Biosorption of Hexavalent Chromium By Paenibacillus Pabuli and Bacillus Cereus Isolated from Alkaline Industrial Contaminated Soil
Received: 30-Jan-2020 / Accepted Date: 18-Feb-2020 / Published Date: 25-Feb-2020 DOI: 10.4172/2155-6199.1000476
Abstract
In the present study we intended to remediate Cr(VI) with alkaliphilic bacterium Paenibacillus pabuli (JX561107) and Bacillus cereus (JX561108) isolated from alkaline industrial contaminated soil, Pondicherry. The bacterium was tested for its chromium removal efficiency at different concentration (50 mg/l, 200 mg/l, 400 mg/l). At 50 mg/l of hexavalent chromium concentration, Paenibacillus pabuli and Bacillus cereus are found to be highly efficient in removing Cr(VI) in 72 hrs at the inoculum rate of 1% of overnight grown bacterial cultures. The bacterium could remove 98% and 74% within 72 hrs at 9.5 pH. Whereas when the Cr(VI) concentration is increased to 400 mg/l, there was no removal. SEM images were done from the pellets of treated bacterium to examine the bacterial cell surface for morphological changes and EDX was also obtained to verify whether the adhered particles are chromium. The Cr(VI) removal efficiency of isolated bacterium from alkaline industrial contaminated soil are compared with efficiency of other bacterial species tested previously. The mechanism behind such adhesion and variation among bacterial population in chromium removal are discussed.
Keywords: Alkaliphilic bacteria; hexavalent chromium; Paenibacillus pabuli; Bacillus cereus; Bioremediation
Introduction
Alkaliphilic microorganisms attract increased attention during the last decades in the context of their great potential for biotechnological applications and research of ecological diversity [1]. Several alkaliphilic bacteria have been isolated from different environments for example deserts, soda lakes, and arid soils [2]. Natural alkaline environments, in particular soda lakes, which are distributed worldwide, contain large numbers of alkaliphiles [3,4]. Most intense and extensive intervals of organisms have been observed generally in “moderate” environments. It would also have been known to have an “extreme” environment on Earth are considered to avoid the endurance of existence. Habitats in these ecological environments, for instance pH, temperature and salinity concentrations are exceedingly elevated or low. The extreme environment is populated by a group of organisms that have been particularly modified to these specific circumstances and these types of intense microbes are frequently referred to as alkaliphiles, halophiles, thermophiles and acidophiles, and reflects an exacting type extreme environment in which they inhabitat [5].
Chromium metal exists under several oxidation states, ranging from 2 to +6. Among them, Cr (VI) and Cr (III) are of major environmental significance because of their persistence, stability and chemical reactivity. Cr (VI) is a strong oxidizing agent, commonly present as hydrochromate (HCrO4)), chromate (CrO4)) or dichromate (Cr2O7) oxyanions, depending on pH (United States, Environmental Protection Agency, US EPA 1998). Because of its high solubility in water Cr (VI) is consider as highly toxic and has been listed as one of the most dangerous substances by the US EPA (1998). Extensive use of chromium in industries such as leather tanning, stainless-steel production, electroplating and wood preservatives have resulted in chromium contaminated soil and ground water in and around production sites [6]. It is understood from the reports of Francisco et al. [7,8] that microorganisms can resist to this stress and are more likely to survive and thus influence chromium speciation. Chromium remediation studies have been carried out with a variety of organisms like Filamentous Cyanobacteria Anabaena [9]. Present study was undertaken to exam the ability of the alkaliphilic bacteria Paenibacillus pabuli and Bacillus cereus isolated from alkaline industrial contaminated soil to remediate hexavalent chromium at different concentration.
Materials and Methods
Collection of soil samples
Soil samples were collected from alkaline industry-contaminated soil from Pondicherry in the area of thavalakuppam. Triplicate sampling at every station was collected in a plastic container during the postmonsoon months from October-December. Before the collection of soil sample pH of the soil sample was measured at the collection site and recorded as 9.5 (Alkaline). The soil samples were collected from five randomly selected spots from the locations with the help of scooper from the top most sediment layer i.e. 0-15 cm layer of sediment samples (approximately 500 g) and transferred into sterile sample container and brought to the laboratory [10].
Isolation of alkaliphilic bacteria
Isolation of alkaliphilic bacteria was carried out from soil samples collected aseptically from alkaline industry effluent contaminated soil using rich alkaline nutrient agar medium containing Sodium Sesquicarbonate Solution [11] and was performed by making serial dilution of the samples and the dilution used for studies were 100 μl of the 10-7 dilution was spread in Alkaline Nutrient Agar. The pH was adjusted to 9.5 for alkaline industry effluent contaminated soil. The plates were incubated at 37˚C for 48 hrs. The isolated colony was subcultured with alkaline nutrient agar to check the purity of the isolates. Identification of the bacterial isolates was carried out by bacteriological methods based on colony morphology, Gram staining, motility and biochemical tests. Bar-coding of bacterial isolates was done by the method described by Marmur [12].
DNA Bar coding
Total genomic DNA isolation: Alkaliphilic bacterial strain was grown in alkaline nutrient broth pH 9.5 at 37˚C. After 12 h of incubation, 1.5 ml of cultured broth was taken and centrifuged at 8,000 g for 6 min. The pellets were re-suspended with 330 μl of GTE solution and incubated at 37˚C for 30 min. The pinch amount of lysozyme was added to the same solution and incubated at 37˚C for 1 h. 10 μl of 20% SDS was added and incubated at 37˚C for overnight. RNase (0.1 mg/ml) was added to the solution to remove the RNA from solution and it was kept at 37˚C. After 3 h of incubation 17 μl of EDTA (0.5M) was mixed and incubated at 50˚C for 10 min. Proteinase K (10 μl) was added and incubated at 37˚C for 3 h. After incubation, 200 μl of phenol: Chloroform (24:1) was added, mixed slowly and centrifuged at 16,000 g for 15 min. After centrifugation, the aqueous phase layer was collected and mixed with equal volume of isopropanol. It was slowly shacked up, down until to see the pool of DNA and centrifuged at 16,000 g for 15 min. The DNA pellet was washed with 1 ml of 95% ethanol and centrifuged at 16,000 g for 15 min. After centrifugation, the pellet was air dried and dissolved in 40 μl of 1X TE buffer. It was confirmed by running the agarose gel electrophoresis.
Agarose gel electrophoresis
The isolated DNA sample was separated on 0.8% agarose gel. 1X TAE buffer was prepared by appropriate concentration of 1 ml of 50X TAE buffer and mixed with 49 ml distilled water. Further 0.4g of powdered agarose was added and mixed well. They were allowed to boil until agarose dissolved completely. Then 3 μl of ethidium bromide (0.5 μg/ ml) was added from stock solution of 10 mg/ml and mixed well. The warmed agarose solution was poured into the gel casting tray and allowed to set for 30-45 min at room temperature. The gel was mounted in the electrophoresis tank. Electrophoresis buffer was added to cover the gel to a depth of about 1mm. The isolated DNA sample was mixed with loading buffer and loaded into the well of the submerged gel using a micropipette. The voltage of 50-60 was applied. After 1-2 h the gel was taken out from the buffer. The gel was examined under UV illuminator (UVI-TEC). The clear band was observed as red orange fluorescence. The molecular weight was measured by using appropriate marker DNA.
Polymerase Chain Reaction (PCR)
The total genomic DNA from the isolates was done by 16SrDNA PCR assay, by using the 16SrDNA universal primers (F-5’AGA GTT TGA TCC TGG CTC AG-3’ and R-5’-CGG TTA CCT TGT TAC GAC TT- 3’). They were amplified using above mentioned PCR mixture. The PCR was run using Eppendorf Gradient thermocycler, the gradient PCR assay was done with using the various annealing temperatures. The PCR cycle used was Intial denaturation 95˚C; 4 min. Denaturation 95˚C; 30 s. Annealing 55˚C; 30 s. Extension 72˚C; 30 s. Final extension 72˚C; 10 min, Cycles 30 cycles. The PCR products were analyzed by 0.8% Agarose gel electrophoresis The PCR-amplified 16SrDNAs were purified using the quick PCR purification Kit from Bangalore Genie. The sequencing was performed in (EUROFINS, BANGALORE) and sequencing was deposited in the NCBI Genbank. The study of arrangement and homology and the construction of a phylogenetic tree for the sequenced nucleotide were carried out by the bioinformatics tools.
Experimental Study
Degradation of Cr(VI)
The experimental study involves three different hexavalent chromium concentrations i.e. 50 mg/l, 200mg/l and 400 mg/l were carried out in 250 ml conical flasks (100 ml in each) at 9.5 pH. 1% of sucrose was added to all the conical flasks and 1% of overnight grown bacterial cultures were seeded in each conical flask. The flasks were kept on rotary shaker at 180 rpm at 37˚C. Hexavalent chromium depletion was estimated at an interval of 24 h till 72 h to depletion by the isolated bacterial strains with different chromium concentration. After biosorption, the mixture was centrifuged at 4000 rpm for 15 min. Remaining metal concentration in the solution was measured. Quantity of metals bound was taken to be the distinction among the initial and final concentrations of metal [13]. Concentration of metal in the solution was estimated with an atomic absorption spectrometer. The experiment was done thrice and the average value was taken for discussion.
Scanning Electron Microscopic study and EDX analysis
Scanning Electron Microscopic (SEM) studies were performed, to examine the changes in surface morphology before and after treatment and elemental composition of the bio sorbent along with EDX was studied using scanning electron microscope. Pellets of bacterial culture obtained through centrifugation are used for these spectral studies. The pellets obtained after centrifugation of treated bacterial culture are processed as. The pellets were washed twofold with 0.1 M phosphate buffered saline (PBS; 15 mM phosphate buffer, 138 mMNaCl, 2.7 mMKCl, pH 7.4 at 25ºC) and fixed overnight in 2% glutaraldehyde(prepared in 0.1 M PBS). The cells were washed with PBS and distilled water earlier to dehydration throughout an ethanol series (10% to absolute), held at each concentration for 30 minutes. Samples were positioned on a brass stub, sputter-coated with gold and examined by Scanning electron microscope [14]. SEM and EDX studies were performed with a Hitachi S-3400N Scanning electron microscope, available at Central Instrumentation Facility (CIF) Pondicherry University.
Results
Identification of isolated alkaliphilic bacteria from alkaline industry-contaminated soil
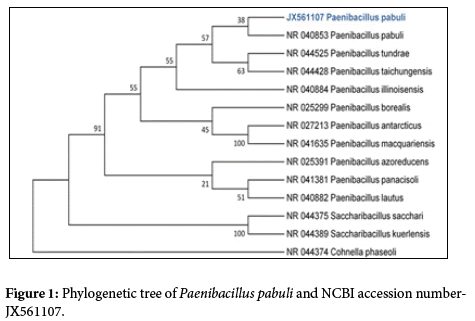
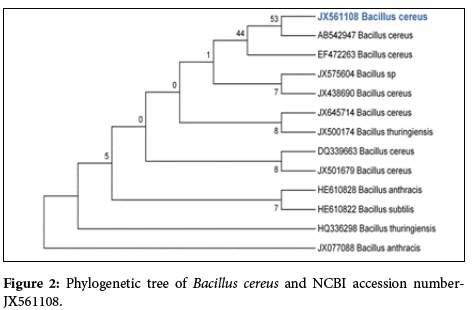
Alkaliphilic bacteria were isolated from alkaline industrialcontaminated soil. And they are exhibiting most favourable growth at pH 9.5. Paenibacillus pabuli, Bacillus cereus were isolated from the alkaline industry-contaminated soil. For which molecular characterizations were done. On the basis of Phylogenetic analyses 16S rDNA gene sequence of isolated bacteria was compared with sequence existing in the Genbank catalogue by BLAST software and sequence were deposited in NCBI Genbank with accession number.
The phylogenetic tree derived from 16S rRNA gene sequence of Paenibacillus pabuli, Bacillus cereus and sequences of the closest phylogenetic neighbors obtained by NCBI BLAST analysis demonstrated the relationships among selected isolates (Figures 1 and 2).
Removal of Chromium from tannery effluent by isolated alkaliphilic bacteria
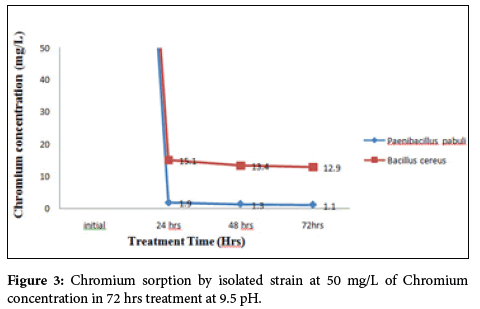
Removal of Chromium from the tannery effluent by alkaliphilic bacterial strains isolated from alkaline industrial soil at pH 9.5 (pH of the alkaline industry effluent contaminated soil from which bacterium was isolated). The degradation efficiencies of Cr(VI) in solution were calculated from the differences between the initial concentration and the residual concentration after 72 hrs of incubation at 37˚C in medium at pH 9.5. The concentration of total Cr(VI) was reduced to 1.1 mg/l from 50 mg/l in 72 hrs of treatment with Paenibacillus pabuli showed 98% efficiency and Bacillus cereus removed Cr(VI) to 12.92 mg/l from 50 mg/l in 72 h with 74% efficiency (Figure 3).
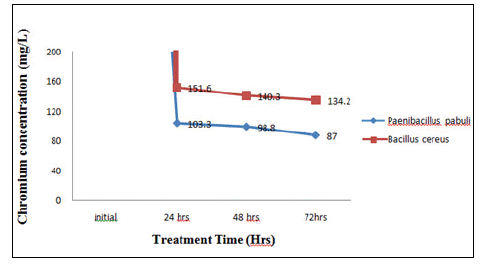
When The concentration of Cr(VI) was increased to 200 mg/l there was a gradual decrease in the Cr(VI) concentration to 57% from 200mg/l in 72 hrs of treatment with Paenibacillus pabuli and Bacillus cereus reduced Cr(VI) to 33% from 200 mg/l in 72 hrs of treatment (Figure 4).
Whereas when the concentration effluent is increased to 400 mg/l, there was no removal indicating the intolerance of bacterial biomass in higher concentration of chromium due to high stress exerted by higher level of metal ions in the medium.
SEM and EDX analysis
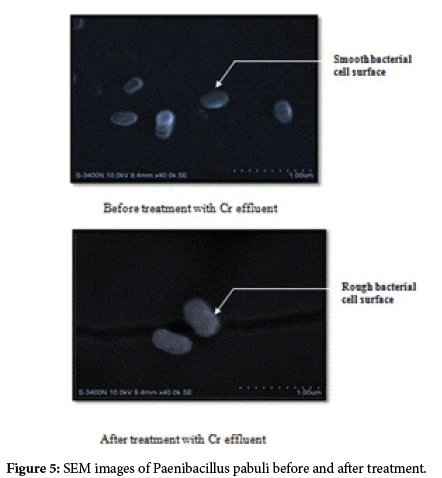
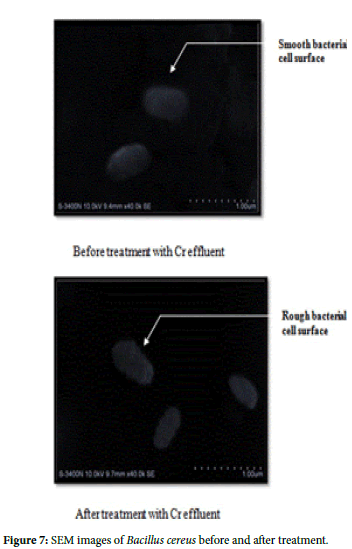
Bacterial strains Paenibacillus pabuli and Bacillus cereus showed high efficiency in removal of Chromium in 72 hrs of treatment. SEM analysis was carried out to distinguish likely morphology changes due to Chromium adsorption. Before the biosorption by the bacterium the cell surface was smooth and even. After the bio sorption, significant changes on the cell surfaces were noticed. Figures clearly indicate that the cell surfaces of bacteria before treatment were smooth without any adhesion whereas the treated one are with rough and wrinkled surface because of metal adhesion on the bacterium (Figures 5 and 6).
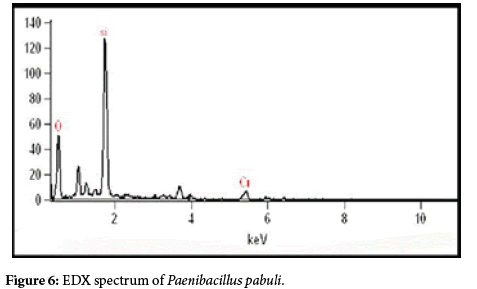
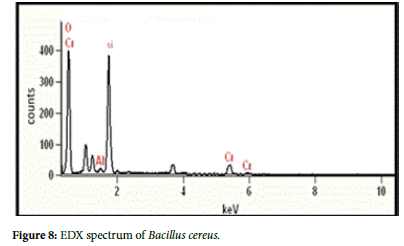
The EDX spectrum confirms the presence of Cr in the pellets of bacteria used in chromium treatment (Figures 7 and 8).
Discussion
Current awareness has been drawn to the expansion of biosorption technique as a method of bioremediation. It has been focused on a relatively inexpensive and easier than using a chemical treatment to overcome the contamination of heavy metals [15]. In addition, many researchers have reported the biosorption of heavy metal in to pure cultures of bacteria and algae and onto natural microbial populations as viable, efficiently and environmental friendly remediation technologies [16].
In the present study, the alkaliphilic bacteria Paenibacillus pabuli, and Bacillus cereus from alkaline industry-contaminated soil were evaluated for their chromium removal efficiency at 9.5 pH (pH of alkaline industry contaminated soil from which alkaliphilic bacteria were isolated). The results are quite encouraging, recording higher adsorption capacity at higher pH. The chromium (VI) efficiency of bacteria isolated from alkaline industrial contaminated soil, namely Paenibacillus pabuli, and Bacillus cereus revealed 98% and 74% efficiency.
Previous studies have demonstrated that exposure to reasonable amounts of heavymetals triggers adaptive responses such as induction of metallothionein, which confers cells with resistance to heavy metalinduced toxicities [17].
Further experimental study on evaluation of concentration based efficiency, 200 mg/l concentration of Cr(VI), and the tested bacteria recorded about 57% removal; but at higher concentration of chromium (400 mg/l), there was no removal by isolated bacterial strain tested. Such observation indicates the intolerance of bacterial biomass in higher concentration of chromium due to high stress exerted by higher level of metal ions in the medium. Up to 200 mg/l of chromium, the bacterium was able to remove more than 57% of Cr(VI) but when the concentration was doubled (i.e. 400 mg/l), there was no removal. Evidently, it has been also reported that high chromate concentration prohibited multiplication of bacteria [18].
Such intolerance of higher concentration of chromium by the bacteria reveals that the ability of a given quantity of bacterial biomass could have played significant role in maintaining its efficiency. In the present experimental study, a loop full of overnight grown pure culture biomass was inoculated in different Cr(VI) concentration. A given quantity of microbial biomass is not capable of tolerating the stress due to high metal concentration in the medium and it is assumed that actual threshold level of each bacterium might be scaled out so as to maintain the high efficiency of the bacterium for future research. Present findings are in close conformity with the observation made by Wang and Xiao (1995) in Bacillus sp. Basu and Yilmaz reported the removal percentage was decreased with increasing chromium concentration [19,20]. This is due to the fact that as the volume of inoculum was constant, relatively less biomass was available for chromium (VI) removal from the media. It is proving to be reported at low concentrations, all the metal ions in the solution will interact with the binding sites and thus facilitates 100% adsorption. At higher concentrations due to saturation of binding sites, more Cr ions are left unabsorbed in solution. Ahalya and Sethuraman stated when concentration increases to 200 mg/l there is a gradual decline in the removal percentage in all bacteria in their capacity to adsorb chromium [21,22]. The higher the concentrations, the sites available for adsorption is less compared to the moles of solute present. Thus, observations made in the experimental studies are in close conformity with previous reports.
On examining the mechanism behind adsorption of chromium to the bacterial cell surface, it has been reported to endure under metalstressed circumstances, bacteria have emerged numerous types of mechanisms to endure and survive through bio mechanism of the uptake of heavy metal ions. These mechanisms comprise the discharge of metal ions outer surface the cell, aggregation and complexation of the metal ions within the cell [23]. Further adhesion of metal to the bacterial cell surface might be due to the presence of an exopolysaccharide production [24]. Cell walls of microorganisms consisting mostly of polysaccharides, protein and lipids are the metal binding group such as carboxyl, sulphate, phosphate and amino groups [25].
To verify the present findings on the adsorption of chromium onto the bacterial cell surface, the SEM images of the cell surface of treated and untreated bacteria clearly show the metal particle adhered on bacterial surface, giving a rough and wrinkled surface morphology in Paenibacillus pabuli and Bacillus cereus. Similar images are also obtained by Jun Guo et al. [26] in Pseudomonas plecoglossicida and reported that before the treatment the cells were rod shaped, and the cell surface was smooth, but and after the treatment, there were significant changes on the cell surfaces. Similarly, Dahl et al.[27] also obtained SEM-EDX images indicating adherence of chromium on the surface of bacterial cells. Further, to confirm whether the adhered material is made of chromium, EDX spectrum was obtained for Paenibacillus pabuli and Bacillus cereus used in treatment studies. The EDX spectrum established the presence of Cr(VI) in the pellets of bacteria treated in Cr(VI) remediation. Silica(Si) peak in the EDXmight be due the smear preparation on glass slides and Al peak might have originated from sample holder.
Conclusion
Concludingly, it is reported that to remediate Cr(VI) bioremediation having less than 200 mg/l of chromium, the alkaliphilic bacteria Paenibacillus pabuli and Bacillus cereus was able to tolerate and degrade high concentrations of Cr(VI) and could be the potential species as bio adsorbents. The suitability of these alkaliphilic isolates particularly for chromium removal would open up new lines of application through suitable biotechnological tools and techniques.
Acknowledgments
We extend our heartful thanks to Central Instrumentation Facility, Pondicherry University, for analysing the sample under the Scanning electron microscope.
References
- Rossi M, Ciaramella M, Cannio R, Pisani FM, Moracci M, et al. (2003). Extremophiles 2002. JBacteriol 185: 3683-3689.
- Li Z, Kawamura Y, Shida O, Yamagata S, Deguchi T, et al. (2002) Bacillus okuhidensis sp.nov., isolated from the Okuhida spa area of Japan. Int J SystEvolMicr 52: 1205-1209.
- Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2: 191-200.
- Martins RF, Davids W, Al-Soud W, Levander F, Rådström P, et al. (2001) Starch-hydrolyzing bacteria from Ethiopian soda lakes. Extremophiles 5: 135-144.
- Horikoshi K (1991) General View of alkaliphiles and thermophiles. In Superbugs: Micro-organisms in Extreme Environments, (eds) K. Horikoshi and W.D. Grant, Springer Verlag. Berlin. 3-13.
- Turick CE, Apel WA, Carmiol NS (1996) Isolation of hexavalent chromium reducing anaerobes from hexavalent chromium contaminated and non contaminated environments. Applied MicrobiolBiotechnol 44: 683-688.
- Francisco CA, Casciotti KL, Tebo BM (2002) Localization of Mn (II)-oxidizing activity and the putative multicopper oxidase, MnxG, to the exosporium of the marine Bacillus sp. strain SG-1. Archives of Microbiology 178: 450-456.
- Branco R, Chung AP, VerÃssimo A, Morais PV (2005) Impact of chromium-contaminated wastewaters on the microbial community of a river. FEMS Microbiology Ecology 54: 35-46.
- Kannan P, Ignacimuthu S, Paulraj MG (2009) Buffering capacity and membrane H+ conductance of protease producing facultative alkaliphilic bacterium Bacillus flexus from mangrove soil. Indian Journal of Biochemistry & Biophysics 46: 261-265.
- Das S, De M, Ray R, Ganguly D, kumar Jana T, et al. (2011) Salt tolerant culturable microbes accessible in the soil of the Sundarban Mangrove forest, India. OpenJournal of Ecology 1: 35-40.
- Horikoshi K (1999) Alkaliphiles. Kodahansa. Harwood Academic Publishers. Australia.
- Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. Journal of Molecular Biology 3: 208-218.
- Gardea-Torresdeya JL, Arenasb JL, Franciscob NMC, Tiemanna KJ, Webbb R (1998). Ability of immobilized cyanobacteria to remove metal ions from solution and demonstration of the presence ofmetallothionein genes in various strains. J Hazard Mater 1: 2-18.
- De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Marine Biotechnology 10: 471-477.
- Nies DH (2000) Microbial heavy-metal resistance. ApplMicrobiolBiotechnol 51: 451-460.
- Gutnick DL, Bach H (2000) Engineering bacterial biopolymers for the biosorption of heavy metals: New products and novel formulations. App MicrobiolBiotechnol 54: 451-460.
- Chen L, Ma L, Bai Q, Zhu X, Zhang J, et al. (2014) Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. Int J BiolChem 289: 22413-22426.
- Bopp LH, Ehrlich HL (1988) Chromate resistance and reduction in Pseudomonas fluorescens strain LB300. Archives of Microbiology 150: 426-431.
- Basu S, Dasgupta M, Chakraborty B (2014) Removal of Chromium (VI) by Bacillus subtilisIsolated from East Calcutta Wetlands, West Bengal, India. Int JBioscibiochem and Bioinformatics 4: 7-10.
- Yilmaz EI (2003) Metal tolerance and biosorption capacity of Bacillus circulans strain EB1. Research in Microbiology 154: 409-415.
- Ahalya N, Kanamadi RD, Ramachandra TV (2005) Biosorption of chromium (VI) from aqueous solutions by the husk of Bengal gram (Cicerarientinum). Electronic. J ofBiotech 8: 258-264.
- Sethuraman P (2013) Biosorption of chromium vi nickel ii Copper ii and cobalt ii from Aqueous solution using Bacillus subtilis, Pseudomonas aeruginosa and Enterobacter cloacae.
- Nies DH (1999) Microbial heavy-metal resistance. Applied MicrobiolBiotechnol 51: 730-750.
- Loaëc M, Olier R, Guezennec J (1997) Uptake of lead, cadmium and zinc by a novel bacterial exopolysaccharide. Water Research 31: 1171-1179.
- Puzon GJ, Petersen JN, Roberts AG, Kramer DM, Xu, L (2002) A bacterial flavinreductase system reduces chromate to a soluble chromium (III)–NAD+complex. BiochemBioph Res 294: 76-81.
- Jun Guo, Zheng XD, Chen QB, Zhang L, Xu XP (2012) Biosorption of Cd (II) from aqueous solution by Pseudomonas plecoglossicida: kinetics and mechanism. Current Microbiology 65: 350-355.
- Dhal B, Thatoi H, Das N, Pandey BD (2010) Reduction of hexavalent chromium by Bacillus sp. isolated from chromite mine soils and characterization of reduced product. J ChemTechnolBiot 85: 1471-1479.
Citation: Anandbabu R, Vignesh T, Subashchandrabose G (2020) Biosorption of hexavalent chromium by paenibacillus pabuli and bacillus cereus isolated from alkaline industrial contaminated soil. J Bioremediat Biodegrad 11: 476. DOI: 10.4172/2155-6199.1000476
Copyright: © 2020 Anandbabu R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1899
- [From(publication date): 0-2020 - Apr 25, 2025]
- Breakdown by view type
- HTML page views: 1155
- PDF downloads: 744