Research Article Open Access
Biosorption and Bioaccumulation of Some Heavy Metals by Deinococcus Radiodurans Isolated from Soil in Basra Governorate- Iraq
Raghad Jaafar1, Amin Al-Sulami1, Asaad Al-Taee2*, Faris Aldoghachi3 and Suhaimi Napes41College of Education for Pure Science, Basra University, Basra, Iraq
2Marine Science Center, Basra University, Basra, Iraq
3College of Science, Basra University, Basra, Iraq
4College of Biotechnology, Putra Malaysia University, Malaysia
- Corresponding Author:
- Asaad Al-Taee
University of Basrah, environmental development, Basra, 61001, Iraq
Tel: 009647801405715
E-mail: amraltaee@yahoo.com
Received date: June 06, 2015; Accepted date: July 13, 2015; Published date: July 20, 2015
Citation: Jaafar R, Al-Sulami A, Al-Taee A, Aldoghachi F, Napes S (2015) Biosorption and Bioaccumulation of Some Heavy Metals by Deinococcus Radiodurans Isolated from Soil in Basra Governorate- Iraq. J Biotechnol Biomater 5:190. doi:10.4172/2155-952X.1000190
Copyright: © 2015 Jaafar R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The bacterium Deinococcus radiodurans has been isolated from soil. On the basis of morphological, biochemical, 16S rRNA gene sequencing and phylogeny analysis revealed that, the isolates were authentically identified as D. radiodurans.
D. radiodurans showed signiï¬cant resistance to high concentrations of Pb and Cd, but it was more tolerant to Cd than Pb. Minimum inhibitory concentration was 400 mgl-1 for Pb, while it was 600 mgl-1 for Cd. The potent bacterium has the optimal bioaccumulation capacity differ according to metal type, concentration, and contact time. In bioaccumulation experiment, the results showed the highest increase in accumulation of Pb in the concentration 50 mgl-1 at 6 h of incubation (0.33 mgg-1), while the lowest accumulation was in concentration 5 mgl-1 (0.029 mgg- 1) at 2h of incubation. For Cd the results showed maximum accumulation at 24h for concentration 100 mgl-1 then decreased at 48 h.
The results of biosorption experiment showed that D. radiodurans has a good ability to absorption both Pb and Cd in considering to the metals concentrations and times. This which can be clarified from the elevated percentage of Pb absorption (63.46%) in concentration 50 mgl-1 and during 2h. For biosorption of Cd the was decreased with the increasing time and the high biosorption noticed during 2h at concentration 50 mgl-1 (31.23%).
Keywords
Deinococcus radiodurans ; Heavy metals; Minimum inhibitory concentration; Bioaccumulation; Biosorption
Introduction
D. radiodurans is one of the most known radiation resistant organisms. It can live in cold, dehydration, vacuum and acid, and is therefore known as a polyextremophile. It is found in habitats rich in organic materials, such as soil, feces, meat, or sewage, in addition to that, it’s isolated from dried foods, room dust, medical instruments and textiles [1].
The manufacturing of energy from nuclear power factories, uranium mining, nuclear weapons production and nuclear accidents are the major cause of release of radionuclide's into the environment. The nuclear wastes typically contain inorganic and organic contaminants that include radionuclide's, heavy metals, acids/base and solvents. The high radiation level, in combination with the chemical hazards cause intensive damage to ecosystem and living organisms. The clean –up of nuclear waste by physiochemical methods is impractical and the cost is prohibitive. As a result a less costly in situ bioremediation technology is being investigated as a potential substitute method for treating such contaminated sites. The development of bioremediation strategies using Deinococcus spp, is therefore vital for the clean up of contaminated sites with radioactive waste. As these sites rarely contaminated by a single chemical, it is necessary to developed strain to be multi- resistant to various toxic agents [2].
Bioremediation is a natural process which depends on bacteria, fungi, and plants to altering pollutants as these organisms perform their normal life functions. These organisms have the ability of exploiting chemical contaminants as an energy source in their metabolic processes. Therefore, bioremediation affords alternative tool to destroy or reduce the risky contaminants through biological activity and this method has an effective cost [3].
Among the different methods, bioaccumulation and biosorption are potential for the removal of metals [4]. The active mode of metal accumulation of living cells is in most cases referred to bioaccumulation which relys on intrinsic biochemical and structural properties, physiological and genetic adaptation, environmental modification of metal specification, availability and toxicity [5]. Bioaccumulation is defined as the uptake of toxicant by living cells and transports it into the cell [6] and is a growth dependent process mediated only by living biomass [7]. The process of biosorption is possible by both living and dead biomasses [8]. The ability of certain species of bacteria in accumulating heavy metals was investigated inclusively [9,10]. Hence the natural organisms, either indigenous or extraneous can be used for bioremediation of heavy metals [11].
The present study aimed to isolate Deinococcus radiodurans from soil and identifying it biochemically and molecularly, in addition to determining their ability to remediation Pb and Cd through bioaccumulation and biosorption processes.
Materials and Methods
Isolation of bacteria
Three soil samples (30 gm each) were collected from Um- Qasr district, south of Basra city- Iraq during the January (2013). The samples were collected using a sterile plastic bag and transferred within 2 h to laboratory for analysis. For isolation of Deinococcus radiodurans, one gram of air dried soil sample was serially diluted using distilled water and spread over Tryptone Glucose Yeast extract (TGY) medium (0.5% tryptone, 0.1% glucose and 0.3% yeast extract) agar solid plates. The plates were incubated at 30ºC for 24 h.
Bacterial characterization
Properties of the isolates that included gram reaction, citrate utilization, indole production, nitrate reduction, catalase, fermentation of D- glucose, arginine, lactose and mannose, hydrolysis of casien, and gelatin liquefaction tests were determined according to Murray [12].
16S rRNA based identification
Bacterial samples were identified by sequencing of the 16S rRNA gene. To determine the identity of bacterial samples, the amplified 16S rRNA gene PCR products obtained from total genomic DNA using primer set 27F (5'-AGAGTTTGATCCTGGCTCAG-3') and 1492R (5'-GGTTACCTTGTTACGACTT-3'), [13] were sequenced commercially. DNA sequences obtained were compared to sequences available online in the Gene Bank database (www.ncbi.nlm.nih.gov). Homology search was performed using Bioinformatics tools available online, BLASTn www.ncbi.nlm.nih.gov/BLA [14].
Determination of minimum inhibitory concentrations (MIC) for Cd and Pb The MIC of Cd and Pb of bacteria were determined by disc diffusion method [15]. The concentrations of Cd and Pb were between 100 to 1000 mgl-1. Filter paper disks were saturated with heavy metals for 30 min, and then added to nutrient agar plates which incubated for 24h at 30ºC. The salts of CdCl2 and Pb (NO3)2 were used to prepare mother solution of these metals in sterile distilled water.
Bioaccumulation of heavy metals by Bacteria
Bacteria were grown in TGY broth containing different concentrations of lead (5, 10, 25, 50) mgl-1 and for cadmium 10, 20, 50, 100 mgl-1 for 2, 4, 6, 24 and 48 h and incubated at 30ºC in a shaker incubator at 180 rpm. Two replicates for each concentration have been done plus, one as a control. The bacterial cells were harvested by centrifugation at 6000 rpm for 15 min and suspended in 1 ml of distilled water, oven - dried and weighed. The dried biomass were then digested as follow: 100 ml beaker containing the dried cells, 5 ml concentrated nitric acid was added; the beaker was placed on a hot plate, stirred continuously, and heated initially at a medium rate for 5 min. Then, the beaker was heated on maximum setting until nitrogen oxide fumes were given off for a short time and a white residue was left. The beaker was left to cool for about 2 min and digestion was repeated with an additional 2 ml of concentrated nitric acid; this time it was heated until brown nitrogen oxide fumes almost ceased to appear. The beaker was cooled again for about 2 min and then 2 ml of 1:1 hydrochloric acid (35- 37%) was added. The mixture was heated at a medium rate for 3 min. After that it was cooled to room temperature and made up to 25 ml or bigger volume with distilled water.
Metal concentrations were measured by an atomic absorption spectrophotometer (Thermo Scientific ICE 3000 Series AA Spectrometer, USA) [16]. The metals accumulation then calculated using this equation:
E. Con. = A*B/D
Where E. Con = the concentration of heavy metals in (mg/g)
A= Concentration of heavy metals from calibration curve
B= final concentration of sample (mg/l)
D= dry weight of sample (gram)
Biosorption experiment
The equilibrium, kinetics data of the biosorbent D. radiodurans were obtained by performing batch experiments. The experiments were carried out in 250 ml flasks to which 100 ml of single ion metals solution of Cd and Pb, and l ml of biomass (exponential phase) was added. The mixture was stirred at 180 rpm at 30ºC and 15 ml of sample was collected at interval times (2, 4, 6, 24, and 48 h) centrifuged at 6000 rpm for 15 min in a centrifuge. The remaining concentration of metals was analyzed by the atomic absorbance spectrophotometer. An experiment was carried out twice and the mean values were reported. The difference between the initial metal ion concentration and the final metal ion concentration was considered as metal bound to the biosorbent [17].
Statistical analysis
The data obtained on the Bioaccumulation and biosorption of different metals at different concentration and time interval, by the bacterium D. radiodurans subjected to Statistical analysis using the SPSS program (SPSS Inc., Chicago, IL Version 15.0). The data were analyzed through analysis of variance (ANOVA). To detect the statistical significance of differences (P<0.05) between means.
Results and Discussion
Characterization and molecular identification of isolated bacteria
The selected bacterium was characterized and identified by using morphological, physiological and biochemical tests (Table 1). Bacteria were presumptively identified as Deinococcus sp. According to Chaturvedi [18]. Also the bacteria were subjected to 16S rRNA gene sequence analysis. The sequence of 16S rRNA of bacteria was submitted to Blastn (database 16S ribosomal RNA sequences (Bacteria and Archaea); Megablast) (www.ncbi.nlm.nih.gov/blast). That indicated a close genetic relatedness of bacteria with the rRNA sequence of D. radiodurans.
| Tests | Characteristics observed |
|---|---|
| Morphology | |
| shape | Cocci |
| pigment | pink |
| Gram reaction | + |
| Biochemical reaction | |
| Oxidase test | + |
| Catalase test | + |
| Indol formation | + |
| Citrate utilization | + |
| Gelatin liquefaction | + |
| Nitrate reduction | - |
| Fermentation | |
| D-glucose | + |
| Arginine | + |
| Lactose | - |
| Mannose | + |
| Casien | + |
| "+"and "-" indicate positive and negative reactions, respectively | |
Table 1: Morphological and biochemical characteristics of D. radiodurans.
Minimum inhibitory concentration
The MIC is the lowest concentration of the heavy metals that completely inhibited bacterial growth [19]. D. radiodurans showed significant resistance to high concentrations of Pb and Cd, but it was more tolerant to Cd than Pb. MIC was 400 mgl-1 for Pb, while it was 600 mgl-1 for Cd. Chaturvedi [18] reported different results in his study, he reported that the different isolated of Deinococcus (GrK2, Grk4, GrK5 and DR1) were sensitive to Cd+2, but they exhibited varying levels of tolerance. Grk2 was being extremely sensitive to Cd+2, DR1 is moderately tolerant, while Grk4 and Grk5 showed comparable tolerance, and also he mentioned that the toxicity of Cd affected by growth state, with stationary phase cells being more sensitive than the exponential phase. Hua et al. [20] reported that D. radiodurans R1 does not exhibit strong resistance to heavy metal Hg (II), Ag (I), Cr (VI) and Pb (IV), and recorded MIC (300 , 1600 300 and 3200 μM) for these metals respectively.
From the results of the present study there is a difference in results recorded in comparison with other studies results recorded previously, and this can be explained as the isolated strains was different and the growing conditions such as growth stage also different. In addition to the high concentration of heavy metals in the study soil, which enhance the ability of tolerant to these bacteria. Those can be supported by the result of Chaturvedi [18], who reported that, the growth phase depending differences in tolerance. Qi and Hulett [21] reported that, the tolerance of growing B. thuringiensis DM55 cells has been shown to vary at different growth stages, supporting the existing evidence that the structural features of gram positive bacterial cell walls are affected by the developmental state of the cell. Another reason for these results differences is the variance in design of experiment, such as growth media composition as clarified by Kumar et al. [22]. Complex media such as LB precipitate Pb as a result of sequestration with organic moieties thus reduce the bioavailability of Pb to the bacterial strains [23].
Bioaccumulation study
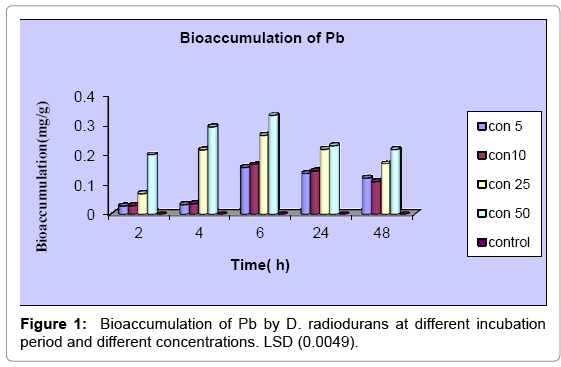
From the results shown in Figure 1 there is an increasing in the accumulation of Pb with the increasing of the concentration. The highest accumulation occurs in the concentration 50 mgl-1 after 6h of incubation was 0.33± 0.0007 mgg-1, while the lowest was 0.029 ± 0.268 mgg-1 after 2h. In addition to that, the rising of incubation period may reduce the accumulation for all concentrations.
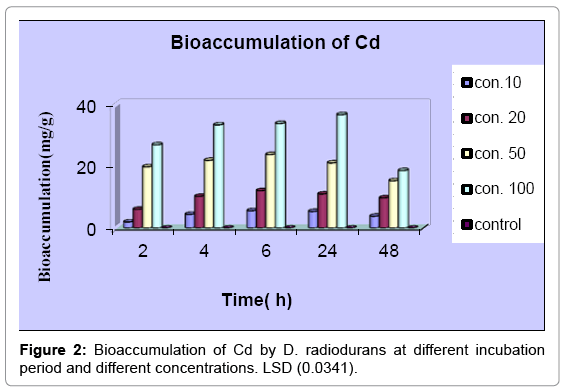
The accumulation of Cd increase in parallel with the increasing of concentration for all studying time (Figure 2). The figure clarifies increasing in Cd accumulation with the times (2, 4 and 6 h) for concentrations 10, 20 and 50 mgl-1, then decrease during 24 and 48h. For concentration 100 mgl-1, accumulation increase within 2, 4, 6, and 24h, then decrease with 48h. The analysis of variance of bioaccumulation of Pb and Cd between time and concentration was significant (P<0.05) in all treatments as show by LSD value.
The results of Pb and Cd accumulation showed that, this bacterium was able to accumulate high amounts of Cd 36.86 ± 0.007 mgg-1 in comparison to 0.33 ± 0.0007 mgg-1 for Pb. This may be due to difference in toxicity of these metals to this bacterium and to its intrinsic properties which help it in occupying varying tolerance, also the history of pollution of the soils from which bacterium isolated by these metals play critical tool in development ability of these bacteria to deal with such contaminations, this which agrees with the results of the present study where it show high MIC value for Cd than this for Pb for this bacterium.
From the results the accumulation increase with time, then start decreasing over specific time. This agrees with Ray et al. [24] who reported that, the accumulation of Pb by B. cereus increased with time, then starts decreasing. It may be concluded that, metal binding sites became saturated after specific time. The maximum accumulation of both of metals by this bacterium occurs in the highest concentration of metals then start decreasing with increasing of the time, this observed by Tunali et al. [25] who reported that, the amount of metal ions accumulated per unit mass of Bacillus sp. (ATS-1) increased first with increasing of the initial metal ion concentration and reached to a saturation value. Then the value changes with the initial metal ion concentration change. Also Ozdemir et al. [26] reported that, the accumulation of chromium (VI), cadmium (II) and copper (II) by Pantoea sp. TEM18 increase with high initial metals concentration, then reach to saturate so the accumulation decrease. Table 2 shows a comparison between different microorganisms and D. radiodurans in the present study.
| Biomass | Heavy Metals | Bioremediation capacity (mgg-1) or % | Heavy Metals | Reference |
|---|---|---|---|---|
| Saccorhizapolyschides | Cd | 95 | Cd | Loderioet al.[32] |
| Enterobactersp. | Pb | 50 | Pb | Lue et al.[33] |
| Bacillus cereus | Pb | 36.71 | Pb | Babaket al.[34] |
| Micrococcussp. | Zn | 84.27 | Zn | Hussein et al.[35] |
| Aspergillus niger | Cr | 133 | Cr | Goyal et al.[36] |
| D. radiodurans | Cd | 36.86 | Cd | Present study |
| Pb | 0.33 | Pb | ||
| D. radiodurans | Cd | 31.23% | Cd | Present study |
| Pb | 63.46% | Pb |
Table 2: Completed facial reconstruction (half-profile, plasticine).
| Concentration (mg/l) | % Biosorption of Pb at different times (h.) | ||||
|---|---|---|---|---|---|
| 2 | 4 | 6 | 24 | 478 | |
| 5 | 22.72±0.007 | 22.85± 0.141 | 23.50± 0.007 | 27.91± 0.007 | 30.43± 0.014 |
| 10 | 28.15± 0.007 | 30.32± 0.021 | 30.40± 0.014 | 30.43± 0.007 | 30.45± 0.007 |
| 25 | 41.8± 0.007 | 43.20± 0.141 | 44.00± 0.141 | 44.20± 0.070 | 44.33± 0 |
| 50 | 63.46± 0.007 | 59.97± 0.007 | 39.33± 0.007 | 33.40± 0 | 31.89± 0.070 |
| LSD= 0.001 | |||||
| Concentration (mg/l) | % Biosorption of Cd at different times (h) | ||||
| 2 | 4 | 6 | 24 | 478 | |
| 5 | 18.97± 0.014 | 18.57± 0.014 | 17.65± 0.014 | 16.10± 0.707 | 15.58± 0.007 |
| 10 | 22.80± 0.070 | 22.76± 0.007 | 21.00± 0 | 20.71± 0.007 | 20.14± 0.007 |
| 25 | 27.64± 0.014 | 27.60± 0.070 | 27.58± 0.021 | 26.57± 0.014 | 26.52± 0.014 |
| 50 | 31.23± 0.014 | 31.12± 0.014 | 30.75± 0.021 | 30.64± 0.014 | 30.32± 0.014 |
| LSD= 0.024 | |||||
Table 3: Biosorption (%) of Lead and Cadmium at different period of incubations and different concentrations by D. radiodurans.
Biosorption study
The results of the present study (Table 2) showed that D. radiodurans has a good ability to absorption Pb and Cd. The highest percentage of Pb absorption was 63.46% in concentration 50 mgl-1 for 2h. Biosorption of Pb increased with the increase of time for concentration 5, 10 and 25 mgl-1, then start decreasing with increase time for concentration 50 mgl-1. Explanations for these results is that at first three concentrations, the increase in biosorption with the time due to the sorbent sites were not saturated, but in concentration 50 mgl-1, the concentration was sufficient to saturate these sites, so the maximum absorption occurs in the first two hours. Tarangini [27], reported that, biosorption of arsenic by mixed cultures of Pseudomonas aeruginosa and Bacillus subtilis was increased with the increase time.
In related to the effect of Pb concentration, results showed sorption increase with the increasing Pb concentration. Taty-Cortodes et al. [28], showed that, the initial ion concentration exhibits quite an interesting effect on the equilibrium sorption capacity of the Pinus sylvestris for Cd (II) and Pb (II). At a fixed biosorbent dose, pH and temperature, the equilibrium sorption capacity, improved with higher initial ion concentration. The ion removal was highest concentrated dependent. The increase in the biosorbents loading capacity as a function of metal ion concentration was believed to be due to a high driving force for mass transfer.
The results of the present study of Cd sorption showed decrease of biosorption with increasing time (Table 2). The high sorption noticed after 2h at concentration 50 mgl-1. This is similar to Anzeze et al. [29], who reported that, the rate of adsorption of Cd by Eichhornia crasippes was very fast at first and over 95 % of total biosorption of Cd (II) ions occurs in the first 5 minutes and thereafter it proceeds at a slower rate and finally no further significant adsorption is noted beyond 20 minutes of contact time. For effect of metal concentration on biosorption, results show increases in Cd sorption with increasing metal concentration. The initial concentration provides an important driving force to overcome all mass transfer resistance of metal between the aqueous and solid phases [30]. The increasing amount of metal adsorbed by the biomass will be increased with initial concentration of metals. Optimum percentage of metal removal can be taken at high initial metal concentration. Thus, at a given concentration of biomass, the metal uptake increases with increase in initial concentration [31].
References
- Battista JR (1997) Against all odds: the survivalstrategies of Deinococcus radiodurans. AnnuRevMicrobiol 51: 203-224.
- http://doesbr.org/http://www.lbl.gov/NABIR.
- Salem IB, Sghaier H, Trif H, Héni S, Khwaldia K, et al. (2012) Isolation and characterization of a novel Micrococcusstrain for bioremediation of strontium in radioactive residues. Afr J MicrobiolRes 64: 851-858.
- Volesky B, Holan ZR (1995) Biosorption of heavymetals. BiotechnolProg 11: 235-250.
- Blackwell KJ, Singleton I, Tobin JM (1995) Metal cation uptake by yeast: areview. ApplMicrobiolBiotechnol 43: 579-584.
- Malik A (2004) Metalbioremediationthrough growing cells. Environ Int 30: 261-278.
- Karna RR, Sajani LS, Mohan PM (1996) Bioaccumulation and biosorption of CO2+ by neurospora crassa. BiotechnolLett 18: 1205-1208.
- Brady D, Stoll S, Duncan JR (1994) Biosorption of heavymetal cations by non- viable yeastbiomas. Environ Technol 15: 429-438 .
- Malekzadeh F, Farazmand A, Ghafourtian H, Shahamat M, Levin M, et al. (2002) Uranium accumulation by a bacteriumisolated from electroplating effluent. World J Microb Biot 18: 295-302.
- Chowdhury S, Mishra M, Adarsh VK, Mukherjee A, Thakur AR, et al. (2008) Novel metalaccumulator and proteasesecretor microbes from East Calcutta Wetland. Am J BiochemBiotechnol 4: 255-264.
- Prescott LM, Harley JP, Klein DA (2002) Microbiology. (5th Edn.), McGraw-Hill, New York.
- Murray RGE (1992) The familyDeinococcaceae. In: The ProkaryotesHandbook of the Biology of Bacteria: Ecophysiology, Isolation Identi?cation, Application. Springer-Verlag, New York.
- Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, et al. (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc NatlAcadSci U S A 82: 6955-6959.
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: A new generation of proteindatabasesearch programs. NucleicAcidsRes 25: 3389–3402.
- Wistreich MD, Lechtman D (1980) Laboratoryexercises in microbiology. (3rdedn), Glencoe Publishing Company, USA.
- Sprocati AR, Alisi C, Segre L, Tasso F, Galletti M, et al. (2006) Investigating heavymetalresistance, bioaccumulation and metabolic profile of a metallophilemicrobial consortium native to an abandoned mine. Sci Total Environ 366: 649-658.
- Sethuraman P, Kumar MD (2011) Bacillus subtilis on Pb2+ ions removal from aqueous solution by Biosorption. Res J PharmaceutBiolChemSci 2: 247.
- Chaturvedi R (2011) Molecular diversity and heavymetal interactions in Deinococcusspp. PhDthesis Maharaja Sayajiraouniversity of Baroda.
- Froidevaux R, Krier F, Nedjar-Arroume N, Vercaigne-Marko D, Kosciarz E, et al. (2001) Antibacterialactivity of a pepsin-derived bovine hemoglobin fragment. FEBS Lett 491: 159-163.
- Hua S, Chang SC, Zongwei L, Yanping W, Guangyong Q (2010) Functional analysis of a putative transcriptionalregulator gene dr2539 in Deinococcus radiodurans. Afr J MicrobiolRes 4: 515-522.
- Qi Y, Hulett FM (1998) Role of Pho-P in transcriptionalregulation of genes involved in cellwallanionicpolymerbiosynthesis in Bacillus subtilis. J Bacteriol 180: 4007-4010.
- Kumar R, Nongkhlaw M, Acharya C, Joshi SR (2013) Growth media composition and heavymetaltolerance behavior of bacteriacharacterized from the sub-surface soil of uranium rich ore bearing site of Domiasiat in Meghalaya. Ind J Biotechnol 12: 115-119.
- Pike R, Stapleton P, Lucas V, Roberts G, Rowbury R, et al. (2002) Effect of medium composition on the susceptibility of oral streptococci to mercuricchloride. CurrMicrobiol 45: 272-276.
- Ray L, Paul S, Bera D, Chattopadhyay P (2006) Bioaccumulation of Pb (II) from Aqueous solution by Bacillus cereus M1. J HazardSubstRes 5: 1-22.
- Tunali S, Cabuk A, Akar T (2006) Removal of lead and copper ion from aqueous solutions by bacterialstrainisolated from soil. Chem Eng J 115: 203-211.
- Ozdemir G, Ceyhan NO, Zturk T, Akirmak FT (2004) Biosorption of chromium (VI), cadmium (II) and copper (II) by Pantoeasp. TEM18. ChemEngineer J 102: 249-253.
- Tarangini k (2009) Biosorption of heavymetalsusingindividual and mixed cultures of Pseudomonas aeruginosa and Bacillus subtilis. National institute of technology, Orissa, India.
- Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinussylvestris. J Hazard Mater 105: 121-142.
- Anzeze DA, Onyari JM, Shiundu PM, Gichuki JW (2014) Equilibrium and Kineticsstudies for the biosorption of aqueous Cd (II) ions onto Eichhorniacrasippesbiomass. OSR J ApplChem 7: 29-37.
- Zouboulis AL, Matis KA, Hancock IC (1997) Biosorption of metals from diluteaqueous solutions. Sep PurifMeth 26: 255-295.
- Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of Heavy Metals: A Review. J ChemSciTechnol 3: 74-102.
- Lodeiro P, Cordero B, Barriada JL, Herrero R, Sastre de Vicente ME (2005) Biosorption of cadmium by biomass of brown marine macroalgae. BioresourTechnol 96: 1796-1803.
- Lue WB, Shi JJ, Wang CH, Chang JS (2006) Biosorption of Lead, Copper, and Cadmium by an indigenousisolateEnterobactersp. J1 Possessing high-heavymetalresistance. J Hazard Mater 134: 80-86.
- Babak L, Šupinova P, Zichova M, Burdychova R, Vitova E (2012) Biosorption of Cu, Zn and Pb by thermophilicbacteria – effect of biomass concentration on biosorptioncapacity. ACTA Universities Agriculture ET SilvictureMedlineBrunsis.
- Hussein H, Ibrahim SF, Kandeel K, Moawad H (2004) Biosorption of heavymetals from waste water using Pseudomonas sp. Elect J Biotechnol 7: 2004.
- Goyal N, Jain SC, Banerjee UC (2003) Comparative studies on the microbial adsorption of heavymetals. Adv Environ Res 7: 311-319.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15929
- [From(publication date):
August-2015 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 11212
- PDF downloads : 4717