Short Communication Open Access
Biosolubilization of Mineral Insoluble phosphates by Immobilized Fungi (Aspergillus Niger) in Fluidized Bed Bioreactor
Y Zeroual*, R Chadghan, A Hakam and A KossirUnité Recherche & Développement Engrais Et Fertilisation, Direction Recherche & Développement, Groupe OCP, Pôle Industriel - Jorf Lasfar, Morocco
- Corresponding Author:
- Youssef Zeroual
Unité Recherche & Développement Engrais et Fertilisation
Direction Recherche & Développement, Groupe OCP
Pôle Industriel - Jorf Lasfar, Morocco
Tel: +212661972881
E-mail: y.zeroual@ocpgroup.ma
Received date: December 24, 2011; Accepted date: February 04, 2012; Published date: February 06, 2012
Citation: Zeroual Y, Chadghan R, Hakam A, Kossir A (2012) Biosolubilization of Mineral Insoluble Phosphates by Immobilized Fungi (Aspergillus Niger) in Fluidized Bed Bioreactor. J Biotechnol Biomaterial S6:004. doi:10.4172/2155-952X.S6-004
Copyright: © 2012 Zeroual Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The authors discuss the ever increasing role of biological renewable resources in energy, nutrition, and pharmaceuticals; specifically those potentially available deep within the oceans. They provide a list of products already gleaned from this vastly untapped marine environment; discuss the innovations in technology required to effectively explore and prospect the deeper reaches of the ocean; expose the impressive contribution to the economy; and expound the paramount importance of protecting the oceans to ensure the future. Already many new proteins, enzymes, and pharmaceuticals are being developed from the fauna and flora of the forests and relatively shallow economic zones of the ocean. With much of the ocean still an unexplored frontier, the authors hope to promote increased interest in research and development in this arena.
Keywords
Solubilization; Aspergillus niger; Phosphatic fertilizers
Introduction
Phosphorus is an essential macronutrient for plants and is added to soil in the form of phosphatic fertilizers. However, the applied quantity of soluble forms of phosphate fertilizers is easily precipitated into insoluble forms and is not efficiently taken up by the plants [1-4]. Use of phosphatic fertilizers has become a costly affair, also environmentally undesirable and there is need for alternative sources [5].
The ability of microorganisms to solubilize different forms of calcium phosphate has been reported [6-8]. The solubilization of inorganic phosphates by microorganisms supplies phosphates for plant nutrition and increases their growth [9,10]. The attractive approach of microbially mediated solubilization of phosphate has been successfully proved in soil conditions which resulted in agriculture production similar or better than achieved with soluble phosphate [11,12].
Phosphate solubilizing microorganisms convert insoluble phosphates into soluble forms through the processes of acidification, by the production of organic acids [13-15], production of acid and alkaline phosphatases [2] and to H+ production [16]. These organic acids can either dissolve phosphates as a result of anion exchange or can chelate Ca, Fe or Al ions associated with the phosphates [17].
A substantial number of microorganism species, mostly those associated with the plant rhizosphere have been isolated and characterized for their ability to solubilize unavailable reduced phosphorus to available forms exerting a beneficial effect upon plant growth [6-8]. It was assumed that phosphate-solubilizing activity was greater for filamentous fungi than for bacteria [18].
Immobilization of living microorganisms has been described by several investigators [19,20] to be useful in several biotechnological applications. It is widely known that immobilized cells offer a lot of advantages: reusability of the same biocatalyst, control of reactions, and the no contamination of products [21].
The main objective of this study was to examine the abilities of Aspergillus niger, isolated from agricultural soil, to solubilize different insoluble phosphate substrates (DCP, TCP and Rock phosphates) at different culture conditions.
Materials and Methods
Microorganisms
Phosphate-solubilizing microorganisms are isolated from rhizospheric samples by plating serial dilutions of rhizospheric soil extracts in NBRIP solid medium (glucose 10 g, MgCl2 .6 H2O 5 g, MgSO4. 7H2O 0.25 g, KCl 0.2 g and (NH4 )2SO4 0.1 g, agar 15 g, H2O 1L) supplemented with 5g/l of TCP [22]. That medium contains insoluble tri-calcium phosphate allowing the detection of phosphate solubilizer microorganisms by the formation of “halos” around their colonies.
All plates were incubated at 30°C for 1 week. Colonies surrounded by solubilized clear zone were picked and streaked onto NBRIP plates containing 5g/l of TCP. Plates were again incubated at the same conditions to confirm their abilities to solubilize insoluble phosphate. Stock cultures were routinely maintained on LB agar supplemented with 1% of glucose.
Among the isolated strains, Aspergillus niger showing higher phosphate solubilization ability (larger solubilization halo surrounding the colony) in NBRIP plate was selected for further study. It was identified based on the visual observation of isolates grown on PDA plates, micro-morphological studies in slide culture [23] at room temperature, and the taxonomic keys described by Hoog and Guarro [24] as well as the compendium of soil fungi [25]. Stock cultures of isolated strain were routinely maintained on a potato dextrose agar (PDA).
Phosphate solubilization in solid media
One fungal isolate with higher phosphate solubilization abilities was selected. Abilities of the isolated fungus to solubilize different kind of insoluble phosphates were investigated. For this, the NBRIP plates supplemented with DCP, TCP or natural rock phosphates PR1, PR2, PR3and PR4 at 5g/l were used. The plates were inoculated with the selected fungus and incubated at 30°C. The diameter of clear zone (halo) surrounding the fungal growth as well as diameter of the colony were measured after 10 days of incubation
Phosphate solubilization in liquid media
Precultures of the fungus were prepared by inoculating plugs (diameter 0.5 cm) from the growing zone of fungus on agar plate to 50 ml of nutrient broth (NB). Then, cells were cultivated statically at 30°C for 3 days. Afterward, the precultures were homogenized aseptically using a homogenizer. Aliquots of 1.5 ml of homogenized precultures were used to inoculate volumes of 150 ml of NBRIP containing 5 g/l of tested insoluble phosphates (DCP, TCP, PR1, PR2, PR3 or PR4) in 250-ml Erlenmeyer flasks. The cultures were incubated aerobically at 30°C on a rotary shaker at 150 rpm for 7 days. At several time intervals 4 ml aliquots of fungal cultures were sampled and centrifuged at 15,000 rpm for 15 min.
The clear supernatant was used for determination of the pH and the soluble phosphorus released into the solution. Phosphorus was determined colorimetrically by using the vanado-molybdate method [26]. The pellets were washed with 0.5 N HCl solutions to dissolve the residual insoluble phosphate and then dried at 105°C for 24 h to determine the biomass dry weight. All experiments were performed in duplicates.
Fungal biomass preparation
Aliquots of 1.5 ml of homogenized preculture of isolated fungus, prepared as described above, were used to inoculate 250-ml Erlenmeyer flasks containing 150 ml of NB. The cultures were incubated aerobically at 30°C on a rotary shaker at 150 rpm for 5 days. After cultivation the fungal biomass was harvested by filtration and then rinsed with sterile sodium chloride water (0.9%).
Fungal biomass immobilization
Entrapment in calcium gel: 100 ml of sterile sodium alginate solution (2% w/v) was mixed, until homogenous, with 2 g of fungal biomass. The mixture was extruded into 150 mM CaCl2, forming beads of 5 mm diameter. The beads were allowed to harden in the CaCl2 solution at room temperature for 30 min, and rinsed with sterile sodium chloride water (0.9%).
Entrapment in polyacrylamide gel: 2 g of fungal biomass were mixed with 78 ml of Tris-HCl buffer (50 mM, pH 7), 20 ml acrylamid-bisacrylamide solution (30-0.8 % wt/vol), and 1 ml ammonium persulfate solution (10 % wt/vol.). The polymerization was initiated adding 100µl of N,N,N’,N’- tetramethyl-ethylenediamine. The polyacrylamide gel was then divided into particles of 0.5 cm diameter and rinsed with sterile sodium chloride water (0.9%).
Solubilization of DCP in fluidized bed bioreactor using free and immobilized fungal biomass
The fluidized bed bioreactors are composed of 500 ml conical flasks containing the immobilized fungal biomass suspended in 200 ml of NBRIP liquid medium and supplemented by 5g/l of DCP. The bioreactors were placed in a rotary shaker at 25°C, and the fluidization was assured by stirring at a rate of 120 rpm. The phosphate solubilization rate was followed according to time in the bioreactor. The same bioreactor have been used for studying DCP solubilization with free cells; thus, 2 g of fungal biomass were suspended in 200 ml of NBRIP liquid medium and supplemented by 5g/l of DCP. Solubilization rate was followed according to time in the bioreactor placed in the same conditions previously cited. For each experiment, a control test without fungal biomass was conducted under the same conditions.
At several time intervals, 1-ml aliquots were collected from the bioreactors and centrifuged at 15,000 rpm for 15 min. The supernatants were analyzed spectrophotometrically to determine the amount of soluble phosphorus and to determine the pH of the reactional media.
Repeated batch operation of phosphate solubilization in fluidized bed bioreactor using free and immobilized fungal biomass
The longevity of solubilization activity of the immobilized fungal biomass was investigated in repeated batch solubilization tests. A fresh reactional medium (NBRIP containing 5 g/l of DCP) was first inoculated with immobilized fungal biomass in the fluidized bed bioreactor, described above, and placed at 30°C in a rotary shaker at 120 rpm. After one week, the reactional medium was discharged and the immobilized fungal biomass were collected, rinsed with sterile NBRIP, and transferred into a fresh reactional medium for the next cycle of solubilization experiment. Solubilization rates were monitored according to time in all bioreactors. For comparison, the repeated batch experiments were also conducted using free fungal biomass under identical experimental procedures.
Results and Discussion
Five bacterial isolates and 2 fungal strains, which are solubilized TCP (forming clear halo surrounding colonies), were isolated from rhizospheric soil samples. Among these strains, one fungal isolate identified as Aspergillus niger with higher DCP solubilization ability (larger solubilization halo surrounding the colony) in NBRIP plate was selected for further study (Figure 1).
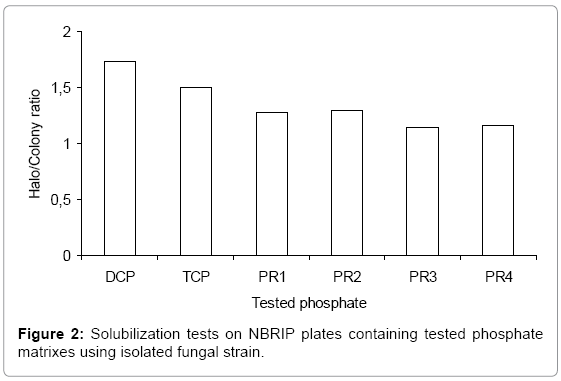
The selected isolate was cultivated, in first time, in NBRIP solid medium containing DCP, TCP or rock phosphates PR1, PR2, PR3 and PR4 as sole insoluble phosphate sources. The obtained results shown as the ratio of halo/colony diameters were represented in (Figure 2).
The obtained results show that the fungal strain Aspergillus niger manage to solubilize all tested phosphate substrates as indicated by the presence of solubilization halo on their culture media. The importance of the solubilization depends of the used phosphate. The highest solubilization halo/colony ratio around 1.73 was observed on plate containing DCP. It is followed by TCP, PR2, PR1, PR4 and PR3 with halo/colony ratios of 1.5, 1.28, 1.16 and 1.14 respectively.
It is generally accepted that phosphate solubilizing microorganisms convert insoluble phosphates into soluble forms through the process of acidification, by producing of organic acids, chelation and exchange reaction [15,27-29]. However, the absence of the halo of solubilization or its reduced diameter can be explained by the diffusion limitation of secreted organic acids [30]. Consequently the phosphate solubilization abilities of isolated fungal strain were screened in NBRIP liquid medium containing the same phosphate substrates at 5g/l. The pH of the culture, the fungal biomass evolution and the concentration of the released orthophosphates were monitored according to time for each tested phosphate.
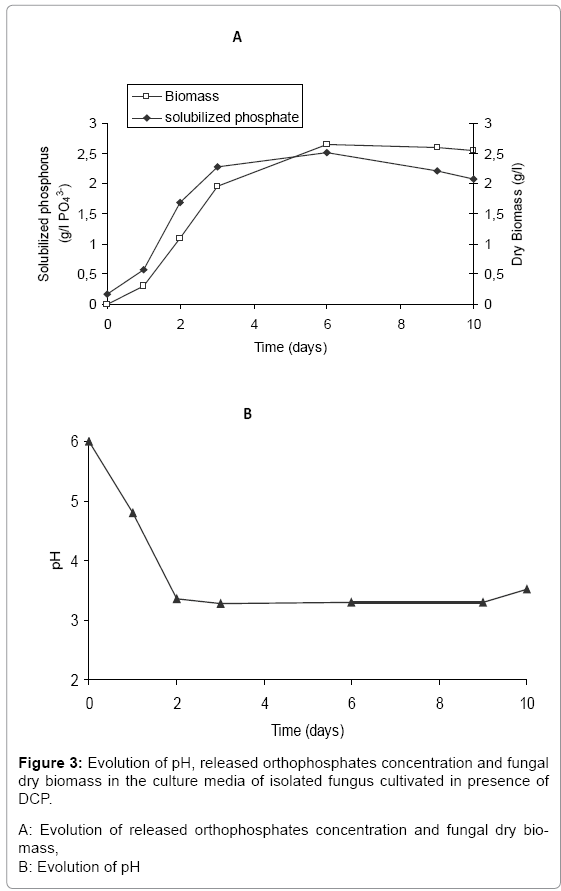
The obtained results, represented in (Figure 3A and 3B), concerning the solubilization of DCP during the cultivation of the isolated fungal strain indicated that this fungus has a high ability to solubilize DCP. The solubilization of DCP was accompanied by significant drop in the pH (to pH= 3.3). During the cultivation of Aspergillus niger, the solubilization rate of DCP was initially low. However more than 79% of insoluble phosphate was released as orthophosphates between the 3rd and the 6th day at which time the fungal biomass began to grow intensively. Conversely, the non-inoculated control presented no solubilization (data not shown).
The solubilization TCP and 4 different kinds of rock phosphates by isolated Aspergillus niger was investigated. The obtained results, plotted in (Table 1), testify of high ability of isolated fungus to solubilize mineral phosphates. Indeed, the use of Aspergillus niger enables a solubilization of all tested phosphates but with different performances that vary according to the used phosphate. Aspergillus niger solubilizes better and more quickly the DCP compared to others tested phosphates with solubilization efficiency and specific solubilization rate of 79.1% and 302.8 mg/g/d respectively. It is followed by TCP with solubilization efficiency and specific solubilization rate of 71.1% and 254.8 mg/g/d respectively. The lowest solubilization was recorded using PR3 with solubilization efficiency and specific solubilization rate of 30.8 and 148mg/g/d respectively. The solubilization of all tested phosphate matrixes was accompanied by significant drop in the pH (inferior to 3.5).
| Final pH value | Solubilization efficiency (%) | Specific solubilization rate (mg/g/d) | |
|---|---|---|---|
| DCP | 3.3 | 79.1 | 302.8 |
| TCP | 3.5 | 71.1 | 254.8 |
| PR1 | 3.3 | 34.4 | 73.5 |
| PR2 | 3.2 | 36.1 | 92.5 |
| PR3 | 3.3 | 30.8 | 58.0 |
| PR4 | 3.2 | 31.7 | 55.1 |
Table 1: Final pH value in reactional medium, Solubilization efficiency and specific solubilization rate of tested phosphate matrixes recorded by isolated fungus in growth condition.
The solubilization of DCP in fluidized bed bioreactor using free and entrapped fungal biomass in alginate and polyacrylamide gels was investigated. The concentration of released orthophosphates in reactional medium was measured at predefined interval time. Thus, free and immobilized fungal biomass exhibited similar solubilization patterns for DCP. The concentration of soluble phosphorus increased progressively in the reactional medium. The recorded solubilization efficiency for free fungal biomass reached 84.7%. Using immobilized fungal biomass, the obtained solubilization efficiencies were 73.4% and 66.1 respectively with alginate and polyacrylamide as entrapment matrixes (Table 2). The lower solubilization rates for immobilized biomass compared to free biomass can be attributed to mass transfer restriction arising from fungal biomass entrapment.
| Entrapment matrix | Solubilization efficiency (%) | Specific solubilization rate (mg/g/d) |
|---|---|---|
| Without (Free biomass) | 84.7 | 399.0 |
| Alginate | 73.4 | 190.5 |
| Polyacrylamide | 66.1 | 179.0 |
Table 2: The Solubilization efficiency and specific solubilization rate of DCP recorded by free and immobilized biomass of isolated fungus.
To investigate the possibility of the reusability of the same fungal biomass in successive cycles of solubilization, repeated batch experiments were performed. As shown in (Table 3), free biomass of Aspergillus niger remind active during all the 4 cycles of solubilization. However, a progressive decrease in solubilization efficiency was observed over the cycles of solubilization. After 4 Cycles of solubilization, the recorded solubilization efficiency and specific solubilization rate dropped from 84.7 to 70.2% and from 399 to 260.5mg/g/d respectively. The immobilization of Aspergillus niger in calcium alginate gel greatly stabilizes the fungal activity for more than 4 cycles. This stability returns to soft polymerization condition of the gel and to a direct role of the calcium in the cells conservation [31]. The use of Aspergillus niger entrapped in polyacrylamide gel in repeated batch fluidized bioreactor has proven to be not interesting. Indeed the phosphate solubilization system lost more than 54 % of its solubilization efficiency at the 4th cycle of treatment, and practically cancelled at the end of the 6th cycle (data not shown). This limitation of the biological activity is due to the existence of a favorable microenvironment inside the gel matrix and the presence of residual monomer that leads to a toxicity of microbial cell [32].
| Free biomass | Alginate | Polyacrylamide | ||||
|---|---|---|---|---|---|---|
| Ts (%) |
Vs (mg/g/d) |
Ts (%) |
Vs (mg/g/d) |
Ts (%) |
Vs (mg/g/d) |
|
| Cycle 1 | 84.7 | 399.0 | 73.4 | 190.5 | 66.1 | 179.0 |
| Cycle 2 | 82.2 | 354.5 | 73.2 | 182.5 | 40.5 | 103.5 |
| Cycle 3 | 78.4 | 290.5 | 73.4 | 185.0 | 36.2 | 98.5 |
| Cycle 4 | 70.2 | 260.5 | 73.3 | 180.0 | 35.7 | 96.5 |
Table 3: Solubilization efficiency (Ts) and specific solubilization rate (Vs) of DCP recorded by free and immobilized biomass of isolated fungus during repeated batch solubilization cycles.
The obtained results indicate that isolated fungal strain is able to mobilize phosphorus from inorganic source and may serve as a good rock phosphate solubilizer when inoculated into soils where rock phosphate is used as phosphatic fertilizers. Encapsulated microbial systems could be adopted for different application: solubilization of inorganic phosphate in bioreactors; preparation of microbial inoculants for introduction in soils enriched with untreated rock phosphate and pre-treatment of ores.
References
- Omar SA (1998) The role of rock phosphate solubilizing fungi and vesicular arbuscular mycorrhiza (VAM) in growth of wheat plants facilitated with rockphosphate. World J Microbiol Biotechnol 14: 211-218.
- Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17: 319-339.
- Igual JM, Valverde A, Cervantes E, Velazquez E (2001) Phosphate-solubilizing bacteria as inoculants for agriculture: use of updated molecular techniques in their study. Agronomie 21: 561-568.
- Vassilev N, Vassileva M (2003) Biotechnological solubilization of rock phosphate on media containing agro-industrial wastes. Appl Microbiol Biotechnol 61: 435-440.
- Reddy MS, Kumar S, Batita K, Readdy MS (2002) Biosolubilization of poorly soluble rock phosphates by Aspergillus tubingenis and Aspergillus niger. Bioresour Technol 84: 187-189.
- Mba CC (1997) Rock phosphate solubilizing Streptosporangium isolates from casts of tropical earthworms. Soil Biol Biochem 29: 381-385.
- Vassileva M, Azcon R, Barea JM, Vassileva N (1998) Application of an encapsulated filamentous fungus in solubilization of inorganic phosphate. J Biotechnol 63: 67-72.
- Ivanova R, Bojinova D, Nedialkova K (2006) Rock phosphate solubilization by soil bacteria. Journal of the University of Chemical Technology and Metallurgy41: 297-302.
- Bhattacharya P, Dey BK, Banik S, Nath S (1986) Organic manures in relation to rhizosphere effect. IV. Effect of organic manures on phosphate solubilizing power of rice and succeeding wheat rhizosphere soils. Zentralbl Mikrobiol 141: 357-365.
- Sahu SN, Jana BB (2000) Enhancement of the fertilizer value of rock phosphate engineered through phosphate-solubilizing bacteria. Ecol Eng 15: 27-39.
- Azcon R, Barea JM, Hayman DS (1976) Utilization of phosphate in alkaline soil by plants inoculated with mycorrhizal fungi and phosphate-solubilizing bacteria. Soil Biol Biochem 8: 135-138.
- Kucey RM (1987) Increased phosphorus uptake by wheat and field beans inoculated with a phosphorus-solubilizing penicillium bilaii strain and with vesicular-arbuscular mycorrhizal fungi. Appl Environ Microbiol 53: 2699-2703.
- Cunningham JE, Kuiack C (1992) Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. App Environ Microbiol 58: 1451-1458.
- Singh CP, Amberger A (1997) Organic acids and phosphorus solubilization in Straw composted with rock phosphate. Bioresour Technol 63: 13-16.
- Lin TF, Huang HI, Shen FT, Young CC (2006) The protos of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-A174. Bioresour Technol 97: 957-960.
- Illmer P, Schinner F (1994) Solubilization of inorganic calcium phosphates, solubilization mechanisms. Soil Biol Biochem 27: 257-263.
- Gyaneshwar P, Naresh Kumar G, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245: 83-93.
- Vassileva M, Azcon R, Barea JM,Vassileva N (2000) Rock phosphate solubilization by free and encapsulated cells of Yarowia lipolytica. Process Biochem 35: 693-697.
- Hyde FW, Hunt GR, Errede LA (1991) Immobilization of bacteria and saccharomyces cerevisiae in poly (tetrafluoroethylene) membranes. Appl Environ Microbiol 57: 219-222.
- Zeroual Y, Moutaouakkil A, Blaghen M (2001) Volatilization of mercury by immobilized bacteria (klebsiella pneumoniae) in different supports using fluidized bed bioreactor. Curr Microbiol 43: 322-327.
- Engasser JM (1988) Biotechnologie: Réacteurs à enzymes et cellules immobilisées. France: Technique et Documentation Lavoisier, Paris.
- Nautiyal CS, Bhadauria S, Kumar P, Lal H, Mondal R (2000) Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol Lett 182: 291-296.
- Riddell RW (1950) Mycologia 42: 265-270.
- Hoog GS, Guarro J (1995) Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Univeesitat Rovira I Virgili: Reus, Spain.
- Domsch KH, Gams W, Anderson TH (1980) Compendium of Soil Fungi. (1stedn), Academic Press, London.
- Afnor (1983) Recueil de normes Françaises des eaux: Méthodes d’essais. (2ndedn), Paris-La De´fense, Paris, France.
- Halder AK, Mishra AK, Bhattacharyya P, Chakrabartty PK (1990) Solubilization of rock phosphate by Rhizobium and Bradyrhizobium. J Gen Appl Microbiol 36: 81-92.
- Kpomblekou-A K, Tabatabai MA (1994) Effect of organic acids on release of phosphorus from phosphate rocks1. Soil Sci 158: 442–453.
- Singh S, Kapoor KK (1999) Inoculation with phosphate-solubilizing microorganisms and a vesicular-arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in a sandy soil. Biology and Fertility of Soils 28: 139–144.
- Johnston HW (1952) The solubilization of phosphate: the action of various organic compounds on dicalcium and tricalcium phosphate. NZ J Sci Technol 33: 436- 444.
- Tamponnet C, Barbotin JN, Calvayrac R (1989) Physical stabilization of Euglena gracilis cells by high extracellular calcium (100mM). Appl Microbiol Biotechnol 32: 211-217.
- Mosbach K, Mosbach R (1966) Entrapment of enzymes and microorganisms in synthetic cross-linked polymers and their application in column techniques. Acta Chem Scand 20: 2807?-2810.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15636
- [From(publication date):
specialissue-2014 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11013
- PDF downloads : 4623