cortex 5 min prior to reconsolidation in the previously drug-paired box or the non-drugpaired box for 15 min (day 14, Figure 1A). Then, the rats were allowed to freely explore the CPP compartments for 15 min without injections, and the time spent in each compartment was recorded. At the same time, locomotors in the CPP apparatus were also recorded by infrared photo beam breaks. Immediately after being re-exposed to the drug-paired compartment (reconsolidation), four animals from each group were randomly selected and decapitated for c-fos immunohistochemistry.
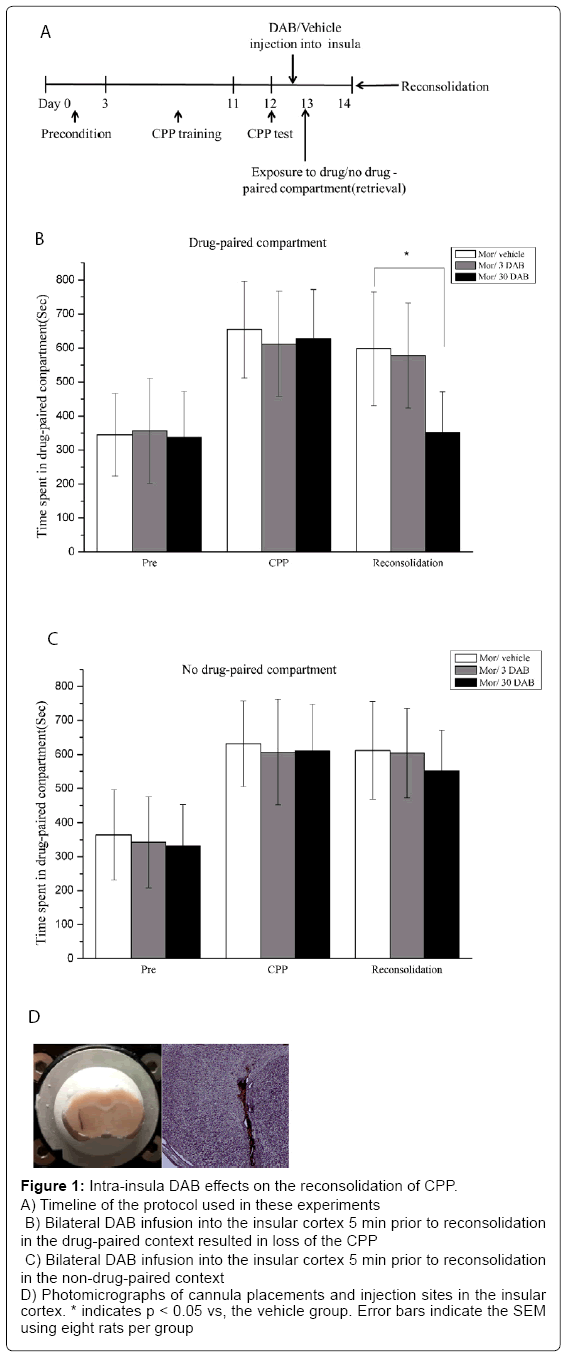
Figure 1: Intra-insula DAB effects on the reconsolidation of CPP.
A) Timeline of the protocol used in these experiments
B) Bilateral DAB infusion into the insular cortex 5 min prior to reconsolidation in the drug-paired context resulted in loss of the CPP
C) Bilateral DAB infusion into the insular cortex 5 min prior to reconsolidation in the non-drug-paired context
D) Photomicrographs of cannula placements and injection sites in the insular cortex. * indicates p < 0.05 vs, the vehicle group. Error bars indicate the SEM using eight rats per group
Experiment 2: Retrieval-independent effects of intra-insula DAB effects on the expression of CPP
To determine whether the effect of DAB on reconsolidation of CPP was retrieval dependent, another set of rats (8-10 rats per group) was trained into extinction of morphine CPP. After a post-condition test, the animals were bilaterally administered DAB (0, 3, or 30 pmol/0.5 μl per side) into the insular cortex 5 min prior to retrieval. On day 14, the time spent in each compartment and the locomotor activity were recorded.
Experiment 3: Effects of co-administration of DAB and L-lactate into the insular cortex on the reconsolidation of CPP
To antagonize the DAB effect, we co-administered DAB with L-lactate to confirm the DAB-induced memory deficit by mono carboxylate transporters (MCTs). Morphine (10 mg/kg, i.p.) or sterile saline (0.5 ml per injection, i.p.) was used to train for CPP in rats after preconditioning. Then, CPP expression was tested (day 12). After retrieval (day 13), the animals were bilaterally co-administered with L-lactate (50 nmol) and DAB (0, 30 pmol/0.5 μl per side) into the insular cortex 5 min prior to reconsolidation in the previously drug-paired box or the non-drug-paired box for 15 min to antagonize the DAB effect (day 14). The dose of L-lactate was based upon a previous study [18]. Then, the rats were allowed to freely explore the CPP compartments for 15 min without injections, and the time spent in each compartment was recorded.
Data Analysis
The data are expressed as the mean ± SEM. The CPP score was defined as the time spent in the drug-paired box. Data sets were compared with either student’s test or as a repeated measure ANOVA followed by post hoc analysis (LSD) by SPSS 13.0 software. We used the origin 8.0 software. The results were considered to be significant at p < 0.05.
Results
Retrieval-dependent intra-insula DAB effects on the reconsolidation of CPP
In experiment 1, we examined the effect of intra-insular infusions of sterile saline and DAB (3 pmol/0.5 μl and 30 pmol/0.5 μl per side) before retrieval on the expression of morphine CPP in six groups of rats (n=8–10 per group). The timeline for preconditioning, CPP conditioning, the CPP test and reconsolidation of CPP is described in Figure 1A. During preconditioning, no obvious difference was found in the time spent in each chamber (p > 0.05). After morphine/ sterile saline conditioning for 8 days, CPP behavior was diminished significantly (p < 0.05; Figure 1B). Vehicle (sterile saline, 0.5 μl per side), 3 DAB (3 pmol/0.5 μl, per side), or 30 DAB (30 pmol/0.5 μl, per side) was bilaterally infused into the insular cortex via an implanted cannula 5 min before reconsolidation of reward memory on day 14 in the previously drug-paired box or the non-drug-paired box for 15 min. On day 14, the CPP was tested without any drug or vehicle administration. Repeated-measure ANOVA revealed that morphine conditioning (F1,44=54.57, p < 0.05) and intra-insular DAB treatments (F2,44=17.43, p < 0.001) had significant effects on the time spent in the drug-paired compartment. Furthermore, there was a significant interaction between morphine conditioning and DAB treatments (F2, 44=11.32, p < 0.001; Figure 1B). Subsequent post hoc tests (LSD) showed that insular infusions of 30 DAB blocked the reconsolidation of CPP (p < 0.05) in the drug-paired compartment, but did not significantly affect the CPP score in the non-drug-paired compartment (p > 0.05, Figure 1C). Thus, intra-insular 30 DAB blocked the reconsolidation of CPP in the drugpaired compartment in a retrieval-dependent manner.
Retrieval-independent intra-insula DAB effects on the extinction of CPP
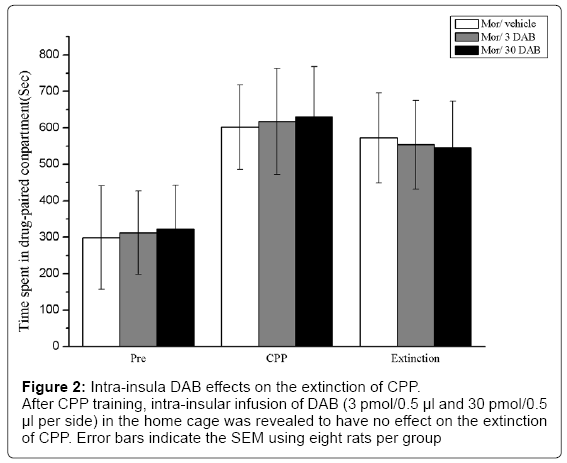
In experiment 2, we examined the effect of intra-insular infusions of sterile saline and DAB (3 pmol/0.5 μl and 30 pmol/0.5 μl per side) on the expression of morphine CPP in three groups of rats (n=8–10 per group) with no retrieval. After CPP training, there was a significant main effect that indicated that morphine/sterile saline conditioning induced significant CPP behavior [F(2,21) = 14.119; p < 0.05]. After intra-insular infusion of DAB (3 pmol/0.5 μl and 30 pmol/0.5 μl per side) in the home cage, no significant effects were identified [exposure × DAB, F(2,42) = 2.753, p > 0.05], indicating that CPP behavior was observed after extinction (p > 0.05) on day 14, as shown in Fig. 2. Thus, the inhibitory effect of DAB on the reconsolidation of CPP was dependent upon the retrieval of drug-paired context and memory reactivation. Furthermore, studies that DAB inhibited reward memory were retrieval dependent (Figure 2).
The effects of the co-administration of DAB and L-lactate into the insular cortex on the reconsolidation of CPP
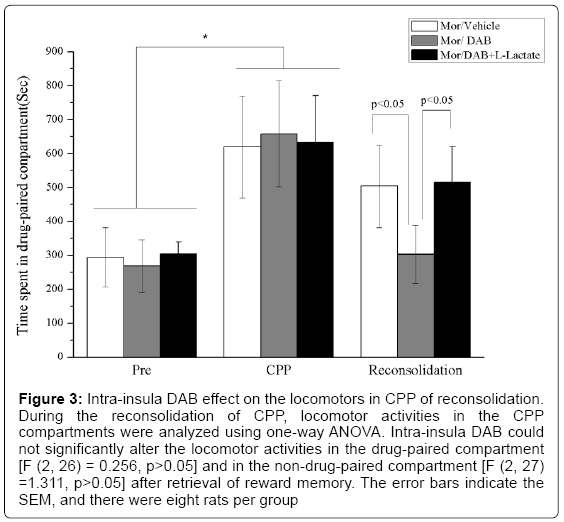
In experiment 3, we examined if the effect of intra-insular infusions of L-lactate can antagonize the DAB effects. After CPP training, there was a significant main effect, indicating that morphine/sterile saline conditioning induced significant CPP behavior [F(2,21) = 56.541, p < 0.05]. After intrainsular infusion of DAB (30 pmol/0.5 μl per side) or L-lactate prior to reconsolidation, significant effects was observed [exposure × groups, F(4,42) = 3.161, p < 0.05]. Subsequent post hoc tests (LSD) demonstrated that L-lactate inhibited the DAB effects on reconsolidation (Figure 3).
Figure 3: Intra-insula DAB effect on the locomotors in CPP of reconsolidation. During the reconsolidation of CPP, locomotor activities in the CPP compartments were analyzed using one-way ANOVA. Intra-insula DAB could not significantly alter the locomotor activities in the drug-paired compartment [F (2, 26) = 0.256, p>0.05] and in the non-drug-paired compartment [F (2, 27) =1.311, p>0.05] after retrieval of reward memory. The error bars indicate the SEM, and there were eight rats per group
Intra-insula DAB effect on locomotors in CPP of reconsolidation
During reconsolidation of CPP, loco motor activities in the conditioning chambers were tracked by infrared photo beam breaks. Locomotors activities were analyzed with one-way ANOVA. Intrainsula DAB could not significantly alter the total locomotors activities after retrieval of reward memory [F (2, 26) = 0.256, p > 0.05].
c-fos immunohistochemistry
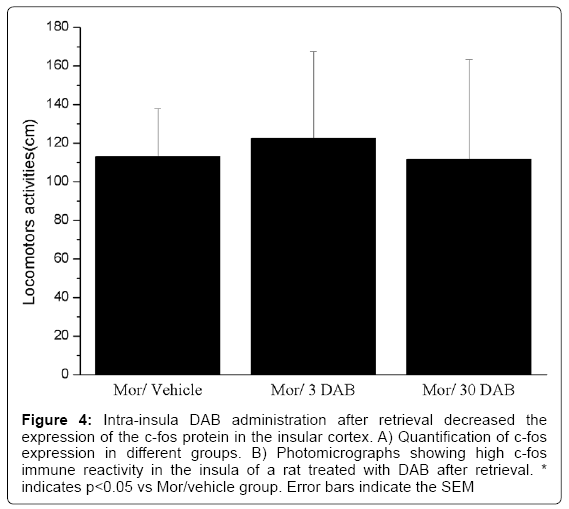
To contrast intra insula DAB effects on reconsolidation, immune reactive c-fos proteins, which are memory activity-related proteins, in the insular cortex were counted [23]. On day 14, rats were killed immediately after the CPP test. In contrast with the rats that were injected with vehicle into the insula plus retrieval (653.7 ± 171.8 c-fos-ir neurons per mm2), the number of c-fos-ir neurons in the DAB plus retrieval group (30pmol/0.5 μl, per side) was decreased ((F (2, 18) = 17.434, p < 0.05, Figure 4). We found that the DAB/retrieval treatment decreased c-fos expression in the insular cortex in comparison with the rats that received vehicle/retrieval treatment.
Figure 4: Intra-insula DAB administration after retrieval decreased the expression of the c-fos protein in the insular cortex. A) Quantification of c-fos expression in different groups. B) Photomicrographs showing high c-fos immune reactivity in the insula of a rat treated with DAB after retrieval. * indicates p<0.05 vs Mor/vehicle group. Error bars indicate the SEM
Discussion
In the present study, we investigated the intra-insula DAB effect on the reconsolidation of reward memory. We found that intra-insula DAB injections prior to reconsolidation could disrupt the morphine reward memories in the drug-paired compartment in a retrieval-dependent manner; however, there were no obvious effects on CPP expression in the absence of retrieval-dependent extinction. This effect could be reversed by the co-administration of DAB+L-lactate, indicating that DAB could disturb glycol genolysis, which results in a decrease in MCT activity. Additionally, intra-insula DAB treatment did not affect the animal response to exposure to the non-drug-paired compartment and loco motor activities in the CPP compartments. Importantly, the number of c-fos-ir neurons was decreased by intra-insula DAB administration. In summary, our data showed that intra-insula DAB could impair the reconsolidation of reward memory, which indicates that these effects were retrieval dependent.
In 2000, Nader et al. reported that the unconditioned stimulus (a tone) reactivated the original memory trace, thereby making it necessary to “reconsolidate” the memory or suffer erasure of the memory [6]. Many studies have supported the existence of a reconsolidation procedure of memory and reported similar results using a variety of manipulations to block memory [24-26]. Therefore, several studies concerning addiction have supported the idea that reconsolidation was also an important node in the CPP model [11,27,28]. The hippocampus, the basolateral amygdala, and the nucleus accumbens were included in the reconsolidation of memory [29-31]. Other studies have agreed that declarative memories were stored in the neo cortex. However, a neocortical region, such as the insular cortex, may be a better hub for linking environmental information with drug effect to become the addition memory.
Conversely, the insular cortex was the intero ceptive hub, which was fundamentally involved in the hedonic and incentive motivational aspects of reward [32]. A previous animal study demonstrated that the insula was activated by presenting a drug-related image to a substance-using individual and were conceptualized as a conditioned stimulus that changes the internal state of the individual towards processing drug-related memories, experiences, and urges to use the drug [33]. Interoceptive information is transmitted by the insular cortex, in which poly sensory region located in environmental cues [34]. Environmental information reaches the insula via brain regions that process spatial information or context, such as the entorhinal cortex and hippocampus [35]. Environmental cues activated representations of the bodily effects of drug use, in which neural activities were enhanced and more energy supply were needed.
Previous studies have suggested that astrocytes have been considered to support the function of neurons, which fulfilled the information processing, signal transmission and regulation need of neural and synaptic plasticity [36]. Recent studies have revealed that astrocytes and neurons actively interacted in response of neuronal activity and that lactate transfer from astrocytes into neurons could induce the molecular changes needed for long-term aversive memory formation [19]. The astrocyte network that is interconnected through gap junctions could form around thousands of synapses, thereby permitting a supply of energy. Disruption of the astrocytic and neuronal lactate transporters was shown to prevent the retention of an inhibitory avoidance task [19]. In addition, in our results, glycol genolysis that was blocked by DAB in the insular cortex could impair the reconsolidation of reward memory, which was inversed by the co-administration of DAB +L-lactate. According to previous studies [18], L-lactate activated MCTs, thereby resulting in enhanced memory. Intra-insula DAB injections inhibited glycol genolysis, thus decreasing the neural energy supplement in the insular cortex to impair the reconsolidation of CPP. c-fos protein, as a marker of activity of the insula, was decreased following retrieval in a paired context, but not the non-paired one [37]. Previous studies have indicated that the insular cortex has an important role in addiction memory [22]. For example, inactivating the insular cortex blunted the signs of malaise that were induced by acute lithium administration and impaired the reward memory [22]. One explanation is that lactate may supply sufficient energy to activate the insular neuron underlying longterm memory formation. Therefore, DAB inhibits the lactate effects that lead to disturbance in insular function.
Conclusion
Our results confirm the importance of astrocyte–neuron metabolic interactions in cognitive functions and demonstrate the key role of the astrocyte–neuron metabolic coupling in positive affective memory storage and retrieval. These findings open novel therapeutic avenues to reducing the long-lasting impact of drug cues on conditioned responses to cocaine.
References
- Hyman SE (2005) Addiction: a disease of learning and memory. Am J Psychiatry 162: 1414-1422.
- Li T1, Yan CX, Hou Y, Cao W, Chen T, et al. (2008) Cue-elicited drug craving represses ERK activation in mice prefrontal association cortex. NeurosciLett 448: 99-104.
- Sara SJ (2000) Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem 7: 73-84.
- McKenzie S, Eichenbaum H (2011) Consolidation and reconsolidation: two lives of memories? Neuron 71: 224-233.
- Sorg BA (2012) Reconsolidation of drug memories. NeurosciBiobehav Rev 36: 1400-1417.
- Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722-726.
- Jarome TJ, Helmstetter FJ (2014) Protein degradation and protein synthesis in long-term memory formation. Front MolNeurosci 7: 61.
- Giese KP, Mizuno K (2013) The roles of protein kinases in learning and memory. Learn Mem 20: 540-552.
- Alberini CM, Milekic MH, Tronel S (2006) Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci 63: 999-1008.
- Milton AL, Schramm MJ, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, et al. (2012) Antagonism at NMDA receptors, but not beta-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology (Berl) 219:751-761.
- Zhao X, Li Y, Peng T, Seese RR, Wang Z (2011) Stress impairs consolidation of recognition memory after blocking drug memory reconsolidation. NeurosciLett 501: 50-54.
- Ribeiro DA, Mello CF, Signor C, Rubin MA (2013) Polyaminergic agents modulate the reconsolidation of conditioned fear. Neurobiol Learn Mem 104: 9-15.
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531-534.
- Mathiasen ML, Hansen L, Witter MP (2015) Insular projections to the parahippocampal region in the rat. J Comp Neurol 523: 1379-1398.
- Magistretti PJ (2006) Neuron-glia metabolic coupling and plasticity. J ExpBiol 209: 2304-2311.
- Perea G, Sur M, Araque A (2014) Neuron-glia networks: integral gear of brain function. Front Cell Neurosci 8: 378.
- Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. ProcNatlAcadSci USA 91:10625-10629.
- Newman LA, Korol DL, Gold PE (2011) Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One 6: e28427.
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, et al. (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144: 810-823.
- Niu H, Zheng Y, Rizak JD, Fan Y, Huang W, et al. (2012) The effects of lesion of the olfactory epithelium on morphine-induced sensitization and conditioned place preference in mice. Behav Brain Res 233:71-78.
- Jian M, Luo YX, Xue YX, Han Y, Shi HS, et al. (2014) eIF2α dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. J Neurosci 34: 10010-10021.
- Contreras M, Ceric F, Torrealba F (2007) Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318: 655-658.
- Hall J, Thomas KL, Everitt BJ (2001) Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci 21:2186-2193.
- Kalaria RN, Andorn AC, Harik SI (1989) Alterations in adrenergic receptors of frontal cortex and cerebral microvessels in Alzheimer's disease and aging. ProgClinBiol Res 317: 367-374.
- Exton-McGuinness MT, Patton RC, Sacco LB, Lee JL (2014) Reconsolidation of a well-learned instrumental memory. Learn Mem 21: 468-477.
- Do Monte FH, Souza RR, Wong TT, Carobrez Ade P (2013) Systemic or intra-prelimbic cortex infusion of prazosin impairs fear memory reconsolidation. Behav Brain Res 244: 137-141.
- Fan YD, Niu HC, Huma T, Li L,Wang GM, et al. (2013) Blockage of glucocorticoid receptors during memory acquisition, retrieval and reconsolidation prevents the expression of morphine-induced conditioned place preferences in mice. DongwuxueYanjiu 34:E26-34.
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L (2008) Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci 28: 5602-5610.
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, et al. (2009) Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci 30:901-912.
- Rehberg K, Bergado-Acosta JR, Koch JC, Stork O (2010) Disruption of fear memory consolidation and reconsolidation by actin filament arrest in the basolateral amygdala. Neurobiol Learn Mem 94: 117-126.
- Ding ZB, Wu P, Luo YX, Shi HS, Shen HW, et al. (2013) Region-specific role of Rac in nucleus accumbens core and basolateral amygdala in consolidation and reconsolidation of cocaine-associated cue memory in rats. Psychopharmacology (Berl) 228: 427-437.
- Paulus MP (2007) Neural basis of reward and craving--a homeostatic point of view. Dialogues ClinNeurosci 9: 379-387.
- Howell LL, Votaw JR, Goodman MM, Lindsey KP (2010) Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 208: 191-199.
- Naqvi NH, Bechara A (2010) The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain StructFunct 214: 435-450.
- Insausti R, Herrero MT, Witter MP (1997) Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus 7: 146-183.
- Henneberger C, Papouin T, Oliet SH, Rusakov DA (2010) Long-term potentiation depends on release of D-serine from astrocytes. Nature 463: 232-236.
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ (2005) Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47: 795-801.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11263
- [From(publication date):
April-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10354
- PDF downloads : 909