Research Article Open Access
Biosorption of Arsenic by Living and Dried Biomass of Fresh Water Microalgae - Potentials and Equilibrium Studies
| Sibi G* | |
| Department of Biotechnology, Indian Academy Degree College, Centre for Research and Post Graduate Studies, Bangalore, India | |
| Corresponding Author : | Sibi G Indian Academy Centre for Research and Post Graduate Studies, India Tel: +91-99864-52875 E-mail: gsibii@gmail.com |
| Received May 23, 2014; Accepted September 01, 2014; Published September 03, 2014 | |
| Citation: Sibi G (2014) Biosorption of Arsenic by Living and Dried Biomass of Fresh Water Microalgae - Potentials and Equilibrium Studies. J Bioremed Biodeg 5:249. doi: 10.4172/2155-6199.1000249 | |
| Copyright: © 2014 Sibi G. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Biosorption of arsenic (III) and (V) from aqueous solutions by living and dried biomass of fresh water microalgae was investigated. A total of five microalgae namely Chlorella, Oscillatioria, Scenedesmus, Spirogyra and Pandorina were identified as arsenic tolerant and used for further studies. Varying conditions of pH, temperature, biomass and contact time were studied for As (III) and As (V) adsorption properties of living and dried biomass of microalgae. Significant biosorption of arsenic was found at pH 4, 32°C and 0.8 g/l levels. Both living and dried biomass were further studied for As (III) and (V) removal from the aqueous solutions containing 30 mg/l under the same experimental conditions. Removal rate of As (III) was higher than the As (V) by the microalgae under the experimental conditions and dried biomass showed higher biosorption rate and faster kinetic than the living ones.
| Keywords |
| Biosorption; Arsenic removal; Microalgae; Scenedesmus, Pandorina |
| Introduction |
| Aquatic ecosystems are mainly affected by heavy metals and represent a potential risk to the health of humans and ecosystems [1]. Heavy metal ions in the environment are biomagnified in the food chain and are accumulated in tissues. Although low concentrations of some heavy metals are metabolically important to many living organisms, at higher levels they can potentially be toxic [2]. |
| Arsenic (As) is a component of many industrial raw materials, products and wastes. Elevated levels of arsenic in drinking water have been implicated in human diseases and mortality [3]. Chronic exposure to arsenic causes neurological and haematological toxicity [4]. Arsenic impacts the major organs and is a potential carcinogen [5-7]. The most common arsenic species observed in the environment are the trivalent form arsenite As (III) and pentavalent form arsenate As (V) in which As (III) is more toxic than As (V) [8]. Because arsenic readily changes valence state and reacts to form species with varying toxicity and mobility, effective treatment of arsenic can be challenging. Arsenic treatment technologies require peroxidation step to form As (V) from As (III) [9] but the cost and secondary product formation during other conventional methods reduce the practices [10]. Use of biological processes provides a means for cost-effective removal of metals for the treatment of metal contaminated waters. |
| Microbes are ideal candidates to decrease the heavy metal ion concentration from ppm to ppb levels [11]. Microalgae are known to sequestrate heavy metals due to their cell wall constituents which act as binding sites for metals [12-17]. Bioaccumulation of metals by algae may create a feasible method for remediating water contaminated with metals [18,19]. It is well established that several marine and fresh water algae are able to take up various heavy metals selectively from aqueous media and to accumulate these metals within their cells [20-22]. Microalgae have been shown to accumulate arsenic and could potentially remediate through adsorption and biotransformation of inorganic arsenic [23-25]. |
| In this study, the emphasis has been laid to know the efficiency of fresh water algal species in removing the arsenic from aqueous solutions. The research was to simultaneously determine the differences in arsenate and arsenite sorption capacities of living and dried algal biomass. |
| Materials and Methods |
| Sample collection and identification |
| Algal samples were collected from Bangalore fresh water habitats (13°04’N, 77°58’E) and washed several times with tap water and then with deionized water before analysed by using microscope. The family and genus identified with reference to the biology of algae [26]. The identified algae were Chlorophyceae (Botryococcus, Chlamydomonas, Chlorella, Gonium, Pandorina, Scenedesmus, Spirogyra, Volvox), Cyanophyceae (Oscillatoria, Spirulina) and Euglenophyceae (Phacus). |
| Chemicals |
| All chemicals used in this study were of analytical reagent grade. Stock solutions of 100 mg/L concentration each of As (III) and As (V) were prepared by dissolving NaAsO2 and Na2HAsO4.7H2O in deionized water respectively. Solutions for adsorption and metal analyses were prepared by appropriate dilution of the freshly prepared stock solution. |
| Cultivation |
| The identified algae were cultivated in appropriate media BG 11 media (Oscillatoria), Bold 1NV media (Spirogyra), modified Bold 3N media (Pandorina, Volvox, Phacus), Proteose medium (Scenedesmus, Gonium, Chlorella), Soil extract medium (Chlamydomonas) and soil water media (Spirulina) [27,28]. |
| Growth inhibition studies |
| Growth inhibition bioassays for the influence of As (III) and As (V) at a concentration of 10 mg/L on algal isolates (10 mg/l) were studied. The final cell density and biomass after arsenic exposure for a period of 15 days were compared to controls in dilution water (media). Samples from each flask was taken and fixed with Lugol’s iodine solution to measure the cell density using haemocytometer. The mean number of cells produced at each concentration after exposing period was expressed as percentage growth reduction with respect to control. The algal biomass was determined by the spectrophotometric transmission of algal suspension (Schimadzu UV-2600) at 550 nm. From the results, arsenic tolerant microalgae were chosen and used for biosorption studies. |
| Arsenic biosorption studies |
| Living biomass: Cells in the exponential growth phase were obtained by centrifuging at 2500 rpm for 7 minutes and inoculated into flasks with fresh media at a concentration of 1 x 104 cells/ml. The flasks were shaken by hand and randomly placed in a growth cabinet (27 ± 1ºC, 12:12 h light/dark cycle, Philips TL 40W cool white fluorescent lighting, 140 μmol photons/m2/s). The quantity of biomass used for the biosorption studies varied from 1- 2x105 cells/ml. |
| Non-living biomass: The algal biomass was sun-dried and then dried in oven at 50°C for 24 h. The dried algal biomass was shredded, ground in a mortar and an average size of 500-600 μm was used for biosorption experiments at a concentration of 0.5 g/l. |
| Arsenic concentration: Both living and non-living algal biomass were inoculated separately into 250 ml conical flasks containing 100 ml of media supplemented with As (III) and As (V) with initial concentrations ranging from 10 to 50 mg/l. The flasks were kept under illumination at 2500 lux for 12 hr light - dark cycle at 24 ± 2°C. Metalfree and algae-free blanks were used as control groups. Test flasks were rotated and shaken twice daily to ensure sufficient gas exchange. Separation of biomass from metal bearing solution after appropriate incubation time was achieved through centrifugation at 10,000 rpm for 10 minutes. |
| pH study: Measurements for the effects of varied pH on Ar (III) and (V) biosorption performance were chosen by adding 0.1M NaOH or 0.1M HCl to achieve pH values of 2.0 through 10.0. |
| Temperature study: Measurements for the effects of increased temperature on arsenic adsorption performance by the algal isolates were studied at temperature between 23.0 to 35.0 ± 2°C. |
| Biomass study: Various biomass concentrations of living and nonliving algal cells in the range of 0.2, 0.4, 0.6, 0.8 and 1 g/l were used to study the adsorption of arsenic. |
| Apparatus |
| All measurements were carried out on Agilent 280FS AA spectrometer (Agilent Technologies, USA). The organic phase was aspirated directly without elution into the flame and the signals related to As (III) and As (V) was recorded at 193.7 and 197.2 nm (lamp current 10mA). The data were analyzed by using Spectra AA software. |
| Biosorption kinetics studies |
| The metal biosorption (q) by the algae and bioremoval efficiency (R) were calculated by the following formulae [29,30]. |
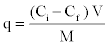 |
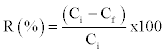 |
| Where q = metal adsorption (mg/g); M = dry mass of a lgae (g); V = volume of i nitial metal ion solution (L); R = bioremoval efficiency (%); Ci= initial concentration of metal in aqueous solution (mg/l); Cf= final concentration of metal in aqueous solution (mg/l). |
| Biosorption equilibrium models: The biosorption equilibrium isotherm was obtained by the Freundlich model (Equation 1) and the Langmuir model (Equation 2) respectively [31]. |
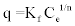 (1) (1) |
| Where, Kf and n are the distribution coefficient and a correction factor, respectively and Ce is equilibrium concentration of heavy metal (mg/l). By plotting the linear form of Equation (1), lnq = 1/n lnCe + lnKf, the slope is the value of 1/n and the intercept is equal to lnKf. |
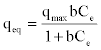 (2) (2) |
| The linear form of Langmuir is |
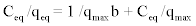 (3) (3) |
| Where qmax is the Langmuir constant (mg/g) reflecting the maximum adsorption capacity of the metal ion per unit weight of biomass to form a complete monolayer on the surface bound at high Ceq. Langmuir constant b(l/mg) represents a ratio of adsorption rate constant to desorption rate constant, which also gives an indication of the affinity of the metal for binding sites on the biosorbent. qmax and b can be determined from the linear form of Langmuir equation (3) by plotting Ceq/qeqvs. Ceq. |
| The specific metal biosorption q was calculated using the following equation |
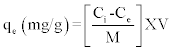 (4) (4) |
| Where qe is the specific metal biosorption (mg metal/g biomass), V is the volume of metal solution (l), Ci and Ce are the initial and equilibrium concentration of metal (mg /l), respectively, and m is the dry weight of the biomass (g). |
| Arsenic biosorption potential |
| Based on the arsenic biosorption equilibriums (mg/g), optimum conditions for pH, temperature and biomass were determined and further studied to determine arsenic biosorption potential of microalgae based on contact time. The living and non-living algal biomass (0.8 g/l) was suspended in 100 ml of Ar (III) and (V) solution (30 mg/l) in 250 ml flasks containing a pH of 4.0. The cell/metal suspensions were shaken (150 rpm) at 32°C. Samples were taken from the solutions at different time intervals from 0-60 hours and analysed for metal biosorption. |
| Results and Discussion |
| Growth pattern of isolated microalgae in the presence of arsenic has revealed that the degree of growth inhibition by arsenic varied widely between the isolates. The growth rate of Spirogyra, Volvox, Phacus, Gonium and Chlamydomonas was inhibited by As (III and V) in 5 days at 10 mg/l concentration. Oscillatoria and Spirogyra reached stationary phase in 8-10 days followed by decline phase. The growth rate of Pandorina was high up to 11 days where as Chlorella and Scenedesmus had exponential phase up to 13 days. These five genera have been considered as arsenic tolerant microalgae for further studies. The arsenic tolerant microalgae were grown in the presence As (III) and As (V) with a initial concentrations of 10-50 mg/l during which increased concentration of arsenic in the growth medium has led to deceased adsorption rate. The environmental setups had varying pH, temperature and biomass which produced variable results irrespective of the species studied. |
| The pH of the solution has played a key role in the biosorption of arsenite and arsenate by the microalgae. Aqueous solutions containing As (III) and As (V) were prepared with varying pH ranging from 2.0 to 10.0. Arsenic sorption decreased with increasing pH and the maximum arsenic removal occurred at pH 4.0 for both living and dried biomass. The highest As (III) uptake (Q) of 23 mg/g was recorded with living biomass of Scenedesmus at a initial concentration of 30 mg/l followed by Pandorina (21 mg/g). Maximum uptake of 22 mg/g As (III) was found with the dried biomass of Scenedesmus which was followed by Chlorella and Pandorina (Table 1). The metal sorption of As (V) was highest in living biomass of Scenedesmus (20 mg/g) and the trend was reflected in dried biomass of Chlorella and Pandorina at 30 mg/g (Table 2) |
| Various temperatures in the range of 23°C - 35°C were used to study the metal uptake by the algal isolates. The results indicated that 32°C was found optimum in which maximum adsorption has taken place and there were no significant changes in the metal uptake at temperature below 29°C and above 32°C. The living biomass of Scenedesmus and Chlorella has adsorbed maximum As (III) from the aqueous solution followed by Spirogyra and Pandorina at 30 mg/l initial concentrations. An uptake of 25 mg/g was recorded with dried biomass of Scenedesmus followed by Chlorella and Pandorina (24 mg/g) under similar conditions (Table 3). The uptake levels of As (V) were comparatively lower than As (III) with a maximum sorption of 16 mg/g by living biomass of Scenedesmus and Oscillatoria. The dried biomass of Scenedesmus and Chlorella has up taken 19 and 17 mg/g of As (V) at 30 mg/l levels (Table 4). |
| The biomass dosage was varied from to 0.2 to 1.0 g/l. Increased biomass has increased the arsenic sorption and the maximum uptake of arsenic was obtained at a biomass concentration of 0.8 g/l. At 30 mg/l initial concentration of As (III), maximum uptake was seen with living biomass of Pandorina (27 mg/g) followed by Scenedesmus (26 mg/g) whereas the dried biomass of Scenedesmus (28 mg/g) has recorded the highest AS (III) uptake over Pandorina (26 mg/g) (Table 5). The trend from temperature variations was repeated in biomass dosage in As (V) sorption with living cells of Oscillatoria and Pandorina has exhibited maximum uptake of 18 mg/g which relatively lower than As (III) uptake under similar conditions. The dried biomass of Pandorina has maximum As (V) sorption (17 mg/g) followed by Oscillatoria (16 mg/g) (Table 6). |
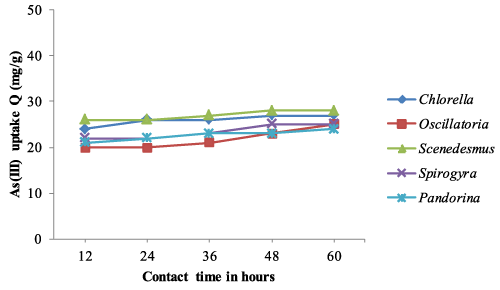
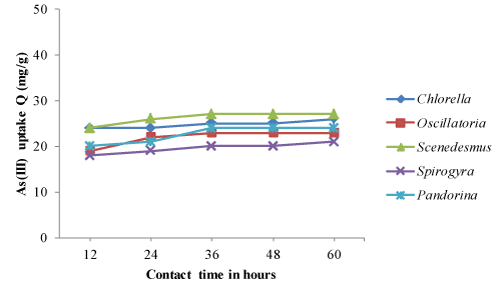
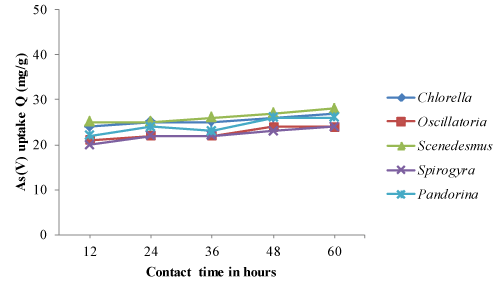
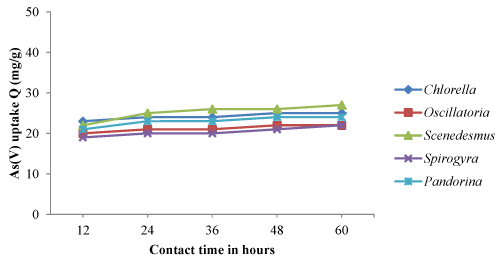
| The optimum pH, temperature and contact time obtained from equilibrium isotherm experiments were used to determine the arsenic biosorption of both living and non-living algal biomass based on time dependent manner. The time dependent biosorption of arsenic by living and dried biomass, samples equivalent to 0.8g/l were placed in solutions of As (III) and As (V) adjusted to pH 4.0 and 32°C temperature. The results of arsenic sorption as a function of time under the controlled conditions have been depicted in Figure 1-4. The kinetic studies revealed that arsenic biosorption increases with time and recorded highest at 36 hrs contact time. However the soption rate decreased progressively and became almost constant during the 60 hrs contact with arsenic. Scenedesmus has showed fast kinetic of As (III) binding and was adsorbed appreciable quantities of As (III) (20 mg/g) during the first 24 -36 hrs from the solution. The second highest uptake of As (III) was observed with Pandorina (21 mg/g) followed by Chlorella and there was no significant changes in the metal uptake after 36 hrs of contact time by the isolates. Similarly highest As (V) was recorded with Scenedesmus with a metal uptake of 20 mg/g followed by Chlorella and Pandorina after 36 hrs of contact time. Oscillatoria and Spirulina have recorded moderate arsenic uptake throughout the study. |
| Algae are known for their capability to accumulate heavy metals as they are required as essential nutrients [32] and have been explored for metal removal [33]. The level of metal removal by a microalga depends on biomass concentrations, pH and contact time [34-36]. In this experiment, arsenic sorption values of dried biomass were high in comparison with living biomass. This could be due to the larger surface area due to the destruction of cell membranes during the dried biomass preparation. Non-living microbial cells have lower sensitivity to toxic metal ions concentrations over living cells which offer to use them at adverse operating conditions [37]. The metal sorption capacity was drastically reduced at pH values above 6.0, which could be due to the formation of insoluble metal hydroxides. Further, it was noted that the arsenic uptake was lowered at pH below 4.0. The temperature of the solution could influence the metal biosorption of living cells [37] and culture temperatures have profound effects on the chemical composition of the algal cells. The biosorption of As increased with temperatures from 23°C-32°C and the results indicated that elevated temperatures tend to increase the biosorptive properties of isolated microalgae with an optimum temperature of 32°C. However the variations in uptake of As (III) and As (V) based on temperature need to be investigated. It was observed that higher biomass levels have reduced the adsorption amount which was influenced by the formation of aggregates at higher concentrations that has ultimately resulted in reduced biosorption area [38]. |
| The accumulation of heavy metals in algae involves and rapid uptake initially followed by slower uptake [37,39-41]. The same trend was observed in the experiments as the metal sorption rate was higher in first 36 hours followed by significantly slower uptake in the next hours. Further, As (III) and As (V) sorption was more at initial time (0- 36 hrs) followed by almost constant after 36 hrs of contact time. |
| Based on the pH studies, As (III) was effectively removed by both living and dried biomass of Scenedesmus and Pandorina whereas As (V) was removed by Scenedesmus and Chlorella. Significant levels of As (III) was removed by the living biomass of Spirogyra at varying pH. Under varying temperatures, Scenedesmus, Chlorella and Pandorina were efficient in removing As (III) and As (V) from the solutions. In general, Scenedesmus, Pandorina and Chlorella were found as potential arsenic removers from the aqueous solutions under varying environmental conditions. Heavy metal removal capacities of Scenedesmus was reported in earlier studies [42-45]. Arsenite and arsneate tolerance levels of Chlorella were studied earlier [23,24,46,47]. Spirogyra was efficient in As (III) removal than As (V) and the previous studies reported the arsenic removal by Spirogyra [48]. Samal et al. [49] has revealed the arsenic removing capacity of Oscillatoria in their studies and in this study dried biomass of Oscillatoria was significant at 30 mg/l of As (V). |
| In this study, removal rate of As (III) was higher than the As (V) by the microalgae under the experimental conditions. The trivalent arsenite is more toxic than the pentavalent arsenate and this study reveals the potential use of microalgae in removing the toxic As (III). The dried biomass was found effective for arsenic sorption than the living biomass. This study emphasizes that microalgae are ideal candidates to be further exploited for the removal of other heavy metal ions. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 17213
- [From(publication date):
November-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12344
- PDF downloads : 4869
