Research Article Open Access
Biosorption of Heavy Metals onto Different Eco-Friendly Substrates
Eman M Fawzy1*, Fatma F Abdel-Motaal2 and Soad A El-Zayat21Chemistry Department, Faculty of Science, Aswan University, Aswan, Egypt
2Botany Department, Faculty of Science, Aswan University, Aswan, Egypt
- *Corresponding Author:
- Eman Mohumed Fawzy
Department of Chemistry
Faculty of Science, Aswan University
Aswan, Egypt
Tel: +201007292350
Fax: +20973480448
E-mail: emanmohumed@hotmail.com
Received date: Febraury 27, 2017; Accepted date: April 06, 2017; Published date: April 10, 2017
Citation: Fawzy EM, Abdel-Motaal FF, El-Zayat SA (2017) Biosorption of Heavy Metals onto Different Eco-Friendly Substrates. J Bioremediat Biodegrad 8:394.doi: 10.4172/2155-6199.1000394
Copyright: © 2017 Fawzy EM, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Fungi play an important role in biosorption of heavy metals in heavily contaminated soils. Five metals-tolerant fungal species were isolated from two different contaminated soils (soil 1 and soil 2). The number of fungal colonies isolated from the contaminated soil 2 was higher than that of soil 1. The most resistant fungal species for the toxic studied metals (Pb, Cd, Cu and Zn) was Rhizopus stolonifier followed by Macrophomina phaseolina. It was established that the metal toxicity was related to the contamination levels, the physico-chemical properties including pH, conductivity, organic matter, and carbonate contents of the soils. This study confirmed the good ability of different chemicals (CaCO3, MO, Zeolite and phosphate) and biological fungal substrates (M. phaseolina and R. stolonifier) in bioremediation of polluted soils and reducing different heavy metals levels compared to the control, especially for fungi. M. phaseolina amendment was superior in reducing the chemically available heavy metals in the studied soils.
Keywords
Heavy metals; Fungal adsorption; Soil remediation; Amendments; Sequential extraction technique
Aims and Background
The basic environmental elements constituting ecosystem is the soil, which is the important material basis of human being to survive and develop. The contaminated soil by heavy metals manifests as concealment, accumulation, irreversibility and protraction. To prevent the heavy metal contamination, sources of contamination should be controlled and remediation of contaminated soils should be enhanced [1]. Recently, decreasing the amount of pollutants and improving the quality of the treated soils have been studied for the development of cheaper and more effective remediation technique. One of the most alternative treatments is adsorption. The adsorbents may be of mineral, organic or biological origin, zeolites, industrial byproducts, agricultural wastes, biomass, and polymeric materials [2]. Microorganisms (bacteria, fungi and algae) are effective heavy metal sequestration for physicochemical methods [3-7]. Removal of potentially toxic metals from polluted industrial and domestic effluents have already been used in large scale using certain microorganisms. These microorganisms have been shown to possess an ability to survive by adapting or mutating at high concentrations of toxic heavy metals. To increase the tolerance of fungi for the bioleaching process, the adaptation of these fungi exposed to heavy metal ions has been examined and developed [8].
Generally, two mechanisms have been proposed for heavy metal tolerance in fungi. The first one is an extracellular (chelation and cellwall binding) sequestration and the second is intracellular physical sequestration of metal by binding to proteins or other ligands to prevent it from damaging the metal sensitive cellular targets. Thus, extracellular mechanisms are mainly implied in the avoidance of metal entry, whereas intracellular systems aim to reduce the metal burden in the cytosol. In the first mechanism, different organic molecules that do not belong to the matrix of the cell wall are excreted by the fungal cell to chelate metal ions. Binding to the cell wall is called biosorption. The presence of various anionic structures, such as glucan and chitin gives negatively charged to the cell surface of microorganisms [9,10], which gives microorganisms the ability to bind metal cations. In the intracellular mechanism, metal transport proteins may be involved in metal tolerance, either by extruding toxic metal ions from the cytosol out of the cell or by allowing metal sequestration into vacuolar compartment [11,12].
In the present study, fungal-tolerant heavy metals will be screened in the two polluted soils. Moreover, although the separation of various chemical forms of heavy metals is very difficult, the use of sequential extraction methods provides an important approach and relevant environmental information on polluted samples. Therefore, the present study using sequential extraction scheme: (1) To predict the metal distribution among different fractions in two contaminated soils, determined periodically by a four-steps chemical fractionation procedure. (2) To compare biosorption capacity of chemical and biological treatments to degrade available metals contaminated soils. (3) To evaluate the changes in the speciation of studied metals in amended soils after treatments.
Experimental
Instrumentation
Metal determination in the extract was carried out by means of atomic absorption spectrophotometer (Model Solaar 969, ATI Unicam Comp.) equipped with a digital direct concentration read out and an air–acetylene burner using single element hollow cathode lamps (ATI Unicam Comp.). When the concentrations were under the detection limit of flame, the AAS external standards in diluted acid were used to calibrate the accuracy of atomic absorption.
Reagents and glassware
All glassware and plastic materials used were previously treated for 24 h in 2M nitric acid and rinsed with double distilled water and then with ultra-pure water. 50 ml of acid washed polyethylene centrifuge tubes was used for extraction, while 50 ml polyethylene vessels were used in the extracting solutions.
Samples collection
Two contaminated soils were selected for this study, one influenced by urban and wastewater (soil 1), and the other is influenced by industrial wastes (soil 2). The surface soils (0-20 cm) of the contaminated sites (ca 20 samples from each site using sterile polyethylene bags) were sampled, air – dried, and then hand-crushed using mortar and sieved through 2 mm stainless steel. Samples were finally homogenized and stored until the analyses.
Analytical procedure
The pH-values of soil samples were measured in 1: 2.5 suspension of sample: bidistilled water using a pH-meter (Orion Research, Model SA520, USA). Electrical conductivity (EC) was measured in the sample suspension obtained in the pH determination using conductivity meter (HANNA Instruments, HI 8033 Italy).
To measure carbonates, two grams of dried sample were weighed into a 250 ml conical flask. 40 ml of 0.5 N HCl was added and swirled gently to mix sediment with acid, and allowed to stand for at least one hour. The excess acid was titrated with a standard solution of 0.5 N NaOH in presence of phenolphthalein as an indicator. The volume of acid consumed was determined, and then the percentage of carbonate (as CaCO3) was calculated. Organic Matter measured by digesting one gram of soil samples with a mixture of K2Cr2O7 and conc. H2SO4. The mixture was shaken vigorously by hand for one minute, and then was gently warmed in a boiling water bath for 30 min. After cooling, the suspension was diluted with distilled water and filtered through whatman No. 1 filter paper. The filter paper and residue were washed with 100 ml distilled water. The excess of K2Cr2O7 was titrated with a standard freshly prepared solution of ferrous ammonium sulphate [(NH4)2SO4. FeSO4.6H2O] in the presence of H3PO4 and diphenylamine as an indicator [13].
Choice of reagents and leaching conditions
In the choice of extracting reagents, particular emphasis was placed on the selectivity, suitability and extracting efficiency of each leaching solution. The extraction methods that are the most informative for environmental purposes are the total element, moderately (reducible and oxidizable) and easily extractable element extraction techniques. The former defines and includes both elements from the rock matrix and the non-residual elements (i.e., those adsorbed from the aqueous medium). The three other extraction techniques show no association with the type of rock farming the soil and give results only for the moderately and weakly hold elements, which include those originating from polluted waters.
Sequential extraction scheme
Sequential extraction method was applied, in triplicate, to 2 gm of soil samples (<2 mm). The reagents and operating conditions for this method is summarized in Table 1. The procedure was conducted in five steps, assuming the forms of Cd, Pb, Zn and Cu extracted were (1) Exchangeable (2) Associated to Fe-Mn oxides (or reducible) (3) Associated to organic matter (or oxidizable) (4) Structurally bound in residual fraction [14].
| Reagent | Shaking time and temperature | Fraction |
|---|---|---|
| 40 ml of 0.11 mol/l acetic acid | 16 h at room temperature | Water-soluble (Available) |
| 40 ml of 0.1 mol/l hydroxylamine oxides hydrochloride (pH 2) | 16 h at room temperature | Occluded in Fe or Mn Reducible) |
| 10 ml 30% H2O2(pH 2) | 1 h at room temperature and 1 h at 85°C |
Organically bound and sulphides (Oxidizable) |
| then 10 ml 30% H2O2(pH 2) | 1 h at 85°C | |
| cool, add 50 ml mol/1 ammonium acetate (pH 2) | 16 h at ambient temperature | |
| Concentrated acid mixture (HCl: HNO3: HF) |
Structurally bound (Residual fractions) |
Table 1: Chemical extraction scheme for metal speciation in soil samples.
Isolation of micro-organisms from polluted sites
The soil samples (10 g) were first suspended in 100 ml of sterilized water; the mixture was agitated for 30 min at room temperature and then diluted (10 to 10000-fold). Aliquots of 100 μl of different dilutions were placed on 2% Malt Extract Agar (MEA) plates (three replicates) to ensure the growth of micro-organisms present in samples. After at least 3 days of incubation at 25°C, developed colonies were randomly picked and isolated. Pure cultures of isolated micro-organisms were identified using the fungal keys [15,16].
Screening and selection of heavy metal-resistant microorganisms
Purified isolates were screened on the basis of their tolerance to Cu, Pb, Zn, Cd. A disk of mycelium was inoculated aseptically onto MEA plates supplemented individually with 1, 10, 30, 50, 100, 300, 600, 1000 ppm of heavy metals. The inoculated plates were incubated at 25°C for 14 days. The effect of the heavy metals on the growth of the isolates tested was estimated by measuring the radius of the colony extension (cm) comparing with the control (medium without metals). The minimum inhibitory concentration (MIC) was calculated which defined as the lowest concentration of metals that inhibit visible growth of the isolate. The isolates which showed resistance to the studied heavy metals were selected for the following experiments.
Different treatments for contaminated soil
Six chemical and biological treatments were used to compare and evaluate the effectiveness of chemical remediation techniques. Seven different slurries of soil (1 gm soil : 25 ml H2O) were put in polyethylene bottles and treated according the following methods: (1) 1 gm calcium carbonate (CaCO3) added to increase soil pH to 7.0; (2) a high quantity of calcium phosphate (10 mg P); (3) 1% manganese oxide (5 gm); (4) 1 gm. of Macrophomina phaseolina (5) 1 gm Rhizopus stolonifier; (6) 1% synthetic zeolite (5 gm., Sigma Chemical Company, USA); and (7) kept as a control. Each treatment was performed in triplicate and incubated for two weeks at room temperature (25°C).
Quality control and analysis
The analyses of the sequential extractions procedure were being replicated three times. A blank was also run at the same time. All glassware and plastic containers were previously soaked in supra pure nitric acid (Merck) overnight, and rinsed with de-ionized water.
Results and Discussion
The monitoring of physico-chemical properties of the studied contaminated soils showed that, a significantly higher organic matter content (25 mg/gm) was observed in soil 2 (Table 2) caused by industrial wastes. This organic matter act as scavenger for metals and may provide sites for cations are due to ligand or groups that form chelates and/or complexes with the metals [17]. These findings tend to support the hypothesis that all geochemical processes leading to recycling and accumulation of trace metals in soils are associated with and influenced by organic matter.
| Location | pH | Conductivity µS/cm |
Organic matter mg/gm | CaCO3 Mg/gm |
Total content (μg/gm dry soil) | |||
|---|---|---|---|---|---|---|---|---|
| Pb | Cd | Cu | Zn | |||||
| Soil 1 | 7.12 | 78 | 19 | 21 | 16.35Â Â Â Â Â Â Â | 5.66Â Â Â | 225.96Â Â Â | 176.07 |
| Soil 2 | 6.75 | 912 | 25 | 2.1 | 41.47Â Â Â Â Â Â Â Â | 2.63 | 124.09Â Â Â Â | 198.49 |
Table 2: The Physico-chemical characteristic of the two studied contaminated soils, soil 1 (Influenced by urban and wastewater) and soil 2 (influenced by industrial wastes).
The electrical conductivity values of the soil samples are 78 μS/cm and 912 μS/ cm for soil 1 and soil 2, respectively (Table 2). The highest conductivity value was observed at soil 2 (912 μS/cm), as a result of the effluent wastes from the factory. These effluents enriched with highly conducting materials, which can be adsorbed on the surface of the suspended matter and are deposited on the bottom and tend to increase the electrical conductance of the soil [17]. On the other hand, the pronounced decrease in the conductivity value obtained at soil 1 (78 μS/cm).
The pH values of the soil samples were 7.12 and 6.75 (Table 2). A minimum pH value that recorded at soil 2, may be ascribed to the increase of organic matter decomposition, which leads to release of CO2 causing a drop of pH value. The hydrogen ion concentration (pH) is probably the most single important factor influencing metal adsorption onto both inorganic and organic surface [18]. The other major difference in soil properties of those contaminated soils was carbonates content (21 mg/gm and 2.1 mg/gm, for soil 1 and 2, respectively).
Total metal concentration provides little indication of metal specification bioavailability, mobility and reactivity in soil samples [19,20]. Total content was the predominant fraction for most of the studied metals (Table 2). Results in Table 2 reflect highest total Cd and Cu concentrations in soil 1 (5.66 μg/gm and 225.96 μg/gm respectively), while soil 2 recorded the highest total Pb (41.47 μg/gm) and Zn (198.49 μg/gm).
Fractionation of heavy metals before treatment
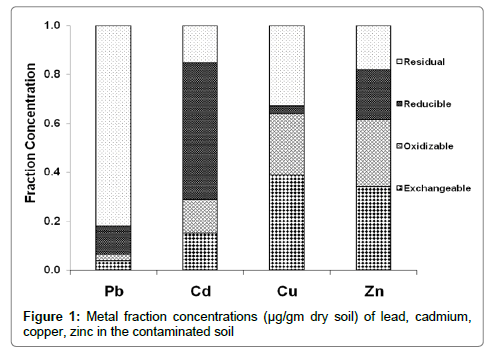
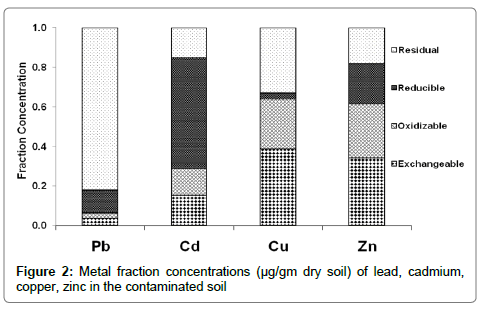
In the sequential extraction scheme used in this study, the mobility and hence possible bioavailability of metals decrease from readily exchangeable to residual. Figures 1 and 2 compared the mobility potential of heavy metals in different forms. It was noticed that Cu and Zn have the highest ability and susceptibility to be released from the soil samples, while Pb and Cd have the lowest mobility.
The presence of acid soluble portion of Pb indicates its sensitivity to the acidic condition and tendency to leach easily. Metal accumulation in the residual fraction prevailed with Pb (10.25 μg/gm and 34.04 μg/gm) mostly present as a major chemical form in soil 1 and 2, respectively (Table 3). But the other three fractions (which are soluble, reducible and oxidisable forms) of Pb are almost equal importance (1.7-1.6 μg/gm, 1.3-1.08 μg/gm, 3.1-4.75 μg/gm of the total content for each fraction) in both contaminated soils 1 and 2, respectively (Table 3). This finding was agreed with previous results [21].
| Soil | Metal Fractionation by Different Reagent μg/g (dry soil) | Sum of Fractions μg/gm(dry soil) |
Residual Metal Concentration μg/gm(dry soil) |
Recovery of a Summation Percentage |
||
|---|---|---|---|---|---|---|
| H2O2+NH4OAC | NH2OH.HCl | HOAC | ||||
| Lead Soil 1 |
3.1 | 1.3 | 1.7 | 6.1 | 10.25 | 17.9 |
| Soil 2 | 4.75 | 1.08 | 1.6 | 7.43 | 34.04 | 37.3 |
| Cadmium Soil 1 |
0.57 | 0.19 | 0.16 | 1.08 | 4.58 | 19.1 |
| Soil 2 | 1.47 | 0.36 | 0.4 | 2.23 | 0.4 | 84.8 |
| Copper Soil 1 |
1.44 | 2.11 | 76.74 | 80.29 | 65.38 | 0.44 |
| Soil 2 | 31.17 | 48.23 | 3.9 | 83.3 | 40.79 | 67.13 |
| Zinc Soil 1 |
13.49 | 4.76 | 96.49 | 114.74 | 61.33 | 65.17 |
| Soil 2 | 40.44 | 54.26 | 67.92 | 162.62 | 35.87 | 81.93 |
Table 3: Mean values sequential fractionation (µg/gm dry soil) of Pb, Cd, Cu, Zn and recovery of summation percentage in the two contaminated soils (Soil 1 and Soil 2).
Moreover, Cd showed a higher residual form concentration (4.58 μg/gm) in the untreated soil 1. The same metal in soil 2 (Table 3) was found in highest concentration in oxidisable fraction (1.47 μg/gm). Beside this, a remarkable reduction of residual fraction was observed in Cd content in soil 2 could be described (ascribed) to the dissolution and decomposition of inorganic and organic compounds, respectively of soil in this fraction leading to an increase in the concentration levels of the other fractions [22]. Cadmium and lead may cause serious problems through food chains [23].
In both control soils, Cu and Zn were displaying the highest potential mobility (Table 3) with values of (0.44-67.13% for Cu) and (65.17-81.93% for Zn) for soil 1 and 2 respectively, respect to total metal concentrations (Table 3). High concentration of heavy metals soils may increase uptake of these elements by crops and potentially affect human health via food chains [21].
Overall Pb and Cd would not be expected to have a high toxicity potential. But the presence of these toxic metals in the environment can be harmful to humans and living species even in low concentration. Since toxic metals do not degrade into harmless end-products, they can accumulate in living bodies and getting concentrated through the food chain [24]. However, Zn and Cu although not usually with high toxicity potential (compared to Pb and Cd toxicity) they should be carefully monitored if these soils are to be reused for agricultural purposes.
Fungal tolerant to Pb, Cd, Cu and Zn which isolated from the two studied soils
In this study, five fungal species were isolated from both soils 1 and 2. Emercilla quadrillineata (100 colonies/gm), Aspergillus niger (650 colonies/gm) and Macrophomina phaseolina (50 colonies/gm) were isolated from soil 1 while Aspergillus niger (2650 colonies/ gm), Rhizopus stolonifier (200 colonies/gm) and Aspergillus fumigatus (1350 colonies/gm) were isolated from soil 2. The number of fungal colonies isolated from (soil 2) contaminated sediment soils by industrial waste was higher than that from the soil 1 contaminated sediments urban and waste water. This variation would be referring to the high sugar content in the soil 2 which refers to sugar cane factory waste. This variation was clear with the number of A. niger colonies (650 and 2650 colonies/gm) isolated from soil 1 and soil 2, respectively.
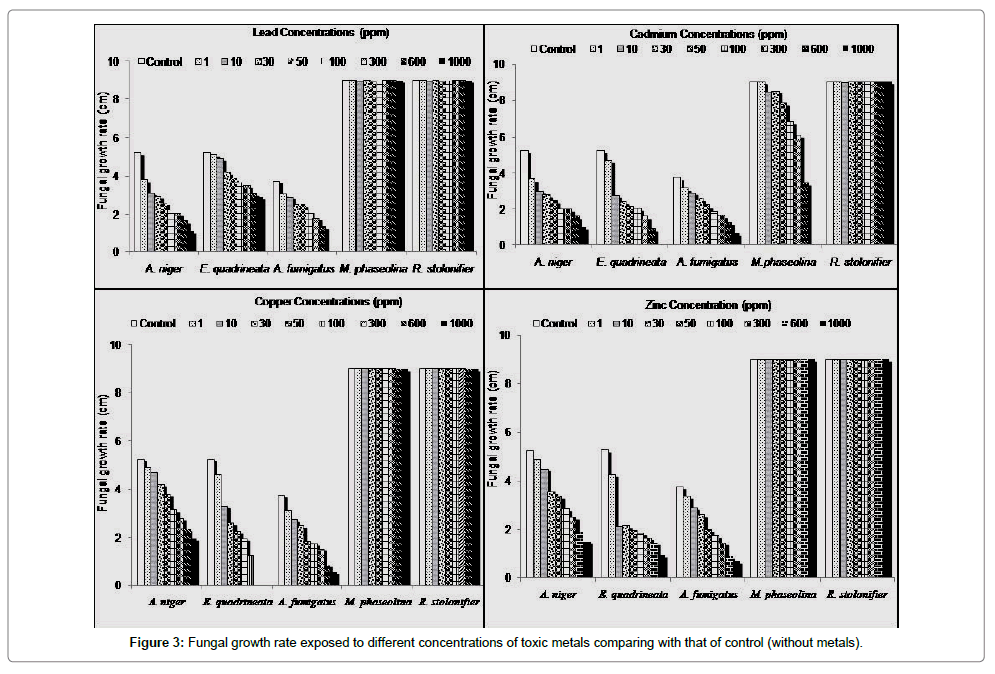
Screening the resistance of these fungal species to Pb, Cd, Cu and Zn has been studied. Generally, the most resistant fungal species to these elements up to 1000 ppm was Rhizopus stolonifier followed by Macrophomina phaseolina which showed resistance with all studied elements even with high concentrations expect cadmium at 1000 ppm recorded MIC100. While the remaining studied fungal species (Aspergillus niger, A. fumigatus and Emercilla quadrillineata) were slightly sensitive to these heavy metals (Figures 3 and 4). E. quadrillineata recorded MIC100 with copper and cadmium at 600 ppm and 1000 ppm respectively while MIC50 with zinc and lead at 10 ppm and 1000 ppm respectively. Cadmium showed similar effect on both Aspergillus species whereas MIC90 observed at 1000 ppm while MIC50 of A. fumigatus and A. niger with zinc 100 ppm and 300 ppm respectively. A. fumigatus showed more sensitivity to copper than A. niger (MIC90 and MIC60 at 1000 ppm) respectively. In the case of lead, A. fumigatus completely inhibited with 1000 ppm. Despite that only 50 ppm was enough to inhibit 50% of A. niger, the fungus showed stable growth rate up to 1000 ppm.
Similar observations about toxic effect of high concentration of heavy metals on growth of fungi have been reported [25-27]. The biomass of Rhizopus sp. had a higher adsorption capacity as compared to Aspergillus sp. Biomass [28]. The differences may be ascribed to the intrinsic ability of organism, its chemical composition of cell wall leading various types of interaction of metals with fungi [4]. According to our knowledge, this is the first study for the resistance of E. quadrillineata for heavy metals.
Fractionation of heavy metals after treatment
Some chemical techniques for immobilizing metals in soils are application into polluted soils to reduce the soluble concentration of heavy metals in soils by precipitation, adsorption, or complexation [21]. Recently, many industrial, agricultural and forestry sources are used as biosorbents such as, red mud [29], Aspergillus niger [30], Echornia speciose [31], Cupressus sempervirens, Eucalyptus longifolia and Pinus halepensis [32] and Pleurotus cornucopiae [33].
Seven substrates to investigate their efficiency to reduce the extraction of heavy metals concentration in a heavily contaminated soil were used [18] and deduced that the most effective treatments in decreasing available metal concentrations were calcium carbonate, zeolite and manganese or iron oxide. The present study was conducted to compare the efficiency of different substrates: calcium carbonate, zeolite and manganese oxide with fungi isolated from the contaminated soils in immobilizing metals in polluted soils.
Table 4 compiles the sequential extraction concentration of the measured metals (Pb, Cd, Cu and Zn) treated with working substrates in different fractions. It is noteworthy that the mobility of the studied metals in treated soils is generally decreases. A significant decrease recorded in exchangeable Pb and Cd fractions of soil 1 treated with Macrophomina phaseolina, Rhizopus stolonifier, carbonate, zeolite, phosphate or manganese oxide (Table 4). This effective role of fungus in reducing mobility of heavy metals due to their ability to accumulate significant amount of metals [34]. The biosorption of Cd (II), Pb (II) and Cu (II) with the filamentous fungus Phanerochaete chrysosporium was studied [35] and reported that the fungal cell walls have a negative charge due to the arrangement of the carboxyl and phosphate groups of the cell walls.
| Metals | Control | M.phaseolina | R.stolonifier | CaCO3 | MO | Zeolite | Phosphate |
|---|---|---|---|---|---|---|---|
| Pb | |||||||
| F1 | 1.7 | 0.87 | 1.05 | 1.1 | 1.3 | 1.1 | 1.2 |
| F2 | 1.3 | 7.62 | 2.04 | 2.8 | 2.14 | 1.81 | 1.16 |
| F3 | 3.1 | 1.62 | 2.69 | 7.2 | 2.03 | 7.4 | 8.3 |
| F4 | 10.25 | 11.22 | 10.63 | 6.85 | 0.56 | 1.17 | 0.11 |
| Cd | |||||||
| F1 | 0.16 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 |
| F2 | 0.19 | 0.08 | 0.11 | 0.34 | 0.22 | 0.26 | 1.58 |
| F3 | 0.57 | 0.17 | 0.2 | 11.73 | 8.7 | 10.43 | 4.34 |
| F4 | 4.58 | 14.3 | 16.98 | 9 | 1.43 | 2 | 5 |
| Cu | |||||||
| F1 | 76.74 | 44.88 | 55.49 | 52.64 | 30.03 | 42.68 | 52 |
| F2 | 2.11 | 2.12 | 1.41 | 7.76 | 20.08 | 5.1 | 10.66 |
| F3 | 1.44 | 6.03 | 8.51 | 4.05 | 6.17 | 23.26 | 22.2 |
| F4 | 65.38 | 13.39 | 37.98 | 34.05 | 14.64 | 62.64 | 14.02 |
| Zn | |||||||
| F1 | 96.49 | 73.72 | 86.67 | 78.9 | 94.02 | 94.51 | 83.41 |
| F2 | 4.76 | 30.18 | 19.2 | 23.13 | 37.34 | 146.46 | 38.76 |
| F3 | 13.49 | 10.19 | 7.88 | 37.11 | 36.86 | 71.99 | 1.46 |
| F4 | 61.33 | 24.86 | 18.27 | 42.81 | 70.91 | 49.28 | 50.67 |
F1: water soluble and exchangeable, F2: bound to Fe and Mn oxides, F3: bound to organic matter, F4: Residual Metal Concentration
Table 4: Distribution fractions of each studied metal (µg/gm) in Soil 1 (Influenced by urban and wastewater).
The more significant function of Macrophomina phaseolina and Rhizopus stolonifier observed to reduce Pb and Cd in both contaminated soils and Zn in soil 1 (Tables 4 and 5) compared with calcium carbonate may be attributed to high capacities of metals binding to cell walls and may exhibit high values of intracellular accumulation [36]. The biosorption of heavy metal by fungi occurs as a result of ionic interaction and complex formation between metal ions and functional group present on the fungal cell surface [37]. These functional groups which may be involved in the biosorption of heavy metals include phosphate, carboxyl, amine and amide groups [38].
| Metals | Control | M.phaseolina | R.stolonifier | CaCO3 | MO | Zeolite | Phosphate |
|---|---|---|---|---|---|---|---|
| Pb | |||||||
| F1 | 1.6 | 0.9 | 1.1 | 1.4 | 1.16 | 1.09 | 0.53 |
| F2 | 1.08 | 8.59 | 7.59 | 7.85 | 2.14 | 7.71 | 0.95 |
| F3 | 4.75 | 2.28 | 4.52 | 3.7 | 5.14 | 15.11 | 6.23 |
| F4 | 34.04 | 12.17 | 12.48 | 15.74 | 15.09 | 18.81 | 15.31 |
| Cd | |||||||
| F1 | 0.4 | 0.23 | 0.23 | 0.34 | 0.19 | 0.8 | 0.37 |
| F2 | 0.36 | 0.04 | 0.004 | 1.03 | 1.03 | 1.64 | 1.13 |
| F3 | 1.47 | 2.02 | 3.02 | 0.04 | 1.03 | 0.49 | 1.12 |
| F4 | 0.4 | 1.03 | 1.03 | 1.63 | 0.043 | 0.18 | 0.23 |
| Cu | |||||||
| F1 | 48.23 | 45.7 | 57.1 | 39.7 | 43.7 | 32.7 | 39.9 |
| F2 | 31.17 | 42.06 | 51.13 | 39.63 | 36.35 | 33.05 | 38.01 |
| F3 | 3.9 | 1.62 | 4.01 | 3.1 | 3.78 | 18 | 6.8 |
| F4 | 40.79 | 47.15 | 31.53 | 42.08 | 61.36 | 22.3 | 25.11 |
| Zn | |||||||
| F1 | 67.92 | 35.2 | 49.8 | 31.34 | 46.01 | 38.92 | 46.65 |
| F2 | 54.26 | 32.72 | 26.92 | 54.05 | 2.47 | 52.28 | 45.73 |
| F3 | 40.44 | 24.3 | 28.25 | 3.87 | 46.35 | 28.13 | 13.64 |
| F4 | 135.87 | 143.98 | 138.01 | 156.57 | 175.52 | 128.78 | 130.1 |
F1: water soluble and exchangeable, F2: bound to Fe and Mn oxides, F3: bound to organic matter, F4: total metal concentration
Table 5: Distribution fractions of each studied metal (µg/gm) in Soil 2 (influenced by industrial wastes).
Table 5 recorded a remarkable role of phosphate, manganese oxide, Macrophomina phaseolina and Rhizopus stolonifier in reducing Pb and Cd concentration in available fraction in soil 2. Microorganism living in a polluted environment became useful to asses toxicity of harmful chemical [39] and fungal biomass showed a remarkable potential to remove Pb, Cd, Mn and Zn from contaminated environment [40]. Application of manganese oxide, carbonate and zeolite reduced the mobility of Cd, Cu and Zn in two studied soils (Tables 4 and 5), this is in agreement with other studies [41,42]. The effects of composts and calcium carbonate on the uptake of cadmium and lead by vegetables grown in polluted soils [43]; they reported that application of calcium carbonate materials significantly reduces the solubility of heavy metals in contaminated soils. Many studied also indicated that application of manganese oxides mixed into contaminated soils could reduce the concentration of soluble Cd or Pb in soils [41]. Mobile phase of Cu content into the contaminated soils (Tables 4 and 5) was can be transformed to unavailable form after amendment with manganese oxide, zeolite and carbonates as substrates.
Conclusion
The application of sequential extraction method to study samples provides relevant information about possible toxicity of heavy metals in contaminated soils and gives valuable information on the mobility of these metals helps in predicting their behavior to the ecosystem. Macrophomina phaseolina and Rhizopus stolonifier recorded significant roles as good biosorbent agents for Pb, Cd, Cu and Zn and showed better uptake capacity for Pb, Zn and Cd compared to Cu. This uptake capacity found increased in M. phaseolina compared to R. stolonifier. The chemical remediation techniques, using calcium carbonate, manganese oxide, zeolite and phosphates can significantly reduce the availability of studied metals and then reducing their toxicity potential. However, these techniques certainly require intensive further improvements and studies in details to optimize the conditions for maximum bioadsorption of heavy metals of contaminated soils.
References
- Zhou HY, Peng XT, Pan JM (2004) Distribution, source and enrichment of some chemical elements in sediments of the Pearl River Estuary, China. Cont Shelf Res 24: 1857-1875.
- Kurniawan TA, Chan GYS, Lo WH,Babel S(2005) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366: 409-426.
- Brierley CL (1990) Bioremediation of metal-contaminated surface and groundwater. Geomicrobiol J 8: 201-223.
- Gadd GM(1993) Interactions of fungi with toxic metals. New Phytol 124: 25-60.
- Niu H, Xu XS, Wang JH (1993)Removal of lead from aqueous solutions by penicillin biomass. BiotechnolBioeng 42: 785-787.
- Wong PK, So CM (1993) Copper accumulation by a strain of Pseudomonas putida. Microbios 73: 113-121.
- Wong PK, Lam KC, So CM (1993) Removal and recovery of Cu (II) from industrial effluent by immobilization cells of Pseudomonas putida II-11. ApplMicrobiolBiot 39: 127-131.
- Yang J, Wang QU, Wang Q, Wu T (2009) Heavy metals extraction from municipal solid waste incineration fly ash using adapted metal tolerant Aspergillus niger.BioresourceTechnol 100: 254-260.
- Maghsoodi V, Razavi J, Yaghmaei S (2007) Production of Chitosan by submerged fermentation from Aspergillus niger. Scientia Iranica, Transactions C, J ChemChemEng 16: 180.
- Bellion M, Courbot M, Jacob C, Blaudez D, Chalot M (2006) Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol Lett 254: 173-181.
- Khan ZT, Maqsood (2007) Critical behavior of Iron (III) with a typical catecholate siderophore. Sci Iran 14: 106-111.
- Le L, Tang J, Ryan D, Valix M (2006) Bioleaching nickel laterite ores using multi-metal tolerant Aspergillus foetidus organism. Miner Eng 19: 1259
- Allen MF (1989) Mycorrhizae and rehabilitation of disturbed arid soils: Processes and practices. Arid Soil Res Rehab 3: 229-241.
- Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate traces metals. Analytical Chemistry 51: 844-851.
- Gilman JC (1957) A Manual of Soil Fungi. Iowa State Univ Press, Ames Iowa, USA, pp: 450.
- Domsch KH, Gams W, Anderson T (1980) Compendium of soil fungi. Acad Press, A Subsidiary of Harcourt Brace Jovanovich Pub, London, New York, USA pp: 859.
- Fawzy EM (2000)Physico-chemical studies on the speciation of heavy elements in the River Nile sediments. M.Sc. Thesis Faculty of Science, South Valley University, Egypt.
- Fawzy EM (2008) Soil remediation using in situ immobilisation techniques. ChemEcol 24: 147-156.
- Sánchez-Martin MJ, GarcÃa-Delgado M, Lorenzo LF, RodrÃguez-Cruz MS, ArienzoM (2007) Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 142: 262-273.
- Babel S, Dacera DM (2006) Heavy metals removal from contaminated sludge for land application: a review. Waste manage 26: 988-1004.
- Chen ZS, Lee GJ, Liu JC (2000) The effects of chemical remediation treatments on the extractability and speciation of cadmium and lead in contaminated soils. Chemosphere 41: 235-242.
- Smith SR (1996) Factors influencing the bioavailability of PTEs to crop plants. Agricultural recycling of sewage sludge and the environment CAB international, Wallingford, pp: 59-85.
- Jackson P, Allowy BJ (1992) The transfer of cadmium from agricultural soils to the human food chain. In: Biogeochemistry of trace metals. Lewis, Boca Raton, FL, USA, p: 109.
- Singh R, Gautam N, Mishra A, Gupta R(2011) Heavy metals and living systems: an overview. Indian J Pharma 43: 246.
- Rama-Rao VSKV, Akhtar N, Maruthi MP(1997) Isolation of a cadmium tolerant Curvularia sp. for polluted effluent. Curr Sci 73: 453-455.
- Malik A (2004) Metal bioremediation through growing cells. Environ Int 30: 271-278.
- Joshi PK, Swarup A, Maheshwari S, Kumar R, Singh N (2011) Bioremediation of Heavy Metals in Liquid Media Through Fungi Isolated from contaminated Sources. Indian J Microbiol 51: 482-487.
- Ahmad I, Zafar S, Ahmad F (2005) Heavy Metal Biosorption potential of Aspergillus and Rhizopus sp. isolated from Wastewater treated soil. J Appl Sci Environ Manag 9: 123.
- Nadaroglu H, Kalkan E, Demir N (2010) Removal of copper from aqueous solution using red mud. Desalin 251: 90-95.
- George B, Kumar JIN, Kumar RN, Sajish PR (2012) Biosorption potentiality of living Aspergillus nigertiegh in removing heavy metal from aqueous solution. Biorem J 16: 195-203.
- Abdel-Halim SH, Shehata AMA,El-Shahat MF (2003) Removal of lead ions from industrial wastewater by different types of natural materials. Water Res 37: 1678-1683.
- Al-Subu MM (2002) The interaction effects of cypress (Cupressus sempervirens), cin-chona (Eucalyptus longifolia) and pine (Pinus halepensis) leaves on their efficiencies for lead removal from aqueous solutions. Adv Environ Res 6: 569-576.
- Danis U (2010) Biosorption of copper (II) from aqueous solutions by Pleurotuscornu-copiae. Desalination Water Treat 22: 117-126.
- Iskandar NL, Zainudin NAIM, Tan SG (2011) Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci 23: 824-830.
- Say R, Denizli A, Arica YM (2001) Biosorption of cadmium (II) and lead (II) and copper (II) with the filamentous fungus phanerochaetechrysosporium. BioresourTechnol 76: 67-70.
- Blanquezet P, Casas N, Font X, Gabarrell X, Sarrà M, et al.(2004) Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res 38: 2166-2172.
- Kapoor T,Viraraghavan (1997) Heavy metal biosorption sites in Aspergillus niger. BioresourTechnol 61: 221-227.
- Akhtar MN, Sastry KS, Mohan PM(1996) Mechanism of metal ion biosorption by fungal biomass. Biometals 9: 21-28.
- Nita-Lazar M, Galaon T, Banciu A, Paun I, Stoica C, Lucaciu I(2016) Screening of various harmful compounds in a new bacterial biological model. JEnvronProtEcol 17: 237-247.
- Bumbac C, Penaleonte E, Dumitrescu C, Ghita I, Stefanescu M (2010) Heavy metals removal using residual fungal biomass. J EnvronProtEcol 11: 822-829.
- Mench MJ, Didier V, Loffler M, Gomez A, Masson P (1994) A mimicked in âÂ?Â?situ remediation study of metal contaminated soils with emphasis on cadmium and lead. J Environ Qual 23: 58-63.
- Lee GY (1996) The assessment of remediation techniques by chemical treatments for soils contaminated with cadmium and lead, Master Thesis. Graduate Institute of Agricultural chemistry, National Taiwan University, Taipei, Taiwan, pp: 130.
- Liu JC, Looi KS, Chen ZS, Lee DY (1998) The effects of composts and calcium carbonate on the uptake of cadmium and lead by vegetables grown in polluted soils. J Chin Inst Environ Eng 8: 53-60.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 4437
- [From(publication date):
May-2017 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 3430
- PDF downloads : 1007