Bioremediation-Waste Water Treatment
Received: 03-Oct-2017 / Accepted Date: 05-Jan-2018 / Published Date: 08-Jan-2018 DOI: 10.4172/2155-6199.1000427
Abstract
The growing population of the world and the progressive adoption of an industrialist lifestyle based, inevitably, have caused an increase in the anthropogenic impact on the biosphere. The textile industries are the most important industries in Asia and their numbers have increased. These industries have shown a significant increase in use of organic synthetic complex dyes as a coloring material. Wastewater printing and dyeing units are often rich in color, which contains residues of reactive dyes and chemicals, and requires proper treatment before being released into the environment. These compounds can be released to effluent water during washing. Examples of these compounds include surfactants, sizing, coatings and additive finishes. Sizing Compounds such as starch contribute to a higher demand for biological oxygen and the demand for chemical oxygen of the wastewater current.
Keywords: Wastewater; Dyes; Bleaching; Textile industries
Introduction
Global consumption of textiles is currently around 28 to 30 million tonnes, with an expected growth of 3% per year [1]. The coloration of this total needs about 8 × 105 tons of dyes [2] and it is estimated that there are 10,000 different types of dyes and pigments are produced every year around the world of which a great name of the dyes are composed azo (-N=N-), which are linked by an azo bridge and are used by a lot of industries. While predominantly used by textile mills, azo dyes can also found in the food, pharmaceutical, paper and printing industries, leather and cosmetics [3]. They have lost synthetic textile dyes used every year during manufacture and transformation and 20 to 22% of these dyes enter the environment through effluents resulting from the treatment of industrial wastewater. Wastewater printing and dyeing units are often rich in color, which contains residues of reactive dyes and chemicals, and requires proper treatment before being released into the environment. The effluent produced by a reactive dye contains non-fixed hydrolyzed reactive dyes, the substrate, which represents 25-35% of the reactive dyes applied, this the residual amount is responsible for the coloration of effluents and cannot be recycled, dyed organic substances, which are not recyclable and responsible for high biology oxygen demand and demand for chemical oxygen of effluent, textile Fibers, 60-100 gL-1 electrolytes, basically sodium chloride and sodium carbonate, that are responsible for the very high content left of the wastewater [4-7]. In addition to dyes, textile wastewater also contains solids, petroleum and halogenated organic from processes such as bleaching. In addition, some compounds can be applied to fibers in processes prior to the final step of the washing to improve the properties of the fibers. These compounds can be released to effluent water during washing. Examples of these compounds include surfactants, sizing, coatings and additive finishes. Sizing Compounds such as starch contribute to a higher demand for biological oxygen and the demand for chemical oxygen of the wastewater current. They are not as biodegradable as starches; they can pass through conventional wastewater treatment system, and are often linked to aquatic toxicity when receiving water. Cotton and polyester represent 76% of global textile demand. After that dyes of tendency, reagents and dispersed are the colors most commonly used to paint cellulose fibers (Table 1). Azo dyes that represent 60-80% of the dyes consumed in the textile. The processing is characterized by being a typical one double azo bond (-N=N-), which is the most common chromophore of reactive inks. Delivering color to the fabric is not an efficient process and up to 40% of the dyes are lost during the dyeing process [8-11]. Dyeing, desizing and rubbing are the main sources of contamination of water in the textile effluent and dye residual waters are characterized by being very visible color, high demand of chemical oxygen, suspended solids and alkaline pH [9-12], therefore, the effluents of these industries are downloaded to the environment is one of the main causes of concern. As the dyes are designed to different colors substances and solutions indefinitely, there is a great potential for these dyes. They accumulate in the environment since many of them are recalcitrant to normal bioremediation [13]. Various physical and chemical methods available are: adsorption, precipitation, coagulation/flocculation, oxidation, electrolysis and membrane extraction. These techniques are effective for color removal, but they do intensive in energy and introduce chemicals that are not wanted, in the first place. They also concentrate the contaminants in solid or liquid side washes that they require additional treatment or elimination, therefore, increase the cost of effluent treatment [14-16]. Biological discoloration is the most common and widespread technique used treatment of textile effluents [17]. There are two types of biological treatment: Aerobic and anaerobic. Aerobic systems require oxygen for fungi and bacteria to perform the degradation process, while anaerobic ones operate in the absence of air and under static conditions. It has been shown that activated mud eliminates moderate control amount (12-25%) of the color by adsorption to the biomass of cells and sludge [18]. The efficiency of biological treatment systems in textile effluents the treatment has stimulated the investigation on the real mechanism behind the process. As such, there has been a growing recognition that enzymes can be used in many remediation processes for specific contaminants for treatment. In this direction, recent biotechnological advances have led to cheaper production and more enzymes easily available through improved insulation and purification procedures [19]. The potential benefits of enzymatic treatment such as compared to conventional treatments are: application on recalcitrant materials, operation at high and low concentrations of contaminants in a wide range of pH, range of temperature and salinity, air conditioning to biomass and easy control process [20].
| Class | Characteristics |
|---|---|
| Basic dyes | Basic dyes work well with acrylic due to strong ionic interactionsuch as a dynamic functional group such as –NR+3 or =NR+2 andnegative reserves in the copolymer. The most common structures are azo, diarylmethane, triarylmethane and anthraquinone. |
| Reactive dyes | Form covalent bonds with groups -OH, -NH or -SH in cotton, wool, silk. The problem of color effluents associated with the use of These dyes is due to the hydrolysis of the reactive groups that are produced during the dyeing process. The most common structures are azo, Azo metal complex, anthraquinone and phthalocyanine. |
| Mordant dyes | Mordants are usually salts of metals such as sodium or potassiumdichromate They act as a "fixing agent" to improve the solidity of the color. They are used with cellulose fibers of wool, leather, silk and modified. The most common structures are azo, oxazine or triarylmethane. |
| Acid dyes | Very soluble in water due to the presence of sulfonic acids.Form ionic interactions between the protonated functionalities of the fibers (-NH3+) and the negative charge of the dyes. Also, Van-der-Values, dipolar and hydrogen links are established. The most common the structures are azo, anthraquinone and triarylmethane. |
| Direct dyes | flat shape and its length allows them to join the cellulose on the side of fibers and maximize the Van-der-Waals, dipole and hydrogen links. Only 30% of the 1600 structures are still in production due to their lack of solidity during washing. The most common structures are almost azo dyes are always sulfonated. |
| Disperse dyes | Nonionic structure, with polar functionality such as -NO2 and -CN which improve the solubility of water, the forces of Van der Waals, the diplomatic forces and the colors are generally used with polyester. The most common structures are azo, nitro, antraquinones or metallic complexes azo. |
| Vat dyes | Vat dyes are water-insoluble, but the alkali can be dissolved. cellulose (Van der Waals forces) and can usually be oxidized back with hydrogen peroxide - its insoluble form. The most common structures are anthraquinones or indigoids. |
| Pigment dyes | These insoluble, non-ionic compounds or salts representing 25% of all commercial dye names retain their crystalline or particulate structure throughout its application. The most common structures are azo or metallic complex phthalocyanines. |
| Ingrain dyes | The term ingrain refers to all dyes that are formed in situ, in or onsubstrate by the development or association of one or more intermediates compounds and a diazotized aromatic amine. In the color index a subgraph labeled Ingrain is limited to tetra-azaphorphine derivatives or precursors. |
| Solvent dyes | nonionic surfactants used for the dyeing of substrates in which they can dissolve as plastics, varnish, ink and waxes. They are not often used for textile processing. The most common structures are diazo compounds which undergo molecular rearrangement,triarylmethane, anthraquinone and phthalocyanine. |
| Other dyeclasses | Food colors are not used as textile dyes. The use of natural colorants in textile processingthe operations is very limited. Fluorescent brighteners mask theyellowish shade of natural fibers by absorbing ultraviolet light and weakly emitting blue light. Not listed in a separate class in the Color Index, Many complex metal dyes can be found. The complex metallic dyes are generally azo compounds. |
Table 1: Classification of Dyes.
Process
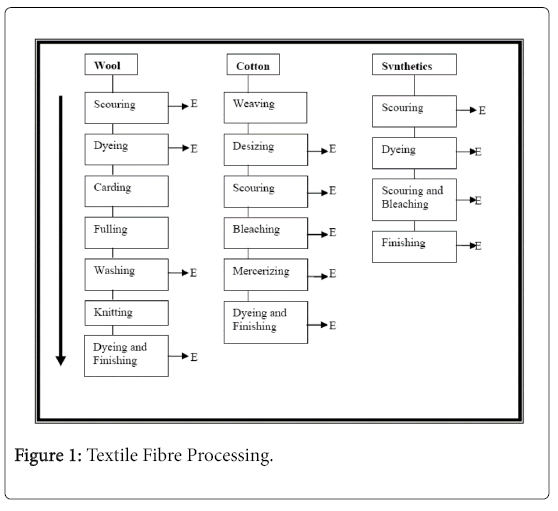
To understand the impact of textile effluents, you need to get a vision in the processes that cause the effluent. The main sources of textile effluents. They are the houses of tincture and the main processes involved are the desizing, cleaning, bleaching, mercerization, dyeing and finishing. Schematic representation of these main stages Implicated in textile fiber processing is shown (Figure 1).
The main stages involved in the coloration of cotton and synthetic ones, using azo dyes are:
Desizing: This is the preliminary phase of the processing of cotton. Basically, that It involves removing the size of cotton with the help of enzymes or detergents to produce a homogenous tissue in preparation for subsequent processing. The carvings are organic compounds such as starch and their derivatives, cellulose derivatives, polyacrylates and polyvinyl alcohol. Therefore, the decomposition effluent is characterized by high organic compounds and chemical oxygen demand [20]. In general, the effluents of size they represent the main component (~70%) of the organic effluent load of the factories of textile finishing [21].
Scouring: The objective of this stage is to eliminate oils, greases, waxes, soluble impurities and any other solid dirt that may have been left to the discouragement. The fabric is treated with detergents. The scouring effluent is characterized by high values of chemical oxygen demand and pH [22].
Bleaching: This process eliminates the natural coloring matter that can’t be eliminated by rubbing in a purely chemical reaction. The general objective of this stage is to improve the whiteness of the textile fabric, through redox reactions. The main chemical used is sodium hypochlorite and, as a result, the effluents have high levels of halogens [23].
Mercerizing: It involves the treatment of cotton fibers with approximately 20% Caustic soda, to increase the tensile strength and improve the affinity of the fabric of the fabric. High pH is the main characteristic of mercerization effluents.
Dyeing: There are three methods of textile dyeing, that is, massive tincture, which consists in dying of synthetic polymer before fiber formation; Dying the pigment where a color insoluble in the fiber adheres surface with the help of a binder; and dyed exhausted from a watery bath with dyes that have an affinity for fiber. Of the three methods, the exhaustion method is the most commonly used [24]. The colors used to distribute color include cationic, non-ionic or anionic. The cationic and anionic dyes are direct dyes, acids and reagents [25]. These reactive dyes and acids are soluble in water more problematic as they tend to go through the conventional treatment systems [26]. Nonionic dyes refer to dispersed dyes because they do not ionize in aqueous media and, if they do not get absorbed into the tissue, they end up being discharged as wastewater [27]. Application of the dyes can be continuous or lots. The volume of effluents containing dyes is higher than that generated from a continuous one process, between four and ten rinses are mandatory after batch dyeing [28]. The finish implies the final rinse of the fabric after dyeing improve quality Therefore, color is the main characteristic notable for dyeing and finishing effluents.
History
The first steps in the textile and fabric world began when the man discovered the use of animal bark and skins to interconnect plant derivatives such as hemp, creeper, manta, sisal and even crust of pulp in water to form a ribbon [29]. This led to the later development of wool, linen, linen, cotton and silk. Once the material became available, you must add color to the material. The art of weaving and the molding of weaves with dyes and pigments dates back to 800 BC [30], but scientists have been able to date black, yellow and white and reddish pigments made from ocher used by primitive man in cave paintings more than 15000 BC [30]. To understand the art and history of dyeing, it is important to understand how to do it. The process of dying began. By definition the term "dyed" refers to the process of colouring fibbers, threads or cloths using a dye that contains liquids for giving nuances to a substance. The substances that were used to decorate or to give a particular tone in the first times can be classified into four categories [31]. The first and most primitive method was to use natural products such as leaves, flowers, fruit, sticks, wood, shells, hair and similar objects without modification These were only interlaced and taken or stuck in the body or cloth with albumin or blood clotted to impart a temporary coloring [30]. The second method meant the physical friction of the desired colors on the canvas. Pigmenting dyes come from crushed or burned lime, plaster and clay give them whites and creams; hematite, ocher, iron oxide of iron ores and soils generated yellow, red and brown; the carbon black and coal were used to get black and gray nuances the pigments were joined to the fabric by mixing with the resins obtained from trees, albumen of eggs, precipitates or saliva. In some cases, the pigments were present roasted with turpentine, glue or wax to increase the persistence of pigments on the canvas. The third method in the dyeing industry was to use the right one shade where the color of the fabric was deposited on the substance in insoluble form. Many of these dyes have been obtained from the milling of fruits, flowers, roots and even the bark produced a fine colored paste that was left to bind to the tissue. These are the right ones dyes are also boiled or added to water to achieve better dye conservation speed [31]. The fourth method uses the sun, fire or smoke to get color pattern on the canvas. The rays of sunlight tend to naturally whiten colored fabrics such as bark, grass and cloth, as well as many dyed fabrics. Added cloth colors were achieved by placing a template on the cloth and expose it to the direct heat of the sun. The soot was saved as another example on a template or the entire fabric would be strongly colored to provide a deep black color, even difficult to obtain from natural dyes. This technique has been widely applied in the East African trunks, where carbon blacks from the collection plate were removed and mixed the grease of the kitchen, the resin, the clay or the soil to the bark fibers to create a beautiful deep black spot [32]. The major disadvantage of all these tissues dyeing methods was the inefficiency of the fixing process. All of these pigments, despite being light-fast, were not fast in the water, moisture or wear. Therefore, there was a great demand in a permanent way fixing dyes on canvas. This stimulated a whole range of experiments that led to the discovery of 'magical' properties found in certain rivers, seawater, saliva and even urine. These provided a more permanent effect on the tissue. As technology progressing, other experiments gradually isolated the "magical" quality that led to discovery of mordant and chemical conversions. Dyes are characterized by their ability to absorb energy from a special part of electromagnetic radiation on which the human eye is sensitive [33]. Dyes provide the color of the material on which they are anchored, selectively retaining some wavelengths of light falls on the surface. However, there are only some organic molecules. They possess this property to selectively absorb light [34]. Therefore, if the color is strongly absorbed at the red end of the spectrum, the light that is reflected is from a blue tone. A compound of colors is constructed from three subsystems, that is, aromatic groups called chromophores that are characteristically molecules and electron recipients Secondly, nucleophiles commonly called auxochromes. These are electrons retiring and containing substituted groups such as amino, hydroxyl, sulfonic and carboxylic groups. The third component of a typical color compound is a system Double conjugated links that unite chromophore and auxochrome to form one Color specific compound known as a chromogen [35]. Besides complementing the chromophore in the production of color, also increase dye solubility and increase its affinity to fibers [36]. Both auxochromes and chromophores move the higher wavelength absorption bands of the system conjugated to longer wavelengths and both can be empirically classified by a growing batoxocratic effect on a particular Conjugated system [37].
Classification
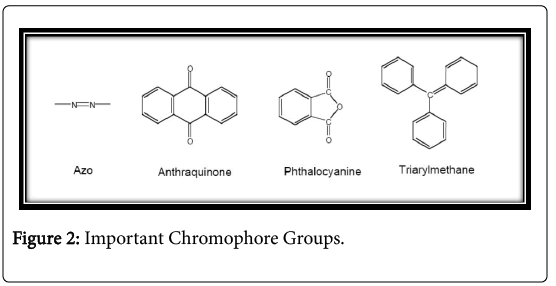
All molecules absorb electromagnetic radiation, but differ from the specific wavelengths are absorbed. Some molecules are able to absorb light visible spectrum and as a result, they will recruit themselves. Dyes are molecules with conjugated delocalized electron systems double bonds containing two groups: chromophore and aux chromium. Chromophore is an atomic group that controls the dye and It is usually an electron pulling group. The main chromosomes is -C=C-, -C=N-, - C=O, -N=N, -NO2 and -NO2. Auxochrome on electron donor substituent which can increase the color of the chromophore by changing the overall energy and solubility of the electron system and color adhesion to the fiber. The main auxochromes are - NH2, -NR2, - NHR, -COOH, -SO3H, -OH and -OCH3. Based on the chemical structure or chromophore, 20 to 30 different toner groups can be identified. Azo, anthraquinones, Phthalocyanine and triarylmethane dyes are quantitatively the most important chromophores (Figure 2).
Most commercial dyes are classified as color, texture or color (C.I.), changed every three years months since 1924 "Dyers and Colorists" "American Association of Textile Chemists and Colorists". Last edition of the color index has approx. 13,000 different tones. Each dye is shown in C.I. Generic name determined by its use and color. 15 color index Different application classes are listed in Table 1.
Azo dye
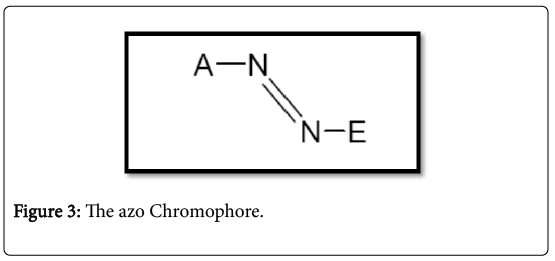
Azo dyes are by far the most important and versatile dyestuffs studied and used more than any other class [38]. They are watersoluble synthetic organic compounds having the characteristic -N=N-, which combines the chromophore and aux chromium to form colored molecules structural diversity. Generally, the azo dyes have one and three azo couplings, which often combine phenyl and/or naphthyl rings functional groups, including triazine amino, chloro, hydroxyl, methyl, nitro and sulphonate [38]. The transformation contains azo dyes (Figure 3) where the junction angle is about 120°. The nitrogen atoms are sp2 hybridized and the markings for the A and E groups are consistent with the color index (C.I.) (Color index, 1971).
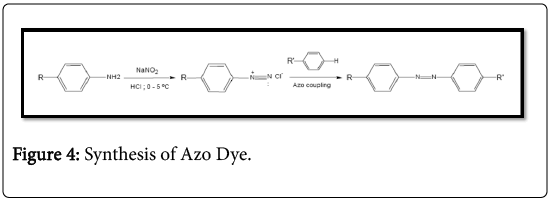
The typical properties of an azo dye are those groups that A represents the acceptance of an electron substitutes particularly hydroxyl and amino groups, while E represents the electron removing groups such as halogens and carboxylic groups. Dyes without any type Substitutes that carry only aromatic groups such as benzene are known as carboxylic azo dyes while those with heterocyclic groups are referred to as azo heterocyclic inks [39]. The fact that I come from Azo can unite several chromophores and auxocromes illustrates the enormous structural diversity that is possible with the azo Dyes that lead to a wide spectrum of nuances, mainly within the red scale. Unfortunately, the disadvantage that limits its function is that none of the azo dyes are green [40]. Azo dyes are the largest group of industrial synthetic dyes and pigments because of their relatively simple synthesis and almost unlimited number and type of substituent [41]. Global production these organic dyestuffs are currently estimated at 450000 tonnes per year, and almost 50000 tonnes/year in wastewater in waste water during application and production. Azo colors contain at least one N=N double bond and many different structures are possible. Mono azo dyes have only one N=N double bond, whereas diazo, triazole and polyacetating dyes contain two, three or more N=N double bonds, respectively. Azo groups are usually linked to benzene and but may also be linked to an aromatic heterocycle or enolated aliphatic groups [42]. General structure of azo-color the molecule can be seen in Figure 4.
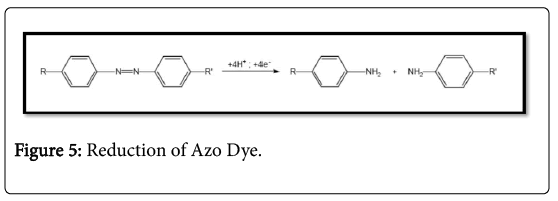
The azo bond is considered to be the most labile part of an azo dye. The bond readily undergoes an enzymatic but thermal or photochemical failure a breakdown may also occur. Degradation of azo dyes can be achieved by reduction or oxidation. Reduction releases colorless components (Figure 5).
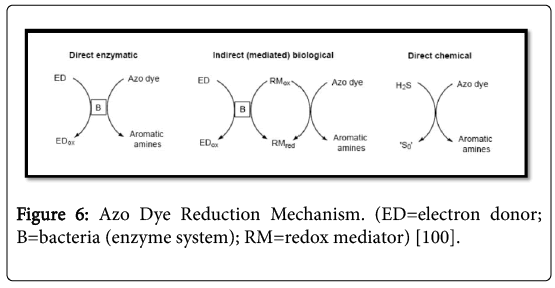
A wide variety of azo dyes can be reduced by many different bacteria, which suggest the non-specific nature of this reaction. The possibility of reducing azo dyes can therefore be considered a universal property of anaerobic bacteria. An accepted distinction between the various mechanisms for reducing azo dyes may be produced in direct enzymatic reduction, indirect enzymatic reduction (requiring mediators) and chemical reduction (Figure 6) [42].
Figure 6: Azo Dye Reduction Mechanism. (ED=electron donor; B=bacteria (enzyme system); RM=redox mediator) [100].
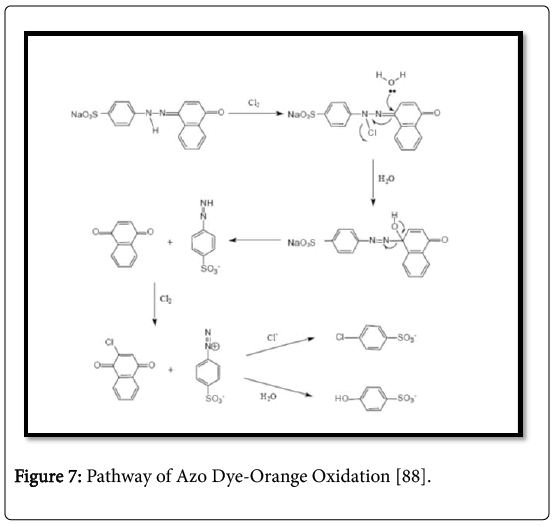
The general oxidation mechanism is more difficult because of the high reactivity of free radicals that are normally involved in the degradation process. The chemical oxidation of the azo dye (orange I) for chlorine in the acid medium is shown in Figure 7 [43]. A similar journey was observed in enzymatic oxidation [44]. Electronic elimination nature of the azo-groups generates a shortage of electrons. That is all causes the compounds to be less prone to oxidative catabolism and the like due to the fact that many of these chemicals tend to persist under aerobics environmental conditions [45].
Figure 7: Pathway of Azo Dye-Orange Oxidation [88].
Azo dye removal techniques
In the water reuse technology various physical, chemical and biological treatments are carried out and subsequent processing can be used to treat waste fabrics from fabrics. Physico-chemical techniques including membrane filtration, coagulation, flocculation, precipitation, flotation, adsorption, ion exchange, mineralization, advanced oxidation, electrolysis and chemicals are known. Biological treatment systems that can effectively remove dyes in large volumes of waste water at a low cost are more advantageous alternatives [44]. Biological techniques involving bio-sorption and biodegradation in aerobic, anaerobic or combined aerobic/anaerobic treatment with bacteria, fungi, plants, yeast, algae and enzymes are known [45,46]. Textile waste dyes are complexes that contain a wide range of dyes, natural impurities extracted from fibers and other products such as acids, bases, salts and the like sometimes heavy metals [42]. Therefore, they consist mainly of a combination of different techniques. When choosing type the treatment must take into an account of several factors such as the type of dye, the composition of the waste water, the cost of the chemical required, and the costs of operation, handling and the cost of the resulting residual product. It is up to the industry to choose which healing it is suitable for the factory. Most physical, chemical and biological color removal techniques work either by concentrating the color in sludge, solid supports or by the total destruction of the dye molecule. It is expected that discoloration prevailing systems that involve destruction technologies, such as the transfer of pollution from a part of the environment is anticipated [47]. Currently, the main textile methods for the treatment of waste water involves physical and/or chemical processes such as membrane filtration, coagulation/flocculation, precipitation, flotation, adsorption, ion exchange, ion-ion extraction, ultrasonic mineralization, Electrolysis, chemical reduction and advanced chemical oxidation [48]. Advanced oxidation processes include chlorination, bleaching, ozonation, oxidation of fenton, photocatalytic oxidation and humid air Oxidation [49]. Biological techniques include biosorption combined aerobic, anaerobic, anoxic or anaerobic/aerobic biodegradation Methods of treatment with bacteria, fungi, plants, yeasts, algae and enzymes [50]. Waste textile dyes are complex, it contains a wide range of dyes, natural impurities extracted from fibers and other products such as dispersants, buffering agents, acids, bases, salts and sometimes heavy metals [48]. In general, the discharge is strongly colored with a high demand for biological oxygen and chemical oxygen demand has a high conductivity and is fundamental in nature. For this reason, the technical and economic aspects determine several factors the viability of any technique of elimination of dyes like types of dyes, wastewater composition, lot and cost of chemical products, operating costs, environmental costs and waste treatment costs products in general, the use of an individual process is not enough to get the color change, as each technique has its limits. Dye removal strategies therefore consist mainly of different combination techniques [51]. The following chapters provide a general overview of important techniques is presented.
Physical treatment
Flocculation: A first common step in the treatment of wastewater is the flocculation, where particles in the water coagulates the flocks, generally improved with additional iron (III) chloride or aluminum chloride. Flocculation studies have showed that the amount of sulfonic and amino groups of the dye molecule they correlate with the ability to flocculate [52].
Adsorption: Adsorption is a way to capture chemical molecules on solid surfaces material and can therefore be extracted from the water phase. One of the most famous adsorbents is active carbon with good adsorption values and also expensive. Natural materials also have a proven ability to absorb various colours. The cheapest method is to use fractions of waste like bark, sawdust, rice shells or other shells. Miscellaneous chemical products parameters of different dyes influence the adsorption efficiency of the materials.
Filtration: Treatment with different filters is very attractive, as this opens the window of possibility to reuse water. How filters work depends on few parameters such as pH and concentrations. Filtration is divided into three categories: Ultra filtration, nanofiltration and reverse osmosis. The membranes are constructed from different polymeric materials (e.g., polyamides, polyester, polycarbonate, fluorcarbon polymers, polysulphons, and polyacrylonitrils). All types of filters work with pressure as a motor and its performance. It depends on the temperature, the concentrations and the type of molecule. Other parameters, such as the available surface to separate and embed pores, too worry separation.
Chemical
Electrolysis: Electrochemical technology is very effective in removing the color of a wide range of dyes and pigments. Biological demand for oxygen and chemical oxygen demand and coagulation the total suspended solids present in the waste water [53]. Process is very simply based on the application of electric current in wastewater by means of a sacrificial iron electrode for the production of ferrous hydroxide in solution. These iron sacrificial electrodes form Fe (II) and -OH ions. Fe (OH)2 is resulting acid dyes, soluble and insoluble, are removed from the waste water. In addition, Fe (II) can reduce azo dyes to arylamines. Water can also be oxidized, leading to O2 and O3 formation. Efficiency the electrochemical pollutant removal system can often reach 90%. However, the process is costly due to high energy demands, limited lifetime electrodes and uncontrolled radical reactions [54].
Ozonation: Ozone is a very strong and fast oxidizing agent that can react most species containing multiple linkages with simple oxidizable ions such as S2- to form oxyanions such as SO3-2 and SO4-2- [55]. Ozone quickly discolours water-soluble dyes, but with insoluble dyes react much more slowly. In addition, waste water from textile processing usually contains other refractory materials which will react with ozone and will increase its demand [56]. Decomposition of ozone requires high pH (pH>10). With alkaline solutions, ozone reacts almost undoubtedly with all the compounds present in the reaction medium [57], which transforms organic compounds into smaller ones biodegradable molecules [58]. Therefore, after ozone use of biological methods to achieve mineralization [59]. Major restrictions the ozone process is the relatively high cost of the ozone generation process together with its very short half-life [55].
Fenton: The oxidation system is based on fenton's reagent (hydrogen peroxide in the presence of iron salt) which has been used for the treatment of organic and inorganic. The process is based the formation of reactive oxidizing species capable of effectively disrupting contamination of the wastewater flow, but the nature of these species remains [60]. The most important reactive radicals include the presence of hydroxyl radicals whereas higher oxidized iron species can be formed [57]. It has been accepted that both hydroxyl and ferryl complexes that depend on the operation conditions, one of which dominates [61]. An oxidation system may be is effectively used for the destruction of biodegradable effluents and are suitable for secondary biological treatment [62]. The oxidation process of Fenton can stain a wide variety of dyes and in relation to the ozonization process is relatively inexpensive and generally leads to greater chemical oxygen demand reduction [63]. Fenton oxidation is limited to the fact Textile wastewater is generally high in pH, while Fenton the process requires a low pH. Higher pH values have large amounts of waste which results from the precipitation of the ferritic salts and the process loses it efficiency [64].
Photochemical: Photocatalytic or photochemical degradation processes are of importance in the field of wastewater treatment because these processes create complete mineralization with smooth operation temperature and pressure conditions. Photoactivated chemical reactions are characterized by the mechanism of free radicals initiated photon interaction of a suitable energy level with molecules of chemical products Types present in solution, in the presence or absence of a catalyst [65]. Radicals can be easily made with ultra-violet radiation and was tested in combination with H2O2, TiO2, Fenton agents, O3 and other solid catalysts to change the color of the dye [66]. While the UV/H2O2 process, it seemed too slow, costly, and ineffective for potential large-scale applications, the combination of UV/TiO2 seems more promising. For UV/TiO2 treatment. A wide range of dyes can be oxidized and generally not only disappear [67]. Because UV radiation in dyeing is limited due to the nature of sewage, the best use of UV technology is the subsequent treatment after ozonization [68].
Biological
There are many reports on the degradation of environmental pollutants by different bacteria.
Many of the bacteria are recognized to eat exclusively on hydrocarbons [69]. Bacteria with the hydrocarbon-degradation bacteria have the potential for hydrocarbon degradation. Biodegradation of hydrocarbons under aerobic and anaerobic conditions, namely nitrate reducing bacterial characteristics of pseudomonas sp. and brevibacillus sp. isolated from petroleum corrupted soil [68]. However, data submitted by Khauni et al. [70] indicates that the anaerobic bio degradation may be more important. 25 gene of hydrocarbon degradation the bacteria were isolated from the marine environment [71]. In addition, among 80 separate bacterial elements are related to 10 species as follows: Bacillus, Corynebacterium, Staphylococcus, Streptococcus, Shigella, Alcaligenes, Acinetobacter, Escherichia, Klebsiella and Enterobacter was the best hydrocarbon degradation bacteria. Bacterial circumstances that can degradate aromatic hydrocarbons are repeated several times, mainly from soil. These are usually the negative gram bacteria, most of which are most generous Pseudomonas. Biodegradable channels were also reported in bacteria from Mycobacterium gene, Corynebacterium, Aeromonas, Rhodococcus and Bacillus [72]. Although many bacteria are capable of metabolism of organic pollutants, there is only one bacteria all of them have enzyme potential to degrade all or even most of the organic compounds in soil pollution. Mixed mixed communities have the most powerful biodegradable capacity because genetic information is needed more than one organism to degrade the complex mixtures of organic compounds present in contaminated areas [73]. Both anaerobic and aerobic bacteria can co-translate PCBs. High chlorinated PCBs are subject to reduced mitigation by anaerobic microorganisms. Lower Chlorinated phenyl is oxidated with aerobic bacteria [74]. To date, there is a focus on Gram-negative issues related to the gene Pseudomonas, Burkholderia, Ralstonia, Achromobacter, Sphingomonas and Comamonas . However, some reports on PCB degradation activity and the characterization of genes there PCB degradation related to PCB's degradation capacity of Gram-positive [73]. Recent results of pesticide degradation bacteria including the degraded bacteria of Clorpyrifos Providencia stuartii isolated from agricultural soil [73] and Bacillus , Staphylococcus and Stenotrophomonas alone from cultivation and non-nutrition soil capable of degrading dichlorodiphenyltrichloroethane [74]. Based on the available literature, it can be It has been concluded that microbial disorders of azo dyes are more effective under anaerobic conditions. On the other hand, these conditions give aromatic amino formation; these are mutagenic and toxic for people who are subsequently required for an oxidative phase for their degradation. In this context, combined anaerobic/aerobic biological biological treatments Mitic effluents are associated with common microbial consortia in literature [75]. In contrast to mixed culture, pure culture use, there are some advantages. These include predictable performance and detailed information on degradation channels for better confirmation of catabolism of the drain endless toxic products under a certain set of environmental conditions. There is another advantage that the bacterial characteristics and their activity can be monitored by the use of culture-based or molecular methods to quantify the population density of bacteria over time. Information on the population the density can be extrapolated with a quantitative analysis of the azo dye eradication kinetics and minerals [76]. Heavy metals cannot be biologically eradicated (without "degradation", nuclear change structure of the elements) but they are only transformed from a single or organic oxidation state caste to another [77]. In addition, bacterium-effective metal bacteria are also effective. The microorganisms developed the capabilities to protect themselves from heavy metals Toxicity through various mechanisms, such as adsorption, acceptance, dilution, oxidation and reduction. Metals can be reduced by extraordinary metal reduction [78], where metal bacteria use electronic terminals receivers for anaerobic respiration. Also, bacteria should not have reduced mechanisms that are not attached to respiratory, but instead they are expected to develop metal resistance. For example, reduce Cr (VI) to Cr (III) aerobic [79] or anaerobic conditions [76], Se (VI) reduction to seamless [76] dimension, U (VI) to U (IV) [80] and decrease Hg (II) to Hg (0) [80]. Microbial metabolism does severe weight plays an important role, because there are frequent metallic compounds volatile. For example, Mercury, Hg (II) can be biomedical by some different bacteria species Alcaligenes faecalis, Bacillus pumilus , Bacillus sp., P. aeruginosa and Brevibacterium iodiniam with glacier methyl mercury [80]. In addition to reox reforms and methylation reagents, acidic acid bacteria such as Acidithiobacillus ferrooxidans [81] and sulfur oxidation bacteria [81] high concentrations of Ag, Cd, Cu, Co and Zn can be facilitated from contamination soils. On the other hand, the metal can be dried as insoluble sulphides indirectly at the bacteria reduction of metabolic activity of sulfate [81].
There are two large groups of enzymes that use bacteria in deformation Azo dyes: reductions or oxidation enzymes [74]. Example of the oxidative enzyme activities reported were lignin and manganese peroxidase, laccase and tyrosinase [72]. The lignolytic enzyme system is longer which was discussed in the rubbish fungus section. The enzymes are classified as azoreductases reductions as a flavin dependent (FMN) or independent flavin, and NADH or NADPH or both can be used as cofactor [75]. Both Azoreductase activities was reported as an intracellular and extracellular degradation, although sulfonated azo Flavin's dependent dyes or reductions should be able to pass on the plasma membrane [80]. At present, accurate mechanisms are not clear. Some bacteria have created a degrading ability and ability to produce azoreductase. Species that are involved in using insoluble materials are used sometimes called extracellular respiration to give an electron outside the cell. Electrical tubes can help to transfer electron to substrate (accepting electron) and enhancing reactions by increasing activity the field of the microscopy. The substrate or the receptor can be oxygen, nitrate, sulphate, metal oxides, halogenated organs or azo salts. The electrons can be donated directly at the transfer, or transferred by a secondary intermediary like quinone, indophenol, humic acids or flavin groups. The environment often acts as secondary electronic tubes [81]. Anaerobic bacterial processes were found to decompose azo dyes. This is probably due to the electrochemical unstable azo bond (- N=N-) which can act as an electron receiver. But oxygen is a better one electron acceptor and the potential for the azo bonds to receive electrons are reduced in the presence of oxygen; thus the azo bond is better degraded in anaerobic environment [82]. From extensive research on pesticides and aromatic hydrocarbon polymers (PAH), information related to microbial degradation of aromatic molecules can be removed. Chemicals based on aromatic ring structure disseminated periodically or enthusiastically in nature, has become an environment a problem due to their recurring structure [82]. Use bacteria similar degradation channels for degradation and use of chemicals such as nitothatic compounds, nitrogenons, nitrobenzoates, nitrophenols, nitrotoluenes [83]. In more complex ring structures, the bacteria were degraded on the complex ring structure the first way and then the same way for benzene [81]. Anaerobic and aerobic bacteria seem to have similarities in their degradation of aromatic hydrocarbons strategy; the different structure is degraded in a number of key structures. Subsequently, the organism of the activated and clone ring is the result of short organism acids [82]. In fact, repeatedly, the microbial the consortium has shown a degradation of the aromatic amino produced by azo cleavage far beyond individual species [84]. There are bacterial strains known to decolorize azo dyes under aerobics conditions [85]. A possible criticism from these studies is that cells can absorb dye on theirs surface and no real degradation takes place. The laccase enzyme could also be identified in the outlet of their reactor; still the stones may have supported the biofilm with anaerobic environment in some parts. Several species have been reported for active degradation of aerosol azo dyes environments. Some species have several azoreductases, for example. Pseudomonas aeruginosa has three azoreductases: PaAzoR1, PaAzoR2 and paAzoR3, known to break down various azo dyes [85]. Today, the biological degradation of textile dyes and especially azo dyes focuses on anaerobic treatment [86]. In this area extensive research is mainly carried out on artificial water with one or more dyes mixed in distilled water. Many researchers have performed interesting experiments with anaerobic degradation. Many of the studies focused on one or more species isolated from an environment that is considered to be interesting, for example, Textile expiration or contaminated soil. Their breakdowns were tested below different conditions [87,88]. These studies were usually performed under sterile laboratory conditions on artificial waters. Different strains of Pseudomonas and Bacillus were found to decompose several dyes [89]. Color reduction is not enough and metabolites and chemicals can still be in water. Dyes are often cleaved in aromatic amines that absorb light wavelengths other than most dyes [90]. The presence of sulphate in dye molecules and in the water will affect degradation process. The presence of sulphate-reducing bacteria can contribute to release sulphide which can reduce some of the dyes chemically [91]. The most studied fungi with regard to dye decomposition are ligninolytic fungi. White rot mushrooms, especially produced enzymes such as lignin peroxidase, manganese peroxidase and laccase that break down many aromatic compounds due to their non-specific activity [92]. There is great literature about the possibility of these fungi to oxidize phenolic, non-phenolic, soluble and insoluble dyes [93,94]. In laccase from Pleurotus ostreatus, Schizophyllum, Sclerotium rolfsii and Neurospora crassa , seemed to increase up to 25% degree of staining individual commercial triarylmethane, anthraquinone and indigoid textile dyes using enzyme preparations [92]. On the contrary, manganese peroxidase was reported as the main enzyme involved in dye capture of Phanerochaete chrysosporium [93] and lignin peroxidase for Bjerkandera adusta [95,96]. Some non-white root mushrooms, which can advantageously dye have also been reported [94]. Mushroom degradation of aromatic structures is a secondary metabolic event that begins when nutrients (C, N and S) become limiting [97]. The influence of the substitution pattern on the dye mineralization rates and between binder and mushroom biodegradability is a question of controversy [98]. However, these difficulties are even greater if you consider that complex mixed wastewater is extremely variable in the composition even from the same factory, as is often the case with the textile industry. Other important factors for cultivation of white rock vapor and expression of ligninolytic activity is the availability of enzyme cofactors and the pH of the environment [99,100]. In spite of the stable operation of bioreactors of continuous addition of fungi for the treatment of solutions of synthetic coloring have been achieved, application of rotten white fungi for elimination of textile wastewater dyes face many problems such as the nature of synthetic dyes, the control of the biomass produced and the large volumes of treatment [101].
Conclusion
This article provides a critical assessment of the available technologies as well as the treatment of textile waste water and informs effectively and economically attractive options. Textile wastewater treatment before it is released It is crucial to reduce the burden of pollution and production costs. Convention technologies for treating textile wastewater include a variety of biological combinations, physically and chemically, but these methods must have high capital and operation cost. So far, there is no attractive individual and economic treatment that can effectively decompose the efficiency of the dyes. In recent years, this was the remarkable achievement made in the use of biotechnological applications for textile waste not only for color but also to achieve total degradation of the dyes. Different microorganisms, therefore such as found aerobic and anaerobic bacteria, fungi and physiological chemical methods dilute paints. Compared to physico-chemical, biological treatment systems that can effectively remove dyes from a large proportion of cheap waste water as they are the best alternatives.
References
- Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco PA, et al. (2000) Decolorization and detoxification of textile dyes with a laccase from Trameteshirsuta. Appl Environ Microbiol 66: 3357-3362.
- Akan JC, Abdulrahman FI, Ayodele JT, Ogugbuaja VO (2009) Impact of tannery and textile effluent on the chemical characteristics of Challawa River, Kano State, Nigeria. Aust J Basic Appl Sci 3: 1933-1947.
- Albuquerque MGE, Lopes AT, Serralheiro ML, Novais JM, Pinheiro HM (2005) Biological sulphate reduction and redox mediator effects on azo dye decolorization in anaerobic–aerobic sequencing batch reactors. Enzyme MicrobiolTechnol 36: 790-799.
- Aleboyeh A, Aleboyeh H, Moussa Y (2003) Critical effect of hydrogen peroxide in photochemical oxidative decolorization of dyes: acid orange 8, acid blue 74 and methyl orange. Dyes Pigm 57: 67
- Ali N, Hameed A, Siddiqui MF, Ghumro PB, Ahmed S (2009) Application of Aspergillus niger SA1 for the enhanced bioremoval of azo dyes in simulated textile effluent. Afr J Biotechnol 8: 3839-3845.
- An TC, Zhu XH, Xiong Y (2002) Feasibility study of photoelectrochemical degradation of with three-dimensional electrode-photocatalytic reactor. Chemosphere 46: 897-903.
- Anastasi A, Parato B, Spina F, Tigini V, Prigione V, et al. (2011) Decolorization and detoxification in the fungal treatment of textile wastewaters from dyeing processes. New Biotechnol 29: 38-45
- Ang EL, Zhao H, Obbard JP (2005) Recent advances in the bioremediation of persistent organic pollutants via biomolecular engineering. Enzyme MicrobiolTechnol 37: 487-496.
- Anjaneya O, Souche SY, Santoshkumar M, Karegoudar TB (2011) Decolorization of sulfonated azo dye metanil yellow by newly isolated bacterial strains: Bacillus sp. strain AK1 and Lysinibacillus sp. strain AK2. J Hazard Mater 190: 351-358.
- APHA (1998) Standard methods for the examination of water and wastewater. 20th edn, American Public Health Association, Washington, USA.
- Arslan I, Balcioglu IA, Bahnemann DW (2000) Advanced chemical oxidation of reactive dyes in simulated dyehouse effluents by ferrioxalate-Fenton/UV-A and TiO2/UV-A processes. Dyes Pigm 47: 207-218.
- Asgher M, Azim N, Bhatti HN (2009) Decolorization of practical textile industry effluents by white rot fungus Coriolus versicolor IBL-04. BiochemEng J 47: 61-65
- Ayed L, Mahdhi A, Cheref A, Bakhrouf A (2011) Decolorization and degradation of azo dye methyl red by an isolated Sphingomonaspaucimobilis: biotoxicity and metabolites characterization. Desalination 274: 272-277.
- Babel S, Kurniwan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater B 97: 219-243
- Babu BR, Parande AK, Raghu S, Kumar TP (2007) Cotton textile processing: waste generation and effluent treatment. J Cotton Sci 11: 141-153.
- Bandara J, Morrison C, Kiwi J, Pulgarin C, Peringer P (1996) Degradation/decoloration of concentrated solutions of orange II. Kinetics and quantum yield for sunlight induced reactions via Fenton type reagents. J PhotochemPhotobio A: Chem 99: 57-66.
- Baughman GL, Weber EJ (1994) Transformation of dyes and related compounds in anoxic sediment: kinetic and products. Environ Sci Technol 28: 267-276
- Beydilli MI, Pavlostathis SG, Tincher WC (1998) Decolorization and toxicity screening of selected reactive azo dyes under methanogenic conditions. Water Sci Technol 38: 225-232.
- Blumel S, Stolz A (2003) Cloning and characterization of the gene coding for the aerobic azoreductase from Pigmentiphagakullae K24. ApplMicrobiolBiotechnol 62: 186-190.
- Bohmer U, Kirsten C, Bley T, Noack M (2010) White-rot fungi combined with lignite granules and lignitic xylite to decolorize textile industry wastewater. Eng Life Sci 10: 26-34.
- Bragger JL, Lloyd AW, Soozandehfar SH, Bloomfield SF, Marriott C, et al. (1997) Investigations into the azo reducing activity of a common colonic microorganism. Int J Pharm 157: 61-71.
- Bromley-Challenor KCA, Knapp JS, Zhang Z, Gray NCC, Hetheridge MJ, et al. (2000) Decolorization of an azo dye by unacclimated activated sludge under anaerobic conditions. Water Res 34: 4410-4418
- Brown JP (1981) Reduction of polymeric azo and nitro dyes by intestinal bacteria. Appl Environ Microbiol 41: 1283-1286.
- Carita R, Marin-Morales MA (2008) Induction of chromosome aberrations in the Allium cepa test system caused by the exposure of seeds to industrial effluents contaminated with azo dyes. Chemosphere 72: 722-725.
- Casieri L, Varese GC, Anastasi A, Prigione V, Svobodova K, et al. (2008) Decoloration and detoxification of reactive industrial dyes by immobilized fungTrametespubescens and Pleurotusostreatus. Folia Microbiol 53: 44-52.
- Cervantes FJ (2002) Quinones as electron acceptors and redox mediators for the anaerobic biotransformation of priority pollutants. Agrotechnology and Food Sciences, Sub-department of Environmental Technology, Wageningen University, Wageningen, The Netherlands, p: 166
- Cervantes FJ, Van der Zee FP, Lettinga G, Field JA (2001) Enhanced decolorization of acid orange 7 in continuous UASB reactor with quinines as redox mediators. Water Sci Technol 44: 123-128.
- Charumathi D, Nilanjana D (2010) Bioaccumulation of synthetic dyes by Candida tropicalis growing in sugarcane bagasse extract medium. Adv Biol Res 4: 233-240.
- Chen BY, Hsueh CH, Chen WM, Li WD (2011) Exploring decolorization and halotolerance characteristics by indigenous acclimatized bacteria: chemical structure of azo dyes and doseresponse assessment. J Taiwan Inst ChemEng 42: 816-825.
- Chen G (2004) Electrochemical technologies in wastewater treatment. Sep PurifTechnol 38: 11-41.
- Chen H, Jin X, Zhu K, Yang R (2002) Photocatalytic oxidative degradation of acridine orange in queous solution with polymeric metalloporphyrins. Water Res 36: 4106-4112.
- Chen H, Wang RF, Cerniglia CE (2004) Molecular cloning, overexpression, purification, and characterization of an aerobic FMN-dependent azoreductase from Enterococcus faecalis. Protein Express Purif 34: 302-310.
- Chivukula M, Renganathan V (1995) Phenolic azo dye oxidation by laccase from Pyriculariaoryzae. Appl Environ Microbiol 61: 4374-4377.
- Chung KT, Stevens SEJ (1993) Degradation of azo dyes by environmental microorganisms and helminths. Environ ToxicolChem 12: 2121-2132.
- Chung KT, Stevens SEJ, Cerniglia CE (1992) The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol 18: 175-197.
- Claus H, Faber G, Koenig H (2002) Redox-mediated decolorization of synthetic dyes by fungal laccases. ApplMicrobiolBiotechnol 59: 672-678.
- Cydzik-Kwiatkowska A (2015) Bacterial structure of aerobic granules is determined by aeration mode and nitrogen load in the reactor cycle. BioresourTechnol 181: 312-320.
- Das D, Charumathi D, Das N (2010) Combined effects of sugarcane bagasse extract and synthetic dyes on the growth and bioaccumulation properties of Pichia fermentans MTCC 189. J Hazard Mater 183: 497-505.
- Dos Santos AB (2005) Reductive decolorization of dyes by thermophilic anaerobic granular sludge. Sub-department of Environmental Technology, Wageningen University, Wageningen, Netherlands, p: 176.
- Dua M, Singh A, Sethunathan N, Johri AK (2002) Biotechnology and bioremediation: successes and limitations. ApplMicrobiolBiotechnol 59:143-152.
- Dubin P, Wright KL (1975) Reduction of azo food dyes in cultures of Proteus vulgaris. Xenobiotica 5: 563-571.
- Dunnivant FM, Schwarzenbach RP, Macalady DL (1992) Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol 26: 2133-2141.
- Duran N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme MicrobiolTechnol 31: 907-931.
- Elisangela F, Rea Z, Fabio DG, Cristiano RM, Regina DL, et al. (2009) Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. IntBiodeterBiodegr 63: 280-288.
- El-Sheekh MM, Gharieb MM, Abou-El-Souod GW (2009) Biodegradation of dyes by some green algae and cyanobacteria. IntBiodeterBiodegr 63: 699-704.
- Enayatizamir N, Tabandeh F, Rodriˇıguez Couto S, Yakhchali B, Alikhani HA, et al. (2011) Biodegradation pathway and detoxification of the diazo dye Reactive Black 5 by Phanerochaetechrysosporium. BioresourTechnol 102: 10359-10362.
- Encinas-Yocupicio AA, Razo-Flores E, Sanchez Diaz F, Dos Santos AB, Field JA, et al. (2006) Catalytic effects of different redox mediators on the reductive decolorization of azo dyes. Water Sci Technol 54: 165-170.
- Erden E, Kaymaz Y, Pazarlioglu NK (2011) Biosorption kinetics of a direct azo dye Sirius Blue KCFN by Trametes versicolor. Electron J Biotechnol 14:1-10.
- Faryal R, Hameed A (2005) Isolation and characterization of various fungal strains from textile effluent for their use in bioremediation. Pak J Bot 37:1003-1008.
- Fernandez JA, Henao LM, Pedroza-Rodriguez AM, Quevedo-Hidalgo B (2009) Immobilisinglignilolytic fungus for removing reactive black 5 dye. Rev ColombBiotecnol 11: 59-72.
- Ferraz ERA, Umbuzeiro GA, de-Almeida G, Caloto-Oliveira A, Chequer FMD, et al. (2011) Differential toxicity of disperse Red 1 and disperse red 13 in the ames test HepG2 cytotoxicity assay and Daphnia Acute toxicity test. Environ Toxicol 26: 489-497.
- Field JA, Brady J (2003) Riboflavin as a redox mediator accelerating the reduction of the azo dye mordant yellow 10 by anaerobic granular sludge. Water Sci Technol 48: 187-193.
- Field JA, Stams AJM, Kato M, Schraa G (1995) Enhanced biodegradation of aromatic pollutants in cocultures of anaerobic and aerobic bacterial consortia. Antonie Van Leeuwenhoek 67: 47-77.
- Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Intl 30: 953-971.
- Fredrickson JK, Kostandarites HM, Li SW, Plymale AE, Daly MJ (2000) Reduction of Fe (III), Cr (VI), U(VI), and TC(VII) by Deinococcusradiodurans R1. Appl Environ Microbiol 66: 2006-2011.
- Fu Y, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. BioresTechnol 79: 251-262.
- Garcia-Montano J, Domenech X, Garcia HA, Torrades F, Peral J (2008) The testing of several biological and chemical coupled treatments for Cibacron Red FN-R azo dye removal. J Hazard Mater 154: 484-490.
- Geelhoed JS, Hamelers HV, Stams AJ (2010) Electricity mediated biological hydrogen production. CurrOpinMicrobiol 13: 307-315.
- Gingell R, Walker R (1971) Mechanism of azo reduction by Streptococcus faecalis II. The role of soluble flavins. Xenobiotica 1: 231-239.
- Glenn JK, Akileswaran L, Gold MH (1986) Manganese-Ii oxidation is the principal function of the extracellular manganese peroxidase from Phanerochaetechrysosporium. Arch BiochemBiophys 251: 688-696.
- Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8: 501-553.
- Guo F, Fu G, Zhang Z, Zhang C (2013) Mustard tuber wastewater treatment and simultaneous electricity generation using microbial fuel cells. BioresourTechnol 136: 425-430.
- Hassan SHA, Kim YS, Oh SE (2012) Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzyme MicrobTechnol 51: 269-273.
- Huang L, Logan B (2008) Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. ApplMicrobiolBiotechnol 80: 349-355.
- Jin XC, Liu GQ, Xu ZH, Tao WY (2007) Decolorization of a dye industry effluent by Aspergillus fumigatus XC6. ApplMicrobiolBiotechnol 74: 239-243.
- Jonstrup M, Punzi M, Mattiasson B (2011) Comparison of anaerobic pre-treatment and aerobic post-treatment coupled to photo-fenton oxidation for degradation of azo dyes. J PhotochPhotobio A: Chem 224: 55-61.
- Keck A, Klein J, Kudlich M, Stolz A, Knackmuss HJ, et al. (1997) Reduction of azo dyes by redox mediators originating in the naphthalenesulfonic acid degradation pathway of Sphingomonas sp. strain BN6. Appl Environ Microbiol 63: 3684-3690.
- Keck A, Rau J, Reemtsma T, Mattes R, Stolz A, et al. (2002) Identification of quinoide redox mediators that are formed during the degradation of naphthalene-2-sulfonate by Sphingomonasxenophaga BN6. Appl Environ Microbiol 68: 4341-4349.
- Khelifi E, Ayed L, Bouallagui H, Touhami Y, Hamdi M (2009) Effect of nitrogen and carbon sources on indigo and congo red decolorization by Aspergillus alliaceus strain 121C. J Hazard Mater 163: 1056-1062.
- Khouni I, Marrot B, Amar RB (2012) Treatment of reconstituted textile wastewater containing a reactive dye in an aerobic sequencing batch reactor using a novel bacterial consortium. Sep PurifTechnol 87: 110-119.
- Kim BH (2007) Challenges in microbial fuel cell development and operation. ApplMicrobiolBiotechnol 76: 485-494.
- Knackmuss HJ (1996) Basic knowledge and perspectives of bio elimination of xenobiotic compounds. J Biotechnol 51: 287-295.
- Konstantinous IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Applied Catalysis B: Environ 49:1-14.
- Kuberan T, Anburaj J, Sundaravadivelan C, Kumar P (2011) Biodegradation of azo dye by Listeria sp. Int J Environ Sci 1: 1760-1770.
- Li Z, Zhang X, Lin J, Han S, Lei L (2010) Azo dye treatment with simultaneous electricity production in an anaerobic–aerobic sequential reactor and microbial fuel cell coupled system. BioresourTechnol 101: 4440- 4445.
- Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38: 2281-2285.
- Liu M, Yuan Y, Zhang LX, Zhuang L, Zhou SG, et al. (2010) Bioelectricity generation by a Gram-positive Corynebacterium sp. strain MFC03 under alkaline condition in microbial fuel cells. BioresourTechnol 101: 1807-1811.
- Long B, Yang CZ, Pu WH, Yang JK, Jiang GS, et al. (2014) Rapid cultivation of aerobic granular sludge in a pilot scale sequencing batch reactor. BioresourTechnol 166: 57-63.
- Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. CurrOpinBiotechnol 19: 564-571.
- Lu N, Zhou SG, Zhuang L, Zhang JT, Ni JR (2009) Electricity generation from starch processing wastewater using microbial fuel cell technology. BiochemEng J 43: 246-251.
- Shah MP, Patel KA, Nair SS, Darji AM, Maharaul SJ (2013) Optimization of Environmental Parameters on Decolorization of Remazol Black B Using Mixed Culture. American Journal of Microbiological Research 1: 53-56.
- Shah MP, Patel KA, Nair SS, Darji AM, Maharaul S (2016) Microbial degradation of azo dye by Pseudomonas spp. MPS-2 by an application of sequential microaerophilic & aerobic process. American Journal of Microbiological Research 1: 105-112.
- Shah MP, Patel KA, Nair SS, Darji AM (2013) An Innovative Approach to Biodegradation of Textile Dye (Remazol Black B) by Bacillus Spp. Int J Environ BioremediatBiodegrad 1: 43-48.
- Shah MP, Patel KA, Nair SS, Darji AM (2013) Microbial decolorization of methyl orange dye by Pseudomonas spp. ETL-M. Int J Environ BioremediatBiodegrad 1: 54-59.
- Shah MP, Patel KA, Nair SS, Darji AM (2013) Microbial Degradation and Decolorization of Reactive Orange Dye by Strain of Pseudomonas Spp. Int J Environ BioremediatBiodegrad 1: 1-5.
- Shah MP (2014) Microbiological Removal of Phenol by an Application of Pseudomonas spp. ETL-: An Innovative Biotechnological Approach Providing Answers to the Problems of FETP. J Appl Environ Microbiol 2: 6-11.
- Min B, Logan BE (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38: 5809-5814.
- Oakes J, Gratton P (1998) Kinetic investigation of the oxidation of aryloazonaphotol dyes in hypoclorite solutions as a function of pH. J ChemSoc Perkin Trans 2: 2201-2206.
- Oh S, Logan BE (2005) Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res 39: 4673-4682.
- Othman I, Anuar AN, Ujang Z, Rosman NH, Harun H, et al. (2013) Livestock wastewater treatment using aerobic granular sludge. BioresourTechnol 133: 630-634.
- Patil SA, Surakasi VP, Koul S, Ijmulwar S, Vivek A, et al. (2009) Electricity generation using chocolate industry wastewater and its treatment in activated sludge based microbial fuel cell and analysis of developed microbial community in the anode chamber. BioresourTechnol 100: 5132-5139.
- Pronk M, de Kreuk, MK, de Bruin B, Kamminga P, Kleerebezem R, et al. (2015) Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res 84: 207-217.
- Rabaey K, Verstraete W (2005) Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol 23: 291-298.
- Ren Z, Steinberg LM, Regan JM (2008) Electricity production and microbial biofilm characterization in cellulose-fed microbial fuel cells. Water Sci Technol 58: 617-622.
- Rezaei F, Xing D, Wagner R, Regan JM, Richard TL, et al. (2009) Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl Environ Microbiol 75: 3673-3678.
- Su B, Cui X, Zhu J (2012) Optimal cultivation and characteristics of aerobic granules with typical domestic sewage in an alternating anaerobic/aerobic sequencing batch reactor. BioresourTechnol 110: 125-129.
- Sun J, Bi Z, Hou B, Cao YQ, Hu YY (2011) Further treatment of decolorization liquid of azo dye coupled with increased power production using microbial fuel cell equipped with an aerobic biocathode. Water Res 45: 283-291.
- Sun J, Hu Y, Bi Z, Cao Y (2009) Improved performance of aircathode single-chamber microbial fuel cell for wastewater treatment using microfiltration membranes and multiple sludge inoculation. J Power Sources 187: 471-479.
- Upadhyayula VKK, Gadhamshetty V (2010) Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: A review. Biotechnol Adv 28: 802-816.
- Van der Zee FP (2002) Anaerobic azo dye reduction. PhD Thesis, Wageningen University, Wageningen, Netherlands.
- Zhang T, Cui C, Chen S, Yang H, Shen P (2008) The direct electrocatalysis of Escherichia coli through electroactivated excretion in microbial fuel cell. ElectrochemCommun 10: 293-297.
Citation: Shah MP (2018) Bioremediation-Waste Water Treatment. J Bioremediat Biodegrad 9: 427. DOI: 10.4172/2155-6199.1000427
Copyright: © 2018 Shah MP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 10196
- [From(publication date): 0-2018 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 8958
- PDF downloads: 1238