BIRC5 Expression Correlated with Immunosuppressive Phenotype and Predicted Inferior Response to Immunotherapy in Lung Adenocarcinoma
Received: 05-Aug-2024 / Manuscript No. DPO‑24‑144491 / Editor assigned: 08-Aug-2024 / PreQC No. DPO‑24‑144491 (PQ) / Reviewed: 02-Sep-2024 / QC No. DPO‑24‑144491 (QC) / Revised: 10-Sep-2024 / Manuscript No. DPO‑24‑144491 (R) / Published Date: 18-Sep-2024 DOI: 10.4172/2476-2024.1000239
Abstract
Background: This study aimed to examine the effects of BIRC5 on the clinical and tumor micro environmental aspects of Lung Adenocarcinoma (LUAD), as well as its predictive and prognostic qualities, given its vital function in carcinogenesis, recurrence and chemoresistance.
Methods: Analysis was conducted on the transcriptome and clinical data of 535 LUAD samples, 59 normal lung samples and 54 patients who were treated with Immune Checkpoint Blockades (ICB) for Non-Small Cell Lung Cancer (NSCLC). To determine the correlation between BIRC5 expression level and tumor micro environmental characteristics, deconvolution analysis was performed. The Log-rank test and Cox regression analysis were also utilized to assess the prognostic and predictive values of BIRC5.
Results: BIRC5 expression was significantly greater in LUAD than in normal lung tissues. Adverse clinical outcomes were significantly correlated with higher BIRC5 expression. Analysis of transcriptome and single-cell sequencing data showed a correlation between various pathways' enrichment and tumors exhibiting high BIRC5 expression. Deconvolution analysis revealed a positive link between BIRC5 expression and regulatory T cells (Tregs) infiltrations, but a negative correlation between BIRC5 expression and CD8+ T cell, dendritic cell and NK cell infiltration levels in LUAD. Significantly, ICB with high BIRC5 expression was given to NSCLC patients and these patients had significantly lower overall survival (3.1 vs. 12.7 months; p=0.005) and progression-free survival (1.2 vs. 4.5 months; p=0.012) than those with low BIRC5 expression.
Conclusion: These findings suggested that high BIRC5 expression was associated with DNA damage/repair, cell invasion and proliferation related pathways enrichment and increased Tregs infiltration, which would result in inferior outcomes in NSCLC received ICB.
Keywords: Non-small cell lung cancer; Bioinformatics; BIRC5; TME; Immunotherapy
Introduction
About 85% of all lung cancers are Non-Small-Cell Lung Cancers (NSCLCs), which also happen to be the primary cause of cancer-related mortality globally [1,2]. Lung Adenocarcinoma (LUAD) is the most common histological subtype and accounts for most lung cancer-related fatalities [3,4]. The overall prognosis for LUAD patients remains unsatisfactory despite significant breakthroughs in treatment, such as immunotherapy, molecular targeted therapy and radiation [5]. Immunotherapies, particularly Immune Checkpoint Blockades (ICB) that target Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) or Programmed cell Death protein 1 (PD-1), its Ligand (PD-L1), or both, dramatically increase Overall Survival (OS) in patients with a variety of cancer types, including LUAD [6,7]. However, a sizable fraction of LUAD patients receiving ICB treatment were unable to produce an objective response, highlighting the need for the identification of predictive biomarkers for selection of patients who may gain from this treatment [8]. Although ICB has shown effectiveness as an oncologic treatment for LUAD [9], distinct genomic and immunological landscapes may influence the response to ICB [10]. Thus, comprehending the correlations between particular gene expression and the immune micro environmental characteristics of tumors should aid in the creation of reliable biomarkers for immunotherapy, improve the clinical response, and increase the population that benefits [11,12]. The smallest but most functionally complex member of the Inhibitor of Apoptosis Protein (IAP) family is BIRC5, commonly referred to as survivin [13]. By physically blocking direct connections with molecules that promote apoptosis and indirectly reducing caspase-9 activation, BIRC5 plays a critical role in protecting cells from both intrinsic and extrinsic apoptotic pathways [14]. By regulating the mitotic apparatus, ensuring appropriate chromosomal segregation and protecting microtubule integrity, BIRC5 functionally supports the growth of malignant cells by promoting safe and effective cell division [15]. Hypoxia stimulates the expression of BIRC5, which is closely associated with angiogenesis and cell division. The majority of human malignancies have high levels of BIRC5 expression, which is closely linked to tumor growth, recurrence, resistance to chemotherapy and a poor prognosis. Nevertheless, BIRC5 expression in LUAD and its prognostic and predictive significance for the results of ICB treatment have not yet been thoroughly examined. Thus, we used information from The Cancer Genome Atlas (TCGA) to do this study in order to describe the expression of BIRC5, its correlation with clinicopathological characteristics in LUAD, as well as its predictive and prognostic values, as well as the protein expression from Human Pathology Atlas (HPA) online database. To clarify the relationship between tumor micro-environmental features and BIRC5 expression, we analyze the bulk RNA and single-cell sequencing data to investigate the connections between BIRC5 expression level and enriched biological pathways and the immune cells’ infiltrations.
Materials and Methods
Patients and databases
The TCGA database provided gene expression data for 535 LUAD and 59 normal lung tissues. An additional 57 LUAD and matched normal lung tissues were grouped for prognostic value and clinicopathological feature analysis. To investigate BIRC5 expression across the two databases, transcriptomic data in Transcripts per Million (TPM) formats from TCGA and GTEx was consistently processed. Furthermore, using the Oncomine database, BIRC5 expression data from adjacent normal tissues as well as pan-cancer tissues were obtained and analyzed. LUAD and normal lung tissues' BIRC5 protein levels were assessed using the Human Protein Atlas (HPA) database.
BIRC5-related functional enrichment analysis
Utilizing the STRING website, we examined BIRC5-binding proteins and set certain conditions, such as a minimum required interaction score of low confidence, active interaction sources from publications and a network type of complete network, to find the binding proteins. We found 20 experimentally verified proteins that bind to BIRC5, after limiting the maximum number of interactors shown to 20 and adjusting the significance of network edges to evidence. Furthermore, we chose the top 100 potential genes by using GEPIA2 to assess genes that showed BIRC5-like expression patterns in LUAD. Next, using the DAVID website, we conducted Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment investigations on the BIRC5, 20 BIRC5-binding proteins and 100 putative genes.
Single-cell sequencing data analysis
We investigated the functional state of cancer cells in relation to BIRC5 expression, with the use of CancerSEA database, a dedicated platform for single cell sequencing data analysis. A heatmap was designed based on the findings of the correlation study that was run to investigate the relationship between BIRC5 expression and several tumor functional features. To assess the connection between BIRC5 and tumor cell functionality even more, we created t-distributed Stochastic Neighbor Embedding (t-SNE) diagrams for every single cell from the Cancer SEA website.
Differential gene expression analysis
To identify Differentially Expressed Genes (DEGs) between high and low SIRPg expression groups, the "edgeR" R program was used. To identify the most significant DEGs, a cutoff gene expression fold change of >1.5 or versus low BIRC5 expression group.
Pathway enrichment analysis
To carry out the pathway enrichment study, we used three different approaches: GO, KEGG and GSEA. The Molecular Signature Database provided the curated gene sets of known signaling pathways. The GO word analysis was performed using the R package "cluster Profiler," and the relevant pathways between the high and low SIRPg expression groups were examined using the GSEA software version 4.1.0.
Infiltration of immune cells
We used the single-sample Gene Set Enrichment Analysis (ssGSEA) approach to measure the amount of immune cell infiltration in tumor tissues. The LUAD expression profile data was exposed to immunological datasets that included 24 types of immunocytes and the R package "GSVA" was used to measure the infiltration levels [16]. Additionally, the relationship between immune cells and the two groups as well as the correlation between BIRC5 and other immune cells were assessed using the Spearman correlation and Wilcoxon rank sum test.
Public datasets of anti-PD-1/PD-L1 treated study
The response data that were accessible, along with the clinical and bulk RNA-seq data of 54 NSCLC patients who received PD-1/PD-L1 inhibition monotherapy, were obtained from other publications [1,17,18]. The Complete Response (CR), Partial Response (PR), Stable Disease (SD), or Progressing Disease (PD) were the clinical responses that were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST). Patients who underwent PD-1/PD-L1 blockade with CR, PR, or SD were classified as responders. Patients who got PD-1/PD-L1 blockade in conjunction with PD were classified as non-responders. The definitions of Overall Survival (OS) and Progression-Free Survival (PFS) were in line with the relevant published research. The survival was assessed using the Kaplan-Meier curves with two-sided log-rank tests and a Cox proportional hazards model with computed Hazard Ratios (HRs) and 95% Confidence Intervals (CIs).
Statistical analysis
BIRC5 expression was examined using the Wilcoxon rank-sum test in both the LUAD and normal lung groups. Based on the median expression of BIRC5, two groups of LUAD patients were created. Using the Wilcoxon rank sum or Kruskal-Wallis tests, the clinicopathological characteristics of the high and low BIRC5 expression groups were compared. A Pearson correlation analysis was performed to assess the relationship between the abundance of immune cells, the expression of immunological markers and BIRC5 expression. Depending on the available cohort, survival outcomes were measured using Overall Survival (OS), Disease-Free Survival (DFS), or Progression-Free Survival (PFS). The prognostic and predictive significance of BIRC5 expression was evaluated through the Kaplan-Meier method, the log-rank test and Cox proportional hazards regression. To determine the predictive importance of genes with differential expression, a Receiver Operating Characteristic (ROC) curve was generated using the "plotROC" tool. The predictive value nomogram of LUAD patients was plotted using the R function "rms." In all two-sided statistical significance tests, p values or FDR q values
Results
Characterization of BIRC5 expression in LUAD
First, we utilized the transcriptome datasets from the TCGA program to examine the different expression levels of BIRC5 between the normal lung and LUAD tissues. Table 1 provided the specifics of 535 LUAD patients along with the expression of BIRC5. The expression level of BIRC5 was significantly greater in LUAD tissues compared to normal lung samples (p<0.001; Figure 1A). In the paired tissue samples, LUAD showed a significantly higher expression level of BIRC5 than normal lung tissues (p<0.001; Figure 1B). We conducted a systematic analysis using transcriptomic datasets from the TCGA program to investigate BIRC5 expression across various cancer types. Our findings revealed that BIRC5 was overexpressed in most cancers, such as glioblastoma multiforme, bladder urothelial carcinoma, bone cancer and cholangiocarcinoma (see Figure 1C). We assessed the expression levels of BIRC5 in LUAD and normal lung tissues using the HPA database. Compared to matched normal lung tissues, we found that LUAD had increased protein levels of BIRC5 (Figure 1D). Additionally, BIRC5 expression was considerably higher in tumors with pathological stages II–IV than in those with pathological stages I (p<0.001) (Table 1 and Figures 1A-1D).
Figure 1: The expression level of BIRC5. (A) Comparison of BIRC5 expression level between LUAD and normal lung tissues; (B) BIRC5 expression level in 57 matched LUAD and normal lung tissues; (C) Pan-cancer analysis of BIRC5 expression in various cancers and adjacent normal tissues; (D) Human Protein Atlas (HPA) database were applied to characterize the BIRC5 protein expression. Note: **p<0.01; ***p<0.001.
| Characteristic | Low expression of BIRC5 | High expression of BIRC5 | p-value |
|---|---|---|---|
| n | 267 | 268 | - |
| T stage, n (%) | 0.001 | ||
| T1 | 108 (20.3%) | 67 (12.6%) | - |
| T2 | 124 (23.3%) | 165 (31%) | - |
| T3 | 24 (4.5%) | 25 (4.7%) | - |
| T4 | 10 (1.9%) | 9 (1.7%) | - |
| N stage, n (%) | 0.002 | ||
| N0 | 190 (36.6%) | 158 (30.4%) | - |
| N1 | 38 (7.3%) | 57 (11%) | - |
| N2 | 27 (5.2%) | 47 (9.1%) | - |
| N3 | 0 (0%) | 2 (0.4%) | - |
| M stage, n (%) | 0.149 | ||
| M0 | 177 (45.9%) | 184 (47.7%) | - |
| M1 | 8 (2.1%) | 17 (4.4%) | - |
| Pathologic stage, n (%) | <0.001 | ||
| Stage I | 169 (32.1%) | 125 (23.7%) | - |
| Stage II | 53 (10.1%) | 70 (13.3%) | - |
| Stage III | 31 (5.9%) | 53 (10.1%) | - |
| Stage IV | 9 (1.7%) | 17 (3.2%) | - |
| Primary therapy outcome, n (%) | 0.005 | ||
| PD | 23 (5.2%) | 48 (10.8%) | - |
| SD | 22 (4.9%) | 15 (3.4%) | - |
| PR | 3 (0.7%) | 3 (0.7%) | - |
| CR | 179 (40.1%) | 153 (34.3%) | - |
| Gender, n (%) | <0.001 | ||
| Female | 164 (30.7%) | 122 (22.8%) | - |
| Male | 103 (19.3%) | 146 (27.3%) | - |
| Race, n (%) | 0.817 | ||
| Asian | 3 (0.6%) | 4 (0.9%) | - |
| Black or African American | 30 (6.4%) | 25 (5.3%) | - |
| White | 210 (44.9%) | 196 (41.9%) | - |
| Residual tumor, n (%) | 0.185 | ||
| R0 | 166 (44.6%) | 189 (50.8%) | - |
| R1 | 7 (1.9%) | 6 (1.6%) | - |
| R2 | 0 (0%) | 4 (1.1%) | - |
| Anatomic neoplasm subdivision 2, n (%) | 1 | ||
| Central lung | 28 (14.8%) | 34 (18%) | - |
| Peripheral lung | 56 (29.6%) | 71 (37.6%) | - |
| Smoker, n (%) | 0.078 | ||
| No | 45 (8.6%) | 45 (8.6%) | - |
| Yes | 215 (41.3%) | 231 (44.3%) | - |
| Age, median (IQR) | 67 (60,74) | 65 (58,71) | 0.015 |
| Number pack years smoked, median (IQR) | 30 (20,50) | 40 (25,54) | 0.007 |
Table 1: Baseline characteristics comparison between high and low BIRC5 expression groups.
Additionally, we found that elevated expression of BIRC5 was linked with increased T stage (P<0.001), N and M stages (p<0.001), smoking history (p<0.05) and young age (p<0.05) (Figures 2A-2F).
We investigated the relationship between BIRC5 expression and patient survival rates in order to ascertain the prognostic significance of BIRC5 in LUAD. Significantly lower OS (HR=1.79, p<0.001; Figure 3A), DFS (HR =1.98, p<0.001; Figure 3B) and PFS (HR=1.58, p=0.001; Figure 3C) were all associated with increased expression of BIRC5. Furthermore, an Area Under the Curve (AUC) value of 0.96 was found using the ROC analysis of LUAD to assess the predictive importance of BIRC5 (Figures 3A-3D).
Figure 3: : Survival analysis of clinical outcomes between patients with high and low BIRC5 expression. (A) Kaplan-Meier plot of overall survival; (B) Kaplan-Meier plot of disease-specific survival; (C) Kaplan-Meier plot of progression-free survival; (D) ROC analysis of LUAD to determine the predictive significance of BIRC5.
Analysis of BIRC5 co-expression network and enriched pathway
Using the STRING website, we determined and experimentally verified the binding proteins of BIRC5 in order to explore the co-expression network and pathways where BIRC5 is enriched in LUAD. Twenty proteins were detected, according to the results, binding to BIRC5:MED1, MED6, MED18, MED30, MED31, LAMTOR5, H3F3A, H3F3B, AURKB, AURKC, HFE, INCENP, CDCA8, HIST3H3, CASP3, CASP7, CASP9, DIABLO, XIAP, and BIRC2 (Figure 4A). Next, we chose the 100 genes that were most closely associated with BIRC5 out of the GEPIA2 database. On the 448 genes, GO and KEGG enrichment analysis were performed. These genes were substantially linked to chromosome segregation, mitotic sister chromatid segregation, nuclear division, ATPase activity, microtubule motor activity, tubulin binding, microtubule binding and other processes, according to GO enrichment analysis (Figures 4B-4D). Furthermore, we observed that BIRC5 was connected to the development of tumors through the p53 signaling pathway, cellular senescence, cell cycle, pyrimidine metabolism, DNA replication and progesterone-mediated oocyte maturation (Figure 4E). When combined, these findings supported the traditional function of BIRC5 as described in earlier studies Figures 4A-4E.
Figure 4: Co-expression network of BIRC5 and enrichment pathway analysis. (A) The binding proteins of BIRC5 by STRING website; (B-D) Gene Ontology (GO) enrichment analysis of biological process, cellular component, molecular function; (E) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analysis based on BIRC5 binding proteins and interactive genes.
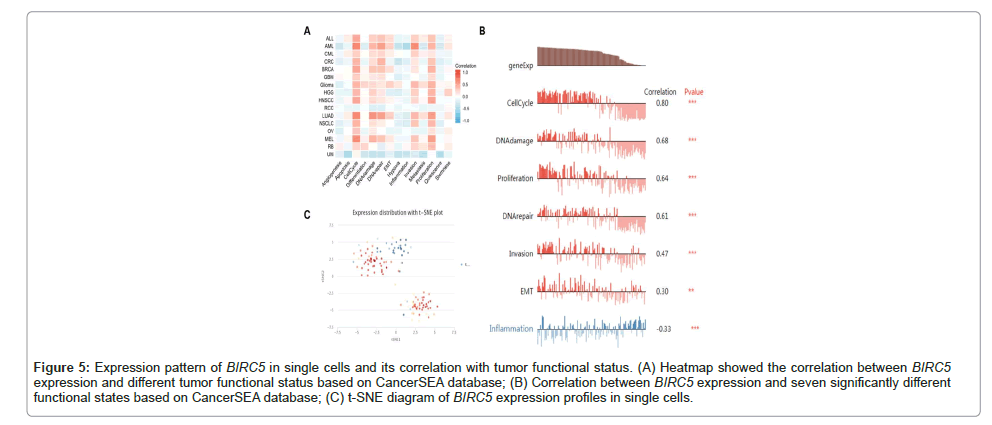
Expression level of BIRC5 in single cells and its effect on functional status
An essential technique for examining the intricate network of tumor, immunological, endothelial and stromal cells in malignancies is single-cell transcriptome sequencing. Using the CancerSEA website, we examined the relationship between BIRC5 expression and tumor function in LUAD at the single-cell level. We found that a number of critical physiological processes, including the cell cycle, DNA damage, repair, hypoxia, inflammation, and proliferation, were strongly linked with the expression of BIRC5 (Figure 5A). Moreover, our study demonstrated a correlation between BIRC5 expression and the following in LUAD: Invasion, proliferation, DNA damage response, cell cycle progression, invasion, Epithelial-Mesenchymal Transition (EMT) and inflammation (Figure 5B). Using t-SNE diagrams, we were also able to see the BIRC5 expression profiles in individual LUAD cells (Figure 5C). These results imply that BIRC5 may play a critical role in the development of LUAD by regulating important biological processes as proliferation, DNA damage/repair, hypoxia, the cell cycle and inflammation (Figures 5A-5C).
Figure 5: Expression pattern of BIRC5 in single cells and its correlation with tumor functional status. (A) Heatmap showed the correlation between BIRC5 expression and different tumor functional status based on CancerSEA database; (B) Correlation between BIRC5 expression and seven significantly different functional states based on CancerSEA database; (C) t-SNE diagram of BIRC5 expression profiles in single cells.
Univariate and multivariate analyses
We next performed the univariate and multivariate analyses by modifying the common clinicopathological characteristics after observing the negative correlation between high BIRC5 expression with the clinical outcomes of LUAD patients. The findings demonstrated that in univariate analyses, tumor status, residual tumor, T, N and M stages, as well as pathological stage, were substantially linked with OS (Supplemental Table 1). Poor OS was also significantly correlated with high BIRC5 expression (HR=1.75, 95% CI 1.30-2.36, p<0.001). The results of multivariate analyses showed an independent relationship between shorter OS and increased BIRC5 expression (HR=1.44, 95% CI 1.01-2.28, p=0.043).
In order to examine the effect of BIRC5 expression on OS based on age, sex, and risk variables for anatomic neoplasm subdivision, we conducted a subgroup analysis. We found that increased BIRC5 expression was substantially linked with inferior survival outcomes in these subgroups stratified by age, gender and anatomic neoplasm classification (Supplemental Figure 1). The results presented above show a substantial correlation between BIRC5 expression levels and LUAD patients' clinical outcomes. In order to quantify the odds of each individual survival, we consequently created a predictive nomogram based on these characteristics (Supplemental Figure 2A). The 1-3 years and 5 years survival estimates closely matched the ideal line, according to the predictive model's calibration curve (Supplemental Figure 2B). These findings demonstrate that BIRC5 expression-based model could provide accurate estimation of overall prognosis.
Enriched pathways in tumor with high BIRC5 expression
Using bulk RNA sequencing data from 535 LUAD patients, we ran GSEA to determine the distinct pathways enriched between high and low BIRC5 expression groups. The findings showed that mRNA splicing, DNA repair, and translation were among the enriched pathways linked to malignancies with elevated BIRC5 expression (Supplemental Figure 3). A further possible mechanistic connection between BIRC5 expression and the cell cycle, DNA damage/repair, hypoxia, inflammation and cell proliferation was discovered using single-cell sequencing data analysis.
Immune infiltration analysis
In order to visualize the immunological infiltration landscape, we deconvoluted the bulk RNA sequencing data of 535 LUAD patients in order to identify the immune profiles of tumors with different degrees of BIRC5 expression. The results of the deconvolution analysis showed that the infiltration levels of various immunosupportive cells, such as B cells, T helper 17 cells, CD8+ T cells, macrophages, dendritic cells and NK cells in LUAD, were negatively correlated with BIRC5 expression. Conversely, the infiltration levels of regulatory T cells (Tregs) were positively correlated with BIRC5 expression (Figure 6). These findings imply that tumors expressing a high level of BIRC5 would have a cool tumor immune milieu.
Predictive value of BIRC5 expression in LUAD received ICB
Generally communicating, immunotherapy is unlikely to be effective for tumors with a large infiltration of immunosuppressive cells. We further postulated that individuals with NSCLC whose pre-treatment tumors had high BIRC5 expression might not respond well to PD-1/PD-L1 inhibition because LUAD with high BIRC5 expression had increased Tregs infiltration. An integrated analysis of the transcriptome and treatment outcome data of patients with advanced Non-Small Cell Lung Cancer (NSCLC) undergoing PD-1/PD-L1 inhibition monotherapy was performed to ascertain the effect of BIRC5 expression on clinical outcomes in patients treated with this medication. Transcriptome and response data were available for three articles involving fifty-four NSCLC patients [1,17,18].
First, we noticed that the expression level of BIRC5 was considerably lower in responders than in non-responders (p=0.029, Figure 7A). The response rate was significantly lower in patients with high BIRC5 expression (≥ median level) compared to those with low BIRC5 expression (<median level) Objective Response Rate (ORR 19.0% vs. 41.1%; p=0.024, Figure 7B). Importantly, PFS was significantly longer in patients with high BIRC5 expression than in those with low expression (median PFS 1.2 vs. 4.5 months; p=0.012, Figure 7C). Also, the median OS was lower in the group with high BIRC5 expression than in the group with low expression (3.1 vs. 12.7 months; P=0.005, Figure 7D). Due to the lack of access to the clinicopathological data from these investigations, we did not do the multivariate analysis (Figures 7A-7D)
Figure 7: Predictive value of BIRC5 expression in LUAD received Immune Checkpoint Blockade (ICB). (A) Responders had significantly lower BIRC5 expression level than non-responders; (B) Patients with high BIRC5 expression (≥ median level) showed a markedly decreased response rate than those with low BIRC5 expression (<median level); (C) Survival analysis of BIRC5 expression in NSCLC patients with PD-1 blockade for PFS; (D) Survival analysis of BIRC5 expression in NSCLC patients with PD-1 blockade for OS.
Discussion
This study demonstrated that LUAD had a considerably greater expression level of BIRC5 than normal lung tissues. We achieved this by integrating the clinical and transcriptome data of 535 LUAD samples, 59 normal lung, and 54 patients with NSCLC treated with ICB from an online database. Adverse clinical outcomes were significantly correlated with higher BIRC5 expression. Tumors exhibiting elevated BIRC5 expression were linked to enhanced pathways relevant to cancer cell invasion and proliferation, such as cell cycle, DNA damage/repair, hypoxia, inflammation and cell proliferation, as demonstrated by transcriptome and single-cell sequencing data analysis.
BIRC5 expression was found to be positively correlated with Treg infiltrations, but negatively correlated with CD8+ T cell, T helper 17 cell, B cell, macrophage, dendritic cell and NK cell infiltrations in LUAD, according to the deconvolution study. Significantly, PFS and OS were notably shorter in PD-1/PD-L1 inhibitor-treated NSCLC patients with baseline high BIRC5 expression than in those with low BIRC5 expression. Apoptosis is an essential process that maintains regular cellular activity [19]. Usually, apoptosis helps eliminate cells with abnormal cell cycles and damaged DNA [20]. The disturbance of the apoptotic pathway by malignant cells leads to tumorigenesis and resistance to therapy because the body is unable to adequately eliminate these cells [21]. A highly conserved family of antiapoptotic proteins, the IAP protein family mainly prevents apoptosis by inhibiting caspase activation and controlling NF-κB signaling [22]. Situated on chromosome 17q25, BIRC5 is the smallest member of the IAP family. It was extracted from the human genome library in 1997 [23]. BIRC5 performs a number of biological tasks, such as promoting cell mitosis by aiding in chromosome division and microtubule movement, inhibiting apoptosis by directly or indirectly suppressing caspase-3 and caspase-7 activity, angiogenesis and acting as a biomarker for tumor diagnosis, treatment and prognosis prediction [24]. As a result, inhibiting BIRC5 function may help stop tumor cells from growing and spreading, making it a promising target for cancer therapy.
Numerous studies have shown that BIRC5 plays a significant role in regulating the cell cycle, proliferation, angiogenesis and growth of cancer cells [25]. It has been demonstrated that cytokines in lymphocytes regulate the expression of BIRC5, which is necessary for hematopoietic cells to proliferate and survive. It has been documented that BIRC5 inhibits cell apoptosis while promoting cell proliferation in the setting of human malignancies [26,27]. Previous studies have shown that BIRC5 anti-apoptotic effects are achieved by inhibiting both caspase-dependent and caspase-independent apoptotic pathways while also promoting cell growth and development.
The current study integrated bulk and single-cell RNA sequencing data. The findings demonstrated that tumors with increased BIRC5 expression were connected to enrichment in pathways related to cell cycle, hypoxia, cell proliferation, DNA damage/repair, and inflammation. Furthermore, BIRC5-expressed LUAD patients did not have good clinical results. All of these results suggested that BIRC5 might be essential for LUAD prognosis and carcinogenesis. A well-researched hallmark of the tumor microenvironment of advanced solid tumors is hypoxia [28]. Hypoxia is a significant stressor that can control the expression of several genes in tumor cells, enabling the cells to adjust to their hypoxic environment [29].
Vascular Endothelial Growth Factor (VEGF) and related factors are influenced by hypoxia and have been shown to contribute to tumor recurrence and stimulate the production of various vascular growth factors. Additionally, hypoxia has been found to upregulate BIRC5, which supports angiogenesis and is closely associated with cell proliferation. In many cancers, the hypoxic tumor microenvironment is strongly correlated with poor prognosis and resistance to immunotherapy [30-32]. Targeting Hypoxia-Inducible Factors (HIFs), essential for the survival of hypoxic cells, offers a potential strategy. Consequently, developing a BIRC5 inhibitor that specifically targets HIFs could provide a promising new therapeutic approach for patients with advanced lung cancer and those who do not respond to immune-based treatments. The significance of immune cell infiltration in the development of cancer has drawn a lot of attention lately [33]. It has been demonstrated that immune gene signatures and the makeup of immune cell infiltrates are reliable indicators of clinical prognosis in a number of malignancies. A better prognosis is linked to the presence of Th1-biased gene signatures, CD8 and memory T cells, while the presence of M2-like macrophages is linked to a worse prognosis [34,35]. In our investigation, we found that the expression of BIRC5 in LUAD was negatively correlated with immune cells, including B cells, T helper 17 cells, DCs, NK cells, CD8+ T cells and macrophages.
We also observed a positive correlation between Tregs and BIRC5 expression. Tregs are known to be associated with poor outcomes in cancer due to their immune-suppressive and anti-tumor effects. This finding suggests that BIRC5 might play a role in modulating the immune environment around tumors. However, the precise mechanisms by which BIRC5 affects tumor immunity and LUAD carcinogenesis remain unclear. A comprehensive understanding of how BIRC5 influences CD8+ T cell phenotype and function, as well as Tregs infiltration, could provide insights for developing new immunotherapeutic targets and combination therapies.
Immunotherapy has emerged as an effective cancer treatment, but its benefits are not universal. Our data showed that LUAD patients with low BIRC5 expression experienced significantly better ORR, PFS and OS compared to those with high BIRC5 expression when treated with PD-1/PD-L1 inhibitors. These findings suggest that BIRC5 expression could serve as a valuable diagnostic marker for predicting treatment response in LUAD patients. More study and larger sample sizes are needed to validate its predictive value.
Conclusion
The current study concluded that patients with LUAD who expressed high levels of BIRC5 had an unfavorable response to PD-1/PD-L1 inhibition, increased infiltration of Tregs, pathways linked to the invasion and proliferation of cancer cells and an enrichment of DNA damage/repair. These results imply that BIRC5 may be a unique potential immunotherapeutic target in LUAD and a promising predictive biomarker for PD-1/PD-L1 inhibition.
Declarations
Acknowledgements
The authors declared no potential conflicts of interest. This work was supported in part by grants from Shanghai Municipal Education Commission (No.16SG18), the Science and Technology Commission of Shanghai Municipality (No.16411964600), the National Natural Science Foundation of China (No. 81772467), the Shanghai Shen Kang Pharmaceutical Development Co. Ltd (No. SHDC 12015314), and the Backbone Program of Shanghai Pulmonary Hospital (NO. FKGG1802).
Authors’ Contributions
All authors contributed to the study conception and design. Xiaohui Chen, Jia Yu, Shengxiang Ren raised the idea. Material preparation, data collection and analysis were performed by Shuoyang, Xiaozhen Liu, Shiqi Mao, Church Shao, Xufei Li and Chao Zhao. The first draft of the manuscript was written by Shuoyang, Xiaozhen Liu, Yan Wang, Qiyu Fang and Bin Chen. Fengying Wu and Xiaoxia Chen revised the manuscript. All authors read and approved the final manuscript.
Ethics statement
This study was reviewed and approved by Institutional Review Board of our center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
References
- Cho JW, Hong MH, Ha SJ, Kim YJ, Cho BC, et al. (2020) Genome-wide identification of differentially methylated promoters and enhancers associated with response to anti-PD-1 therapy in non-small cell lung cancer. Exp Mol Med 52:1550-1563.
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS, et al. (2021) Lung cancer. Lancet 398:535-554.
[Crossref] [Google Scholar] [PubMed]
- Chen P, Liu Y, Wen Y, Zhou C (2022) Non-small cell lung cancer in China. Cancer Commun (Lond) 42:937-970.
[Crossref] [Google Scholar] [PubMed]
- Succony L, Rassl DM, Barker AP, McCaughan FM, Rintoul RC, et al. (2021) Adenocarcinoma spectrum lesions of the lung: Detection, pathology and treatment strategies. Cancer Treat Rev 99:102237.
[Crossref] [Google Scholar] [PubMed]
- Kuhn E, Morbini P, Cancellieri A, Damiani S, Cavazza A, et al. (2018) Adenocarcinoma classification: Patterns and prognosis. Pathologica 110:5-11.
[Google Scholar] [PubMed]
- Reck M, Remon J, Hellmann MD (2022) First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol 40:586-597.
[Crossref] [Google Scholar] [PubMed]
- Suresh K, Naidoo J, Lin CT, Danoff S (2018) Immune checkpoint immunotherapy for non-small cell lung cancer: Benefits and pulmonary toxicities. Chest 154:1416-1423.
[Crossref] [Google Scholar] [PubMed]
- Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, et al. (2019) Immunotherapy in nonsmall cell lung cancer: Facts and hopes. Clin Cancer Res 25:4592-4602.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Herbst RS, Boshoff C (2021) Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med 27:1345-1356.
- Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI, et al. (2022) Immunotherapy in lung cancer: Current landscape and future directions. Front Immunol 13:823618.
[Crossref] [Google Scholar] [PubMed]
- Keenan TE, Burke KP, van Allen EM (2019) Genomic correlates of response to immune checkpoint blockade. Nat Med 25:389-402.
- Miao D, Margolis CA, Vokes NI, Liu D, Taylor-Weiner A, et al. (2018) Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet 50:1271-1281.
- Frazzi R (2021) BIRC3 and BIRC5: Multi-faceted inhibitors in cancer. Cell Biosci 11:8.
[Crossref] [Google Scholar] [PubMed]
- Li F, Aljahdali IAM, Zhang R, Nastiuk KL, Krolewski JJ, et al. (2021) Kidney cancer biomarkers and targets for therapeutics: Survivin (BIRC5), XIAP, MCL-1, HIF1alpha, HIF2alpha, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. J Exp Clin Cancer Res 40:254.
[Crossref] [Google Scholar] [PubMed]
- Li F, Aljahdali I, Ling X (2019) Cancer therapeutics using survivin BIRC5 as a target: What can we do after over two decades of study? J Exp Clin Cancer Res 38:368.
[Crossref] [Google Scholar] [PubMed]
- Restifo NP (2013) A "big data" view of the tumor "immunome". Immunity 39:631-632.
[Crossref] [Google Scholar] [PubMed]
- Jung H, Kim HS, Kim JY, Sun JM, Ahn JS, et al. (2019) DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat Commun 10:4278.
- Prat A, Navarro A, Pare L, Reguart N, Galvan P, et al. (2017) Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res 77:3540-3550.
[Crossref] [Google Scholar] [PubMed]
- Bertheloot D, Latz E, Franklin BS (2021) Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol Immunol 18:1106-1121.
[Crossref] [Google Scholar] [PubMed]
- Ketelut-Carneiro N, Fitzgerald KA (2022) Apoptosis, pyroptosis, and necroptosis-oh my! the many ways a cell can die. J Mol Biol 434:167378.
[Crossref] [Google Scholar] [PubMed]
- Morana O, Wood W, Gregory CD (2022) The apoptosis paradox in cancer. Int J Mol Sci 23:1328.
[Crossref] [Google Scholar] [PubMed]
- Fulda S, Vucic D (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 11:109-124.
[Crossref] [Google Scholar] [PubMed]
- Wheatley SP, Altieri DC (2019) Survivin at a glance. J Cell Sci 132:223826.
[Crossref] [Google Scholar] [PubMed]
- Lin TY, Chan HH, Chen SH, Sarvagalla S, Chen PS, et al. (2020) BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy 16:1296-1313.
[Crossref] [Google Scholar] [PubMed]
- Di X, Jin X, Xiang L, Gao X, Peng L, et al. (2023) Survivin (BIRC5) regulates bladder fibrosis in a rat model of partial bladder outlet obstruction. Chin Med J (Engl) 136:117-119.
[Crossref] [Google Scholar] [PubMed]
- Cheng SM, Lin TY, Chang YC, Lin IW, Leung E, et al. (2021) YM155 and BIRC5 downregulation induce genomic instability via autophagy-mediated ROS production and inhibition in DNA repair. Pharmacol Res 166:105474.
[Crossref] [Google Scholar] [PubMed]
- Chen ZX, Li GS, Yang LH, Liu HC, Qin GM, et al. (2021) Upregulation of BIRC5 plays essential role in esophageal squamous cell carcinoma. Math Biosci Eng 18:6941-6960.
[Crossref] [Google Scholar] [PubMed]
- Bhandari V, Hoey C, Liu LY, Lalonde E, Ray J, et al. (2019) Molecular landmarks of tumor hypoxia across cancer types. Nat Genet 51:308-318.
[Crossref] [Google Scholar] [PubMed]
- Khouzam RA, Brodaczewska K, Filipiak A, Zeinelabdin NA, Buart S, et al. (2020) Tumor hypoxia regulates immune escape/invasion: Influence on angiogenesis and potential impact of hypoxic biomarkers on cancer therapies. Front Immunol 11:613114.
[Crossref] [Google Scholar] [PubMed]
- Apte RS, Chen DS, Ferrara N (2019) VEGF in signaling and disease: Beyond discovery and development. Cell 176:1248-1264.
[Crossref] [Google Scholar] [PubMed]
- Jafarzadeh A, Bazargan N, Chatrabnous N, Jafarzadeh S, Nemati M, et al. (2023) Contribution of survivin to the immune system, allergies and autoimmune diseases. Hum Immunol 84:301-310.
[Crossref] [Google Scholar] [PubMed]
- Graham K, Unger E (2018) Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomedicine 13:6049-6058.
[Crossref] [Google Scholar] [PubMed]
- Li B, Severson E, Pignon JC, Zhao H, Li T, et al. (2016) Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol 17:174.
[Crossref] [Google Scholar] [PubMed]
- Locati M, Curtale G, Mantovani A (2020) Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol 15:123-147.
[Crossref] [Google Scholar] [PubMed]
- Pittet MJ, Michielin O, Migliorini D (2022) Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol 19:402-421.
[Crossref] [Google Scholar] [PubMed]
Citation: Yang S, Xiao L, Mao S, Shao CC, Li X, et al. (2024) BIRC5 Expression Correlated with Immunosuppressive Phenotype and Predicted Inferior Response to Immunotherapy in Lung Adenocarcinoma. Diagnos Pathol Open 9:239. DOI: 10.4172/2476-2024.1000239
Copyright: © 2024 Yang S, et al. This is an open‑access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.