Bleaching Edible Oils Using Clay from Kangole, Moroto District, North Eastern Uganda
Received: 09-Mar-2016 / Accepted Date: 25-Apr-2016 / Published Date: 02-May-2016 DOI: 10.4172/2155-9872.1000320
Abstract
The search for natural clays that can be used to provide industrial bleaching earths is worldwide; Uganda’s rich volcanic clays from North Eastern region remain unutilized. The aim of the study was to evaluate the use and efficiency of clay mined locally at Kangole to bleach vegetable oils when activated by leaching in hydrochloric and sulfuric acids of varying concentrations. The effectiveness of raw and acid activated clays developed from local Ugandan clay from Kangole, Moroto District of North Eastern Uganda for bleaching cotton and sunflower seed oil was studied. Hydrochloric and sulfuric acid of varying strengths 0, 10, 20 and 30% was used for the activation. Mixture of the degummed, neutralized oil and appropriate clay powders placed in Pyrex glass flasks, fitted with a magnetic stirrer was placed in an iso-electric mantle thermostated at 90°C in a nitrogen atmosphere for a duration ranging from 10 to 60 minutes before being cooled and filtered to record absorbance. Minerals were identified using IR, XRD, and elemental chemical composition of fusion mixture following Hutchinson’s method. The clay was montmorillonite in character with subordinate Kaolinite and Illite and unaltered tuff in form plagioclase and feldspars. Samples were subjected to hydrochloric and sulfuric acid activation with 10, 20 and 30% acid at 105°C. Bleaching efficiency for cotton and sunflower oils were. Study revealed that maximum decrease in absorbance of bleached oil was attained with clay leached in 30% sulfuric acid when the oil was in contact with clay for 30 to 40 minute at 90°C. Results obtained in which the performance of locally prepared clays was expressed in terms of percentage decrease in absorbance of oil showed that, the acid-activated samples were more effective in the bleaching of oils than raw clay. The percentage decrease in absorbance of sunflower oil of 80% was achieved with clay leached in 20% hydrochloric acid. Yet cotton oil attained highest percentage absorbance of 55% during the bleaching step. This study revealed for the first time the use Kangole clay in bleaching oils. It’s the first time the clay minerals in admixture mined at Kangole has been shown to contain montmorillonite, illite, kaolinite, feldspars and plagioclase which contribute to its bleaching activity.
Keywords: Adsorptive bleaching; Acid-activated clay; Edible oil refining; Degumming; Neutralization
304521Introduction
Chemical analysis of clay
Clay sample solution in nitric acid can be analyzed by ICP-MS or ICP-OES. Analysis of clays by X-ray fluorescence (XRF) is often more appropriate for many geological materials where total elemental analysis is required, such as many rocks and soils. Some samples are often difficult to take in solution in a matrix suitable for analysis by ICP, so XRF analysis is more appropriate when knowledge of total elemental concentrations is needed. Clays contain many components some of which may be contaminants and/or natural ingredients which impart color and other properties. It is important to determine the metals present in the clays as well as devising means to eliminate other contaminants before using the clay in bleaching edible oils. Methods used to quantify can be difficulty requiring extraction of certain components which may complicate interpretation of results obtained. Knowledge of the composition of clays includes elemental, mineralogical and biological constituents. Once heavy and trace metals in clays have been determined, risk of contamination of products on which the clay may be used to act can be explained easily [1].
The leaching of clays
Leaching of clays in acid or alkalis is commonly used to activate it. Activation of clay is a chemical or physicochemical treatment applied to clay to develop capacity to adsorb coloring matter and other impurities in vegetable, animal or petroleum oils [2]. The activity of clay denotes surface chemical and physicochemical reactivity leading to increase in surface area of solid [3]. Activation modifies surface of material for use as adsorbent for liquid, solid or gas or obtain high rate of reaction or dissolution. Acid activation involves removal of exchangeable interlayer ions as well as dissolution of tetrahedral and octahedral ions from alumina and silica layers [4] and replaces them with hydrogen causing structural deformations. The composition and texture of clay changed when leached [5,6]. Activation of clay may cover a wide range of chemical treatments that produce modifications on the surface of the clay mineral crystals. The classes of methods used to enhance the properties of natural clay for several industrial uses include physical alteration of particle size (specific surface area), thermal alteration of chemical composition and/or crystalline structure by the effect of temperature, chemical alteration which is usually limited to ionic exchange. While heating modifies clay through calcining, dehydration, sintering and dehydroxylation, leaching with acids removes octahedral and tetrahedral cations leaving a silica skeleton [7]. This creates of Lewis and Broensted acidity, which increases the ability to clarify oils [8]. Clays adsorb carotenoids, amines and other olefins via surface reactions. The capacity of clay to bleach was reported to increase with concentration of acid and temperature [9]. Freundlich (1926) developed equation relating adsorption to residual solute concentration given below.
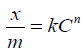 1.0
1.0
where C is the amount of residual substance, k and n are constants.
As absorbance measurements are taken in most experiments involving the bleaching processes, the relative quantity of pigment adsorbed, x and the residual relative quantity at equilibrium, Xe are obtained from equations 2.0 and 3.0 [10].
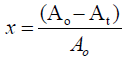 2.0
2.0
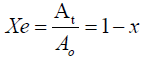 3.0
3.0
where Ao is the absorbance of unbleached crude oil and ‘At’ is absorbance of bleached oil at time t.
Equation 1.0 was rearranged to equations 4.0 and 5.0 [11].
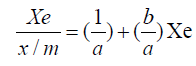 4.0
4.0
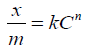 5.0
5.0
where x is the amount of solute adsorbed by m grams of the adsorbents at concentration C in the solution, k and n are constants.
Taking logarithms of equation 5.0 gives equation 6.0.
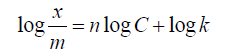 6.0
6.0
A plot of log x/m against logC gives a straight line known as the Freundlich isotherm has a vertical intercept as logk and slope n [12].
The presence of Broensted and Lewis centers and kaolin, illite and nontronite phases in the material was cited as being responsible for the gain in decolorizing power as concentration of acid and temperature increased [8]. When the concentration of impurities on the adsorbent exceeded what the clay could hold, the Freundlich and Langmuir isotherms deviated from linearity because it became saturated with the adsorbate [13]. A number of attempts have been made in the refining of edible oils using local clays from Uganda as adsorbents [14-18] in much the same way as Local Nigerian clays were used to bleach palm oil [19-22].
Visible spectra of the neutralized cottonseed oil and bleached oils gave five absorption maxima due to presence compounds namely, chlorophyll-a, β-carotene and their relative compounds, respectively [23,24]. Some absorption maxima disappeared in the absorption spectra of oil bleached using bentonite leached in 25% acid showing that almost all coloring bodies had been removed [25].
The effectiveness of clays serving as adsorbents can be expressed as a measure of percentage decrease in absorbance of the bleached cotton and sunflower oil, because absorbance is related to concentration in the Beer-Lambert law. The variations in efficiency of clays at bleaching oil can be improved by acid and heat activation [26]. The growing of vegetable oil seeds and pressing oils out of them is on the rise in Uganda and part of the local population in Uganda use unbleached oils for food. There is increasing manufacture of oils and edible products from vegetable oils in Uganda envisaged by the recent development oil mills in many towns in Uganda. Imported bleaching earths make oil production expensive yet local materials can be used if properly prepared. So there is need to find and characterize a Ugandan bentonite. In this work, however, local clays mined from pitch black soils at Kangole, Moroto District in North Eastern Uganda have been prepared as raw and acid-activated earths and used to bleach cotton and sunflower oil. This study has aimed at developing local clay with effective adsorptive capacity for impurities in cotton and sunflower oils.
Materials and Methods
Sample collection and preparation
Composite sampling method was used because the average value of clay properties was needed at low analytical costs. A number of field samples representing the clay from Kangole, Moroto District in North Eastern Uganda were thoroughly mixed to form a composite which was used in the laboratory. This sampling plan was used because physical distance does not alter chemical properties of the clay. It measures the mean. There was no need of estimating of variance. In all cases the clays were dug by removing the top soil and clays scooped from the underlying horizons. The samples were collected through removing the top soil from the surface to minimize the effect of weathering and contamination and scooping 1 kg of clay from the layers below. A total of ten samples weighing nearly 1 kg each were scooped from the ten spots at Kangole. All the samples were put in one polythene bag as a composite and transported to the laboratory for purification and analysis [27].
Sunflower seeds, cotton seeds and date palms were collected from Kampala’s St Balikuddembe market and Jinja oil mills, pressed to collect the different oils.
Purification of samples
Clay powder (30 g) was suspended in distilled water (700 ml) in a 1000 mL large plastic beaker. Using an ultrasonic probe the clay was disaggregated for approximately 20 minutes. The beaker containing the suspended sample was removed from the ultrasonic probe enclosure and covered the beaker. The beaker was placed on a vibrationally stable surface and to allow the sample to settle gravitationally without interruption for 47 minutes per cm depth of water. The supernatant solution was decanted off to other plastic beaker and allowed to stand for 2 days. The remaining supernatant solution was decanted off and allowed to evaporate to dryness, ground to powder and used in experiments. The sediment in the beaker was discarded [28,29].
Elemental chemical analyses of clays
The chemical analyses of elements like aluminium, iron, calcium, sodium and potassium in clays were carried out three times for clay and done by decomposition using sodium carbonate fusion method [30] in platinum crucibles. Silica was determined by gravimetry and the other elements were analyzed using the Perkin-Elmer 3030 model Atomic absorption spectrometer after dissolution of the sample in the hydrofluoric acid-perchloric acid digestion mixture.
Infrared spectroscopic studies
Infra-red spectroscopy (IR) gives chemical overview of the clay, with all chemicals present contributing to spectrum. IR identifies and characterizes clays by comparison of absorption peaks and bands in a plot absorption versus frequency. The bands on the spectrum are determined by strength of the change in dipole moment taking place in a particular inter-atomic vibration. The spectra of bentonite differ from those of kaolinites [16,17,31]. Clay powder (3 mg) was mixed with KBr (100 mg), ground to powder and pressed to discs. The IR spectra were run using B10RD FT 540 Fourier Transform IR spectrophotometer [32].
X-ray diffraction analyses
The mineralogy of clays was determined using Philips diffractometer with PW1710 control unit operating at 40 kV and 30 mA using the Ni-filtered Cu Kα radiation. The diffractograms were automatically matched with JCPDS-cards in the computerized XRD CD-rom. Bulk mineralogy was studied with randomly oriented air-dried samples [33]. XRD hot stage analysis was reported to have identified thermally stable polymorph of the kaolinite [34].
Activation of samples
For preparation of the activated clays, varying concentrations (0, 10, 20 and 30%) of hydrochloric and sulfuric acids were used. The purified clay (200 g) was placed in a 500 mL beaker water (200 mL) added and pasty, slurry was made. Activation was carried out by adding the acid of a known concentration to the clay slurry and the mixture was boiled for 2 hours at a regulated temperature of 105°C. After slow cooling, the slurry was filtered. The residue clay was washed to neutrality with distilled water. The activated sample was dried to constant weight in thermostated oven at 80°C. The lumps of the prepared clays were ground using a porcelain mortar and pestle, sieved to 200 μm and stored for use in the bleaching process.
Degumming of vegetable oils
Crude oil (100.0 g) was placed in a flask, 85% phosphoric acid (1.0 g, 0.1 mmol) added, the mixture heated at 90°C while stirring at 900 revolutions per minute for 10 minutes under nitrogen blanket. The oil was filtered under nitrogen [35,36].
Neutralization of oil
Mixture of the degummed oil (200.0 g, 0.85 mol) and 0.1M sodium hydroxide (10.0 cm3) was placed in 250 cm3 Pyrex glass flasks, fitted with a magnetic stirrer. The mixture was stirred vigorously for 10 minutes at room temperature and filtered.
Bleaching vegetable oils
Bleaching of oils is a process whereby the clay adsorbent mixed with oil under specified conditions removes unwanted color bodies and contaminants [37]. The primary function of the bleaching process is to remove pigments, gums, trace metals, peroxides and secondary oxidation products [38]. Four basic steps are used to refine oil: neutralization and separation, bleaching and deodorizing [39,40]. Diatomaceous earth, clays, peroxides or carbon is added to bleach and adsorb the dark colored impurities in the oil in order to give it a clear color [19,22,41,42]. The bleaching process combines catalytic action such as peroxide decomposition and equilibrium adsorption of pigments from oil [43]. The peroxides decompose to volatile aldehydes and ketones due to further oxidation which get adsorbed on clay [44]. Bleaching is carried out under steam, nitrogen or vacuum to minimize oxidation of oils by oxygen at elevated temperatures [43].
Mixture of the degummed, neutralized oil (200.0 g, 0.85 mol) and appropriate clay powders (4.0 g, 1.24 mmol) was placed in 250 cm3 Pyrex glass flasks, fitted with a magnetic stirrer. The flask was immersed in a thermo-stated iso-electric mantle at various temperatures. The mixture was heated while stirring continuously for a further two hours at the set temperature under high vacuum [45]. The hot oil and clay mixture was filtered in nitrogen atmosphere and tested by measuring absorbance. The absorbance of bleached oil was determined using ultra violet-visible spectrophotometer, Shimadzu, 1201. The absorbance of the bleached oils at 550 nm was determined for each oil sample obtained at the different temperatures of activation. The percentage decrease in absorbance, BE, was calculated as given in equation 7.0.
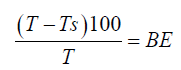 7.0
7.0
where,
T=absorbance of unbleached oil
Ts=Absorbance of bleached oil
To investigate the effect of mass percent of clay on the percentage decrease in absorbance of bleached oils, the procedure above was repeated using different clay dosages of 2, 4, 6 and 8%; the operating temperature was 90°C and contact time was 30 minutes.
Results and Discussion
Characterization of clay
Table 1 shows the results of the analysis of mineralogy content of the clay composite sample investigated. Result showed the presence of SiO2, Al2O3, MgO, K2O, CaO and Na2Oin slightly varying amount may be due to errors in forming the composite studied.
| Element% | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | LOI | Total |
|---|---|---|---|---|---|---|---|---|
| A0 | 45 | 18.3 | 5.7 | 2.3 | 3.5 | 2.4 | 9.7 | 86.9 |
| F0 | 44.3 | 19.4 | 6.2 | 2.4 | 3.4 | 2.6 | 9.9 | 88.2 |
Table 1: Chemical composition of Kangole clay.
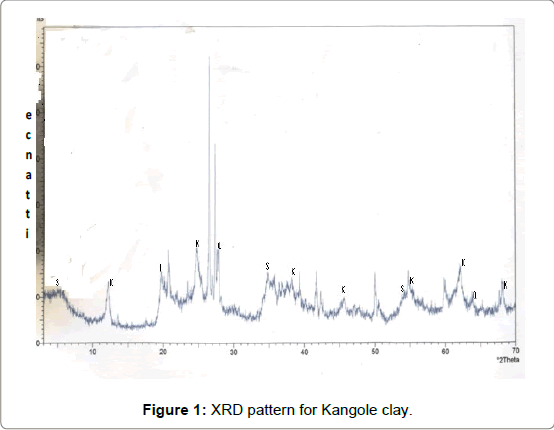
The chemical analyses presented in Table 1 showed the Kangole clay had high content of Na2O (3.4%) and Na2O/ CaO ratio of 1.41 is greater than 1.00, indicating the presence of swelling smectite clay. This has been used as indicating presence of montmorillonite. The presence of iron oxides and aluminium oxide in Kangole deposits in high concentrations of 6 and 20% respectively may be responsible for their high bleaching capacities when acid-leached. The percentages of iron, aluminium and silicon among bentonites worldwide are approximately 11, 18, and 60% respectively [46]. Basing on this, clay from Kangole ressembled bentonites and can substitute commercial bleaching earths and cracking catalysts. On the basis of relative percentages of aluminium, silicon and alkaline metals or alkaline earth metals [47], the clays studied have been found to satisfy the formulae (1/2Ca,Na)0 .33(Mg,Fe+2)3(Si,Al)4O10(OH)2·4H2O and Ca.5(Si7Al.8Fe.2)(Fe3.5Al.4Mg.1) O20 (OH)4 for montmorillonite and nontronite respectively [47]. The dominant clay mineral in clay deposits at Budadiri, Chelel, Siron and Mutufu was published as being nontronite, a smectite and this coincides with studies on clays formed from volcanic sediments [15- 17]. The presence of bentonite among volcanic sediments is well documented in literature [48-51]. The main smectite mineral present in the Kangole clay deposits was nontronite, a di-octahedral clay as shown by the X-ray diffractograms in Figure 1. Tri-octahedral smectites were not detected.
Figure 1 shows that Kangole clay contains smectite (S), illite (I), kaolinite (K), K-fedspars (Kf), plagioclase (Pc) and quartz (Q) [31]. The presence of feldspars and plagioclase in these clays showed that the parent rock has not yet completely metamorphosed.
Using Reynold’s quantitative method, the mineral composition of Kangole clay was determined as follows: feldspars, 20%, illite, 5%, Kaolinite, 7%, plagioclase, 5%, quartz, 10% and smectite, 50%. This indicated the dominant clay in the Kangole sample is smectite. Presence of different clay phases like illite, kaolinite, feldspars, plagioclase and montmorillonite was used to predict that the clay would develop high bleaching capacity when leached in acid [52,53]. The Kangole clay was heterogeneous because it contained different phases of clays, such as kaolinite, montmorillonite and illite layers, which have active centers on their surfaces. It was reported that heterogeneity of Cyprus bentonite was attributed both to different active centers on the smectite surface (Broensted and Lewis centers) and to the different phases present in bentonite, such as illitic layers and clinoptilolite, which also have active centers on their surfaces as the clay was a mixture of illite and smectite [8,53].
Raw clays contained lower silica content than acid-leached clay. Because silica did not dissolve during leaching in acid, its content increased progressively with increase in acid concentration because of removal of ions from the interlayer and octahedral sheets of the clay. Exchangeable ions like calcium, sodium and potassium ions were dissolved the acid activation process leading to decrease in the percentages in Table 2. The changes in calcium and potassium content as concentration of acid increased appear insignificant in terms of magnitude but are large if percentage change is computed. As seen in Table 2 the effect concentration of hydrochloric acid changing respectively from 0, 10, 20 to 30% caused the following percentage decrease in metal content of clay: in iron there was 59.8, 73.9 and 78.5%; for calcium there was 73.9, 78.3 and 80.4%, for aluminium there was 10.8, 14.7 and 38.7%, for magnesium there was 7.4, 25 and 39.7% ; for sodium there was 81.7, 83.1, and 88% yet for potassium there was 64.5, 70.2 and 73.6% when clay was leached with hydrochloric acid. The effect concentration of sulfuric acid changing respectively from 0, 10, 20 to 30% caused the following percentage decrease in metal content of clay: in iron there was 73.4, 78.8, and 82.5%; for calcium there was 55.2, 55.6 and 56.5%, for aluminium there was 15.8, 28.7 and 32.8%, for magnesium there was 23.5, 92.6 and 95.6%; for sodium there was 85.1, 85.7 and 90.1% yet for potassium there was 65.2, 67.8 and 70.2%. Following this analysis, all ions are affected by leaching but aluminium decreases to the least extent probably due to its low chemical activity. Removal of aluminium, iron (III) and magnesium ions from the clay layer must have left unoccupied octahedral sites which may lead to increased adsorptive capacity and surface area of the clay [26]. The surface area rises due to the vacant octahedral spaces form which metal ions dissolved. The loss on ignition increased with increase in concentration of acid used because metal ions were replaced by hydrogen and hydroxyl groups. On heating, hydrogen and hydroxyl groups decompose to give water. So the amount of water lost from the leached material increased. Complete or partial dissolution calcium, iron and magnesium increases pore size for adsorption and surface area because removed ions leave free sites for adsorption [19,21,54].
| %oxide | A0 | A10 | A20 | A30 | F0 | F10 | F20 | F30 |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 45.21 | 54.16 | 60.28 | 68.10 | 44.3 | 59.76 | 64.48 | 67.31 |
| Al2O3 | 18.32 | 16.34 | 15.63 | 11.23 | 19.42 | 15.42 | 13.06 | 12.30 |
| Fe2O3 | 5.71 | 2.41 | 1.49 | 1..23 | 6.21 | 1.52 | 1.21 | 1.00 |
| MgO | 0.68 | 0.63 | 0.51 | 0.41 | 0.63 | 0.52 | 0.05 | 0.03 |
| CaO | 2.30 | 0.60 | 0.50 | 0.45 | 2.41 | 1.03 | 1.02 | 1.00 |
| K2O | 2.42 | 0.86 | 0.72 | 0.64 | 2.66 | 0.84 | 0.78 | 0.72 |
| Na2O | 3.5 | 0.64 | 0.59 | 0.42 | 3.4 | 0.52 | 0.50 | 0.33 |
| LOI | 9.71 | 11.84 | 12.30 | 12.90 | 10.84 | 12.49 | 12.90 | 13.11 |
A is Kangole clay leached in hydrochloric acid yet F is leached in sulfuric acid. The numeral 0, 10, 20 or 30 designates the mass percent of acid used to leach the clay.
Table 2: Composition of raw clay and acid-leached clay from Kangole.
Effect of bleaching time on adsorption
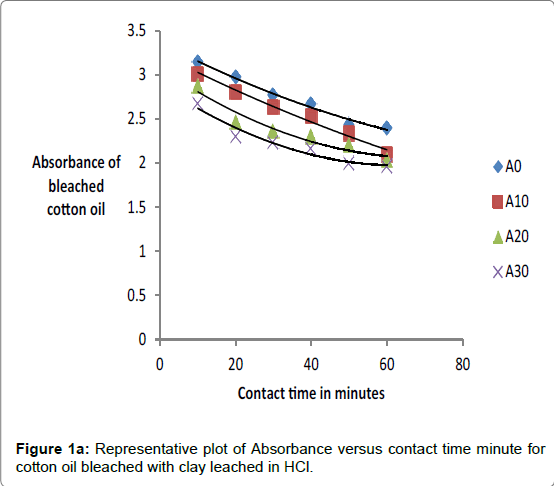
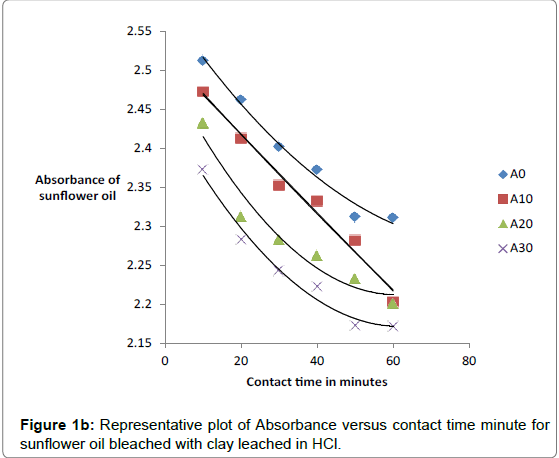
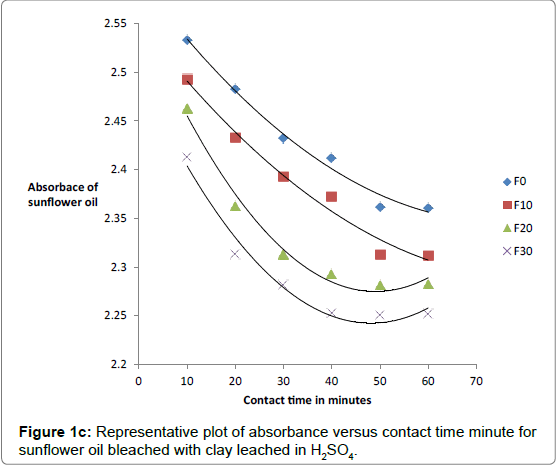
The effect of contact bleaching time for both raw and acid-activated samples was investigated by measuring absorbance of oils bleached at differing temperatures and times. The percentage decrease in absorbance was observed to increase with contact time, concentration of acid and temperature of activation of clay. The data on absorbance of bleached oils bleached at 90°C is given in Tables 2a and 2b.
| Contact time (min) | 10 | 20 | 30 | 40 | 50 | 60 |
|---|---|---|---|---|---|---|
| A0 | 3.147 | 2.977 | 2.774 | 2.673 | 2.436 | 2.402 |
| A10 | 3.011 | 2.808 | 2.632 | 2.537 | 2.334 | 2.097 |
| A20 | 2.876 | 2.470 | 2.368 | 2.300 | 2.199 | 2.030 |
| A30 | 2.673 | 2.300 | 2.233 | 2.165 | 1.999 | 1.962 |
| F0 | 3.214 | 3.045 | 2.909 | 2.774 | 2.632 | 2.571 |
| F10 | 3.079 | 2.876 | 2.704 | 2.639 | 2.470 | 2.436 |
| F20 | 2.977 | 2.639 | 2.470 | 2.402 | 2.334 | 2.300 |
| F30 | 2.808 | 2.470 | 2.334 | 2.667 | 2.199 | 2.233 |
| 2a) Cotton oil | ||||||
| Time(min) | 10 | 20 | 30 | 40 | 50 | 60 |
| A0 | 2.513 | 2.463 | 2.403 | 2.373 | 2.312 | 2.311 |
| A10 | 2.473 | 2.413 | 2.353 | 2.333 | 2.282 | 2.203 |
| A20 | 2.433 | 2.313 | 2.283 | 2.263 | 2.233 | 2.201 |
| A30 | 2.373 | 2.283 | 2.243 | 2.223 | 2.173 | 2.172 |
| F0 | 2.533 | 2.483 | 2.432 | 2.412 | 2.362 | 2.361 |
| F10 | 2.493 | 2.433 | 2.393 | 2.372 | 2.313 | 2.312 |
| F20 | 2.463 | 2.363 | 2.313 | 2.293 | 2.282 | 2.283 |
| F30 | 2.413 | 2.313 | 2.282 | 2.253 | 2.251 | 2.252 |
| 2b) Sunflower oil. | ||||||
Table 2a and 2b: Absorbance of bleached oils.
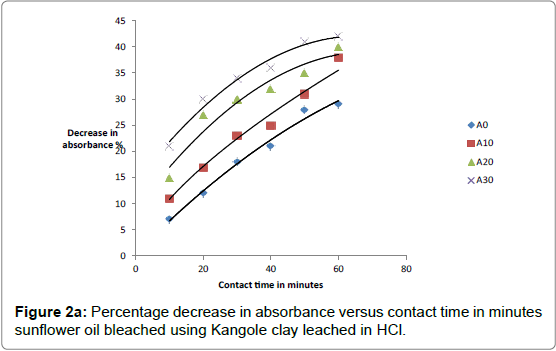
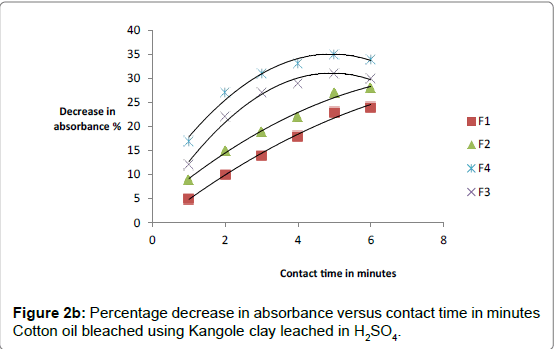
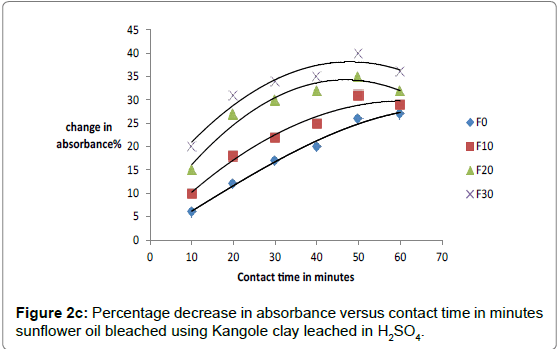
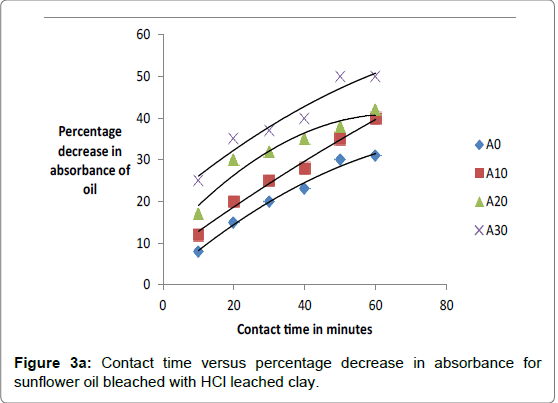
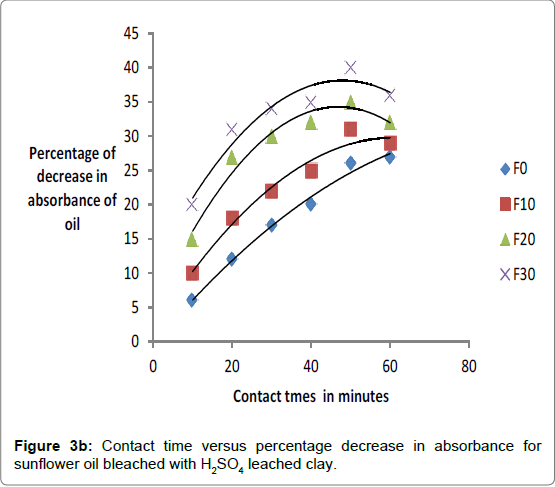
The respective absorbance of cotton and sunflower oils were established in this study to be 2.583 and 3.383 at 550 nm before the oils underwent any treatment to improve their colors. However, when the oils were treated with clays the absorbance of the bleached oils decreased. The decrease in absorbance of oils is illustrated in Figures 1a, 1b and 1c. The representative graphs on variation of absorbance with temperature and time show that absorbance of bleached oils decreased as the contact time, temperatures and concentration of leaching acid increased. This showed that the efficiency of the clay to bleach the oils increased with contact time, temperature and concentration of leaching acid [55,56].
As bleaching is bleaching is strongly associated with acidity of clays, the increase in capacity to bleach was a result of removal of interlayer cations as they were replaced by surface hydrogens and hydroxyls [57,58]. For cotton and sunflower seed oils, the relative changes in pigment adsorbed, x, mass of clay used, m, and the residual relative quantity in equilibrium, Xe, indicated that effective bleaching occurred as shown in Figures 2a-3b.
No significant percentage change in absorbance was observed when the sample was heated for more than 40 minutes. However, shorter bleaching times were observed while using acid-clays. The highest bleaching time established for both hydrochloric and sulfuric acid-leached clays were 30 and 40 minutes respectively. No significant increase in bleaching efficiency of both cotton and sunflower oil when those contact times were exceeded. Earlier researchers using palm oil stated that the contact time for effective bleaching ranges from 15 to 45 minutes [59-61] and this has been confirmed by this work on cotton and sunflower oils. The percentage decrease in absorbance for oil bleached using clay leached in hydrochloric acid was greater than for that leached in sulfuric acid due to the fact that the decrease in metal ion content of the clay was larger for hydrochloric acid than for sulfuric acid. So the activated clay formed had more adsorptive sites than that formed using sulfuric acid [21,54]. The higher sorption efficiency reflected increased pore size and surface area. This showed that hydrochloric acid was better at activating the clay than sulfuric acid.
Effect of dosage on percentage decrease in absorbance of oils
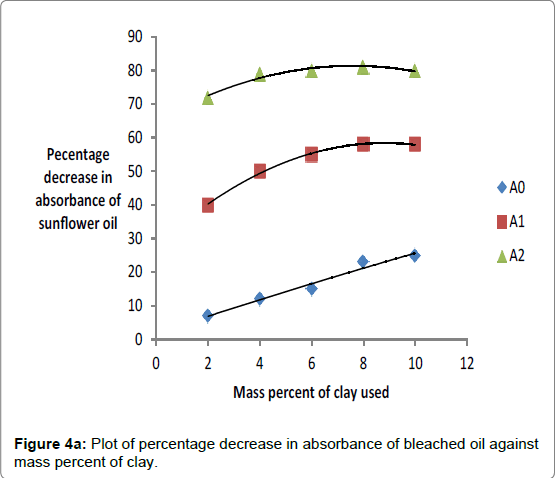
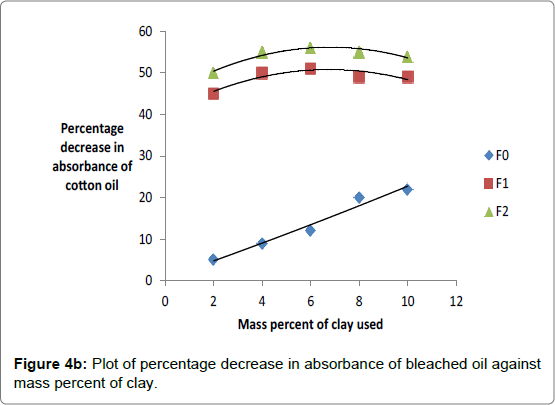
The effect of adsorbent dose expressed in percentage weight of clay dose to weight of oil was also investigated with the aim of establishing the most favorable dose of clay to bleach oil optimally. The data obtained was plotted in Figures 4a and 4b. The data indicated that the percentage decrease in absorbance of oils bleached using Kangole leached with hydrochloric and sulfuric acid increased to an optimum value at adsorbent dosage of 4%. Beyond 4% increase in percentage of clay did not cause significant decrease in percentage decrease in absorbance of bleached oils.
For all activated clays used, there was increase in percentage decrease in absorbance of oil as mass percent of clay increased showing that effective bleaching was increasing as mass of adsorbent was increased.. However, when the percentage was increased beyond 4% there was no significant increase in the bleaching power of the activated clay except raw clay. The failure for the decrease in absorbance of oils to increase after adding clay that is greater than 4% by mass was explained by the fact adsorption equilibrium had been attained between the activated clay and oil mixtures, thereby, preventing further color removal by the excess adsorbent dosage [12]. Earlier reports showed that palm oil required as much as 2 to 4% or more to meet final color requirements [4]. Clays leached in 20% hydrochloric or sulfuric acid were optimally effective in decreasing absorbance of oils because in Figures 4a and 4b, they showed the highest efficiency. Percentage color reductions of 80% for sunflower oil and 55% for cotton oil were obtained with Kangole clay activated using 20% acid respectively as deduced from Figures 4a and 4b.
Adsorption isotherm
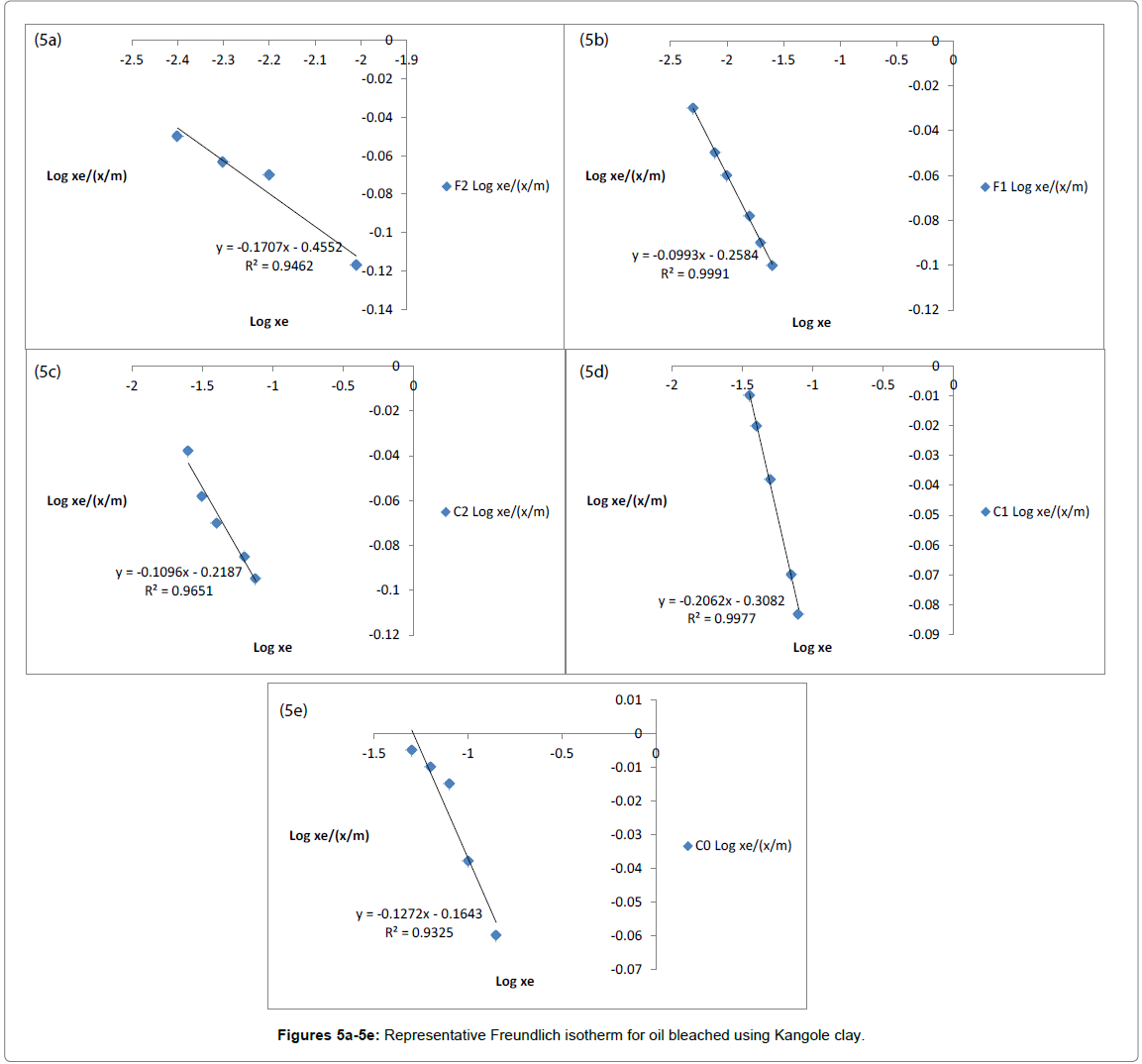
The adsorptive and bleaching capabilities of clays were estimated using Freundlich isotherms [56,62]. The linearity of isotherms obtained by plotting log xe/(x/m) versus logxe was used to show that adsorption occurred to monolayer capacity [8,9,56]. The vertical intercept is k and slopes of the linear graphs are, n characterizing the bleaching power of the clay and the manner in which impurities adsorb on the clay respectively [63]. The constants k and n indicate the adsorption capacity and the adsorption intensity. The linearity of the plots in Figures 5a- 5e showed the representative nature of adsorption on the clay. From the Freundlich adsorption isotherms, the amount of color pigments adsorbed was proportional to change in concentration of coloring bodies in the vegetable oil as measured before and after bleaching has occurred. And this was reflected by the decrease in absorbance of oils as bleaching took place. The value of n depicted that the clay was a very good adsorbent. As Freundlich adsorption isotherms assume monolayer adsorption capacity complications arise when clays and clays minerals exhibit multi-layer adsorption tendencies and this causes deviation from linearity of the isotherms. Clays with low sorption capacities deviate from Freundlich isotherms because they easily get saturated with impurities from oils leading to steric interactions between the adsorbed and unadsorbed impurities [13]. The values of the Freundlich constant k and n were obtained from plots of log Xe(x/m) against log Xe. The Freundlich isotherms developed on Kangole clays are shown in Figures 5a-5e. Figures 5a-5e are highly linear depicting effective adsorption of coloring bodies occurred during the bleaching of oils. The linearity of Freundlich isotherms developed using Kangole clays followed the following equations;
Log xe/(x/m)=-0.1707xe - 0.4552 with linearity coefficient of R2=0.9462 for F2;
Log xe/(x/m)=-0.0993xe - 0.2584 with linearity coefficient of R2=0.9991 for F1;
Log xe/(x/m)=-0.1096xe - 0.2187 with linearity coefficient of R2=0.9651 for C2;
Log xe/(x/m)=-0.199xe - 0.2972 with linearity coefficient of R2=0.9751 for C1;
and Log xe/(x/m)=-0.1272x - 0.1643 with linearity coefficient of R2=0.9325 for C0.
The isotherms for sunflower and cotton seed oils followed the Freundlich equation in a manner similar to the adsorption isotherms of decolorization of maize oil. This indicated the existence of heterogeneous adsorption sites on the solid’s surface [64].
This showed that the acid leached clay matrices did not have limitations resulting from overcrowding, steric interference, thermodynamic instability at high impurity concentrations [65]. The clays were strongly adsorbing as depicted by the high linearity of Freundlich isotherms. These strongly adsorbing clays are better described by the Freundlich isotherms within lower concentration ranges [66].
Freundlich isotherms assume monolayer adsorption capacity, complications arise when clays and clays minerals exhibit multi-layer adsorption tendencies and this causes decrease in linearity coefficients of the isotherms. It was the raw clay that had the least linearity coefficient showing that steric hindrance may have affected it due to its low capacity to adsorb. Clays with low adsorption capacities deviated from Freundlich isotherms because they easily got saturated with impurities from oils leading to steric interactions between the adsorbed and unadsorbed impurities [13]. The amount of impurities in vegetable oils that get adsorbed on clays change with small changes in the equilibrium bulk concentration and this is reflected in the deviation from linearity of Freundlich adsorption isotherms. Commonly, if the clay becomes saturated with impurities, repulsion sets in and data obtained after the clay is saturated gives non-linear Freundlich isotherms. Kangole clay showed high degree of linearity of Freundlich adsorption isotherms because it was smectite-rich clays; had high increase in surface acidity and surface area [67]. Acid activation of clays changes the crystal structure through loss of octahedral ions, thereby increasing pore sizes in the clay matrix. So highly linear Freundlich isotherms were obtained for acid activated Kangole clays.
The values of the Freundlich constant k and n were obtained from plots of log Xe(x/m) against log Xe. The vertical intercept is k and slopes of the linear graphs is, n characterizing the bleaching power of the clay and the manner in which impurities adsorb on the clay respectively [8,56,68,69]. The values of these constants were as follows;
n=-0.0974 and k=0.285 for C40;
n=-0.0993 and k=0.2584 for C30;
n=-0.1096 and k=0.2187 for C20;
n=-0.199 and k=0.2972 for C10;
and n=-0.1272 and k=0.1643 C0.
The larger the value of Freundlich constant, k, the better the clay at removing impurities from the vegetable oils. Generally, the magnitude of values of k for Kangole clays increases with increase in strength of the acid used to the clay. The values of n obtained in this study are less than those obtained by Topallar [56] due to lower purity of Kangole bentonite than bentonite EY-09 was supplied by Bensan Co. Ltd., Edirne (Enez), Turkey that Topallar used. Leaching with more concentrated acids resulted in creation of more impurity adsorption sites in the resulting activated clay. The highest ‘k’ value obtained with Kangole clay which was leached with 40% acid, which in turn gave acid-leached clay with highest bleaching strength. This is attributed to the Kangole clay being a smectite, which made the clay applicable as bleaching agent. When the value of n is below 0.5, it depicts that the clay is a very good adsorbent [70]. If the n is high, the clay will be effective for removing the first portions of color but less efficient for reaching highest bleaching degree [71]. The value of k determines the decolorizing power or activity of the adsorbent for a specific solute [56]. Freundlich isotherm model adequately described the adsorption data for all the clay samples investigated with regression coefficient values greater than 0.80. The same conclusion was reported by Nwabanne and Ekwu [20].
The value of the Freundlich constant n is used to determine the range of decolorization within which the adsorbent is most effective. Adsorbent clay with a higher value of n will be relatively effective at binding impurities in oils, but inefficient at bleaching oil to a low color value. The opposite is true for an adsorbent with a low n value. A high n value is desirable but not at the expense of k [11,56]. Basing this, clay leached in 10 and 20% would satisfactorily bleach.
Conclusion
The use of bleaching earths developed using local clay from Kangole in North Eastern Uganda in bleaching of cotton and sunflower oil has been studied. The results have indicated that this clay has great potential in the removal of color pigments from oil. The percentage decrease in absorbance of sunflower oil of 80%was achieved with clay leached in 20% hydrochloric acid. Yet cotton oil attained highest percentage absorbance of 55% during the bleaching step. The overall performance of hydrochloric acid leached clay was found higher than those activated using sulfuric acid. Also, the overall performance of clays activated with dilute acids was higher than those activated with concentrated acids. The values of Freundlich constants k and n showed that the acid-activated clays were very effective in bleaching cotton and sunflower oils.
Recommendation
The results produced in this research need to be tested in industrial settings.
Acknowledgements
We thank Mr. Edward Ssekubunga for heating the clay samples studied in the furnace, Prof. M. Ntale for availing us chance to use the spectrophotometers in the analytical laboratories and Prof Ludwig, of Institut Minerlagie in Federal Republic of Germany for running XRD patterns of clays.
References
- Ryan PC, Wall AJ, Hillier S, Clark L (2002) Insights into sequential chemical extraction procedures from quantitative XRD: a study of trace metal partitioning in sediments related to frog malformities. Chem Geol 184: 337-357.
- Gregg SJ, Sing KSW, Salzberg HW (1992) BET surface area study and SEM morphology characterization. J Electrochem Soc 42-44: 587-593.
- Valenzuela-DÃaz FR, Souza-Pérsio de S (2001) Studies on acid activation of Brazilian smetitic clays. QuÃm Nova 24.
- Franus W, Klinik J, Franus M (2004) Mineralogical characteristics and textural properties of acid-activated glauconite. Miner Polonica 35: 53-63.
- Rozic L, Novkovic T, Petrovic S (2010) Modeling and optimization process parameters of acid activation of bentonite by response surface methodology. Appl Clay Sci 48: 154-158.
- Beneke K, Lagaly G (2002) ECGA (European Clay Group Association). Newsletter 5: 57-78.
- Ujeneza E, Njenga HN, Mbui ND, Kariuki DN (2014) Optimization of Acid Activation Conditions for Athi River Bentonite Clay and Application of the Treated Clay in Palm Oil Bleaching. IOSR Journal of Applied Chemistry (IOSR-JAC) 7: 29-38.
- Boki K, Kubo M, Wada T, Tamura T (1992) Bleaching of alkali refined vegetable oils with clay minerals. J Am Oil Chem Soc 61: 233-236.
- Boki K, Hidehito M, Kawasaki N (1994) Bleaching rapeseed and soybean oils with synthetic adsorbents and attapulgites. J Am Oil Chem Soc 71: 595-601.
- Achife J, Ibemesi J (1989) Applicability of Freundlich and Langmuir Adsorption Isotherms in Bleaching of Rubber and Melon Seed Oils. Journal of the American Oil Chemists’ Society 66: 247-252.
- Mustapha SI, Mohammed AA, Zakari AY, Mohammed HA (2013) Performance evaluation of local clays from northern Nigeria for the refining of palm oil. Journal of Chemical Engineering and Materials Science 4: 58-66.
- Nodvin SC, Driscoll CT, Likens GE (1986) Simple Partitioning of Anionsand Dissolved Organic Carbonin Soil. Soil Science 142: 27-36.
- Dandy AJ (1965) Bleaching vegetable oils with Kajansi, Koki and Mutaka clays. Proceedings of the East African Academy 3: 2-8.
- Mukasa-Tebandeke IZ, Ssebuwufu PJM, Schumann A, Kirsch N (2001) The Bleaching Clays of Central and Eastern Uganda. Uganda journal of geology 1: 72-80.
- Mukasa-Tebandeke IZ, Ssebuwufu PJM, Nyanzi SA, Nyakairu GW, Ntale M, et al. (2015) The Elemental, Mineralogical, IR, DTA and XRD Analyses Characterized Clays and Clay Minerals of Central and Eastern Uganda. Advances in Materials Physics and Chemistry 5: 67-86.
- Mukasa-Tebandeke IZ, Ssebuwufu PJM, Nyanzi SA, Nyakairu GW, Ntale M, et al. (2015) Adsorption Behavior of acid-Leached Clays in Bleaching of Oil. American Journal of Analytical Chemistry 6: 495-512.
- Mukasa-Tebandeke IZ, Ssebuwufu PJM, Lugolobi F, Nyanzi S, Schumann A, et al. (2006) The Bleaching vegetable oils using acid and alkali-leached clays. International Journal of Environmental Issues 2: 87-93.
- Salawudeen TO, Dada EO, Alagbe SO (2007) Performance Evaluation of acid treated clays for palm oil bleaching. J Eng Appl Sci 2: 1677-1680.
- Nwabanne JT, Ekwu FC (2013) Decolourization of palm oil by Nigerianlocal clay: A study of adsorption isotherms and bleaching kinetics. Int J Multidiscipl Sci Eng 4: 20-25.
- Regina OA, Okechukwu DO (2012) Adsorptive removal of colorpigment from palm oil using acid activated Nteje clay: Kinetics, equilibrium and thermodynamics. Physicochem Probl Miner Process 49: 369-381.
- Usman MA, Ekwueme VI, Alaje TO, Mohammed AO (2012) Characterization, acid activation and bleaching performance of Ibeshe clay. ISRN Ceram.
- Sarier N, Güler C (1988) ß-Carotene adsorption on acid activated montmorillonite. J Am Oil Chem Soc 65: 776-779.
- Pradas EG, Sanchez MV, Viciana MS, Campo AG, (1994) Adsorption of chlorophyll-a from acetone solution on natural and activated bentonite. J Chem Tech Biotechnol 61: 175-178.
- Low KS, Lee CK, Kong LY (1998) Decolorisation of crude palm oil by activated spent bleaching earth. J Chem Tech Biotechnol 72: 67-73.
- Salawudeen TO, Arinkoola AO, Jimoh MO, Akinwande BA (2014) Clay Characterization and Optimisation of Bleaching Parameters for Palm Kernel Oil Using Alkaline Activated Clays. Journal of Minerals and Materials Characterization and Engineering 2: 586-597.
- Crepin J, Johnson RL (1993) Soil Sampling and Methods of Analysis. In: Carter MR (Ed) Soil sampling for environmental analysis. Alberta, Canada.
- Bain JA, Morgan DJ (1989) Laboratory separation of clays by hydrocycloning. Clay Miner 18: 33-47.
- Kirabira JB, Jounson S, Byaruhanga JK (2005) Powder characterization of high temperature ceramic raw materials in the Lake Victoria region. Silicate industriels 70: 127-134.
- Hutchison CS (1974) Laboratory Handbook of Petrographic Techniques. John Wiley & Sons Inc., New York, USA, pp: 234-240.
- Christidis GE, Scott PW, Marcopolous T (1995) Origin of bentonite deposits of Eastern Milos Islands, Greece: Geological, chemical and geochemical evidence. Clays and Clay Minerals 43: 63-77.
- Russell JD (1979) Instrumentation and techniques. Infrared spectra of minerals. London mineral society, London.
- Reynolds RC (1989) Principles and techniques of quantitative analysis of clay minerals by X-ray powder diffraction. Clay minerals Society 4: 36.
- Ip KH, Stuart BH, Thomas PS, Ray AS (2008) Thermal characterization of the clay binder of heritage Sydney sandstones. J Thermal Analy & Calorimetry 92: 97-100.
- Richardson LL (1978) Use of Bleaching Clays in Processing Edible Oils. Journal of the American Oil Chemists’ Society 55: 777-780.
- Wiederman LH (1981) Degumming, refining and bleaching soybean oil. J Am Oil Chem Soc 58: 159-166.
- Siddiqui MKH (1968) Bleaching earths. Pergamon Press, Oxford, pp: 32-55.
- Zschau W (1983) Bleaching of Palm Oil. Paper presented at the International Conference on Palm Oil Product Technology, Kuala Lumpur.
- Adekeye, Bale RB (2008) Bleaching performance of a Nigerian (Yola) Bentonite. Latin Amer Appl Res 38: 45-90.
- Naeem M, Ahmad N (2008) Characterization and activation studies on Azad Kashmir clays. Geol Bull Punjab Univ 43: 59-68.
- Reddy KK, Subramanian R, Kawakatsu T, Nakajima M (2001) Decolorization of vegetable oils by membrane processing. Eur Food Res Technol 213: 212-218.
- Subramanian R, Nakajima M, Kawakatsu T (1998) Processing of vegetable oils using polymeric composite membranes. Journal of Food Engineering 38: 41-56.
- Patterson HBW (1992) Bleaching and purifying fats and oils: theory and practice. Amer Oil Chem Soc Press pp: 69-78.
- Al-Zaharani AA, Alhamed YA (1996) Regeneration of spent bleaching clay by calcination cum-acid treatment. J Indian Institute of Chem Eng 38: 71-75.
- Gates K (2002) Mineralogy of bentonites. Clays and Clay Minerals 50: 223-239.
- Christidis GE, Scot H, Dunham G (1997) Acid activation and bleaching capacity of bentonite from Milos and Chios, Greece. Applied clay science 12: 329-347.
- Hamza A (1966) An Investigation on the Utilization of Egyptian Clays in Bleaching of Cotton Seed Oil. MSc Thesis, Alexandria University, Alexandria, Egypt.
- Mills GA, Holmes J, Cornelius EB (1950) Acid activation of some bentonite clays. J Phys Colloid Chem 54: 1170-1185.
- Nutting PG (1933) The bleaching earths. United States Geology Survey Circular, p: 3.
- Pawloski GA (1985) Quantitative determination of mineral content of geological samples by X-ray diffraction. Amer Mineralogist 70: 663-667.
- Etnier EL, Travis CL (1981) A survey of sorption relationships for reactive solutes in soil. J Environ Quali 10: 8-17.
- Proctor A, Palaniappan S (1989) Soil Oil Lutein Adsorption by Rice Hull Ash. J Am Oil Chem Soc 66: 1618-1621.
- Rich AD (1964) Some basic factors in bleaching of fatty olis. J Am Oil Chem Soc 41: 315-321.
- Topallar H (1998) Bleaching kinetics of sunflower seed oil. J Am Oil Chem Soc 75: 531-533.
- Temuujin J, Jadambaa T, Burmaa D, Erdenechimeg S, Kenneth JDM (2006) Characterization and bleaching properties of acid-leached montmorillonite. J Chem Technol & Biotechnol 81: 688-693.
- Balaras PK, Lezou P, Seiragakis G (1999) Mineralogical Society of London. Clay Miner 34: 221-312.
- Berbesi R (2006) Achieving Optimal Bleaching Performance. Oil Mill Gazetteer 112: 1-5.
- Ajemba OR, Onukwuli OD (2014) Assessing influence of hydrochloric acid leaching on structural changes and bleaching performance of Nigerian clay from Udi. Physicochem Prob Miner Process 50: 349-358.
- Hymore FK, Iyayi AF (1989) Use of local clays in the refining of palmoil. NS Che J 8: 161-171.
- Jimoh OT, Muriana M, Izuelumba B (2011) Sorption of Lead (II) and Copper (II) ions from Aqueous Solution by acid modified and unmodified Gmelina arborea (Verbenaceae) leaves. J Emerg Trends Eng Appl Sci 2: 734-740.
- Bijay KDJDP, Jignesh BP, Vijay KP, Vinay RP (2000) Activated Clay. J Oleo Science 58: 257-263.
- Christidis GE, Kosiari S (2003) Decolorization of vegetable oils: A study of the mechanism of adsorption of ß-carotene by an acid-activated bentonite from Cyprus. Clays and Clay Miner 51: 327-333.
- Hundal HS (1988) Clays in soils of Maharana Partap. J Agric Sci 111: 155-158.
- Kothawala DN, Moore TR, Hendershot WH (2008) Adsorption of Dissolved Organic Carbon to Mineral Soils: A Comparison of Four Isotherm Approaches. Geoderma 148: 43-50.
- Alemdaroglu T (2003) Investigation of the Surface Acidity of a Bentonite Modified by Acid Activation and Thermal Treatment. Turkish Journal of Chemistry 27: 675-681.
- Boyd SA, Shaobai S, Lee JF, Mortland MM (1988) Pentachlorophenol Sorption by Organo-Clays. Clays and Clay Minerals 36: 125-130.
- Norris FA (1982) Bailey's Industrial Oil and Fats Products. 4th edn., Wiley-Interscience, New York, USA.
- James OO, Mesubi MA, Adekola FA, Odebunmi EO, Adekeye JID, et al. (2008) Bleaching performance of a Nigerian (Yola) bentonite. Lat Am Appl Res 38: 45-49.
- Rossi M, Gianazza M, Alamprese C, Stanga F (2003) The role of bleaching clays and synthetic silica in palm oil physical refining. Food Chem 82: 291-296.
Citation: Mukasa-Tebandeke IZ, Wasajja-Tebandeke H, Schumann A, Lugolobi F (2016) Bleaching Edible Oils Using Clay from Kangole, Moroto District, North Eastern Uganda. J Anal Bioanal Tech 7:320. DOI: 10.4172/2155-9872.1000320
Copyright: © 2016 Mukasa-Tebandeke IZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14120
- [From(publication date): 6-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 12985
- PDF downloads: 1135