Research Article Open Access
Bioremediation of Soil Contaminated with Tannery Effluent by Combined Treatment with Cow Dung and Microorganisms Isolated from Tannery Effluent
Akpomie Olubunmi O and Ejechi Bernard O*
Department of Microbiology, Delta State University, PMB 1 Abraka, Nigeria
- Corresponding Author:
- Ejechi Bernard O
Department of Microbiology, Delta State
University, PMB 1 Abraka, Nigeria
Tel: (662)846-4675
E-mail: ejechiben@gmail.com
Received date: April 29, 2016; Accepted date: May 05, 2016; Published date: May 23, 2016
Citation: Akpomie Olubunmi O, Ejechi Bernard O (2016) Bioremediation of Soil Contaminated with Tannery Effluent by Combined Treatment with Cow Dung and Microorganisms Isolated from Tannery Effluent. J Bioremed Biodeg 7:354. doi:10.4172/2155-6199.1000354
Copyright: © 2016 Akpomie Olubunmi O, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Bioremediation potential of a combination of cow dung and a microbial consortium (Pseudomonas aeruginosa, Penicillium chrysogenum and Aspergillus niger) in soil contaminated with tannery effluent, was investigated in the laboratory. Concentrations of phenol, sulphide and ammonium nitrogen in contaminated soil, were significantly reduced (P=0.000) to permissible levels after treatment with microbial consortium, cow dung or combination of microbial consortium and cow dung. Reduction of these compounds was best with combination of microbial consortium and cow dung. Cr (VI) level (139.2 mg/kg) was significantly reduced (P=0.000-0.050) by 33.2, 96.9 and 99.9% after treatment with microbial consortium, cow dung, and combination of consortium and cow dung, respectively. The treatments elevated soil pH from 5.8 to 6.9-7.2. Growth of maize in soil treated with the combination of microbial consortium and cow dung was not significantly different from growth in uncontaminated soil. Cr (VI) concentrations in the maize tissues were very low (0.002-0.006 mg/g). Thus combination of microbial consortium and cow dung may have potential application in bioremediation of soil polluted by tannery wastes.
Keywords
Tannery effluent; Effluent-contaminated soil; Bioremediation; Cow dung; Microbial consortium
Introduction
Tanning process generates wastes that are of serious environmental impact particularly in countries where environmental regulations are not enforced. Pollutants associated with tanning activities include chloride, formaldehyde, sulphuric acid, manganese, sulphide, phenols, synthans, tannins, chromium, lead, zinc, non-collagenous protein wastes, and tanned and untanned collagen [1,2]. Less than 5% of the tannery industries in the world have adopted adequate measures for treatment of effluents because of the cost implication [3]. Even the present conventional method of treatment (preliminary, primary and secondary treatment) has been found to be a secondary source of pollution [4].
The tanneries in Nigeria are mainly located in the northern part of the country and investigations have shown that the wastes are mostly dumped untreated on water and land surfaces. For example a report [5] showed that untreated tannery wastes were regularly discharged into land surfaces in Sokoto, Northern Nigeria. These researchers reported concentrations of sulphide, ammonium and chromium in Sokoto soil to be far above permissible levels. The integrity of contaminated soils would need to be restored by appropriate remediation treatments.
Bioremediation is an ecologically-friendly strategy that involves the use of living organisms particularly microorganisms, to degrade pollutants to innocuous compounds. Several reports have shown that microorganisms have been extensively researched for their ability to detoxify tannery pollutants [6-9]. However, these reports focused on tannery wastes and not on remediation of soil contaminated with tannery wastes per se. Reports on remediation of soil contaminated with tannery effluent tended to focus more on removal of chromium. These include, but not limited to leaching of chromium in polluted soil in a bioreactor-biosorption system [10] phytoremediation with biological wastes and non-edible crops with high chromium tolerance [11] and remediation by electrokinetic technique [12]. Several other chemical remediation methods have been used as shown in the review by Dhal et al. [13], but environmental concern compelled industries and researchers to turn to bioremediation as an ecologically-friendly approach.
However, there is paucity of information on the use of microorganisms and biological or animal wastes to remedy soil contaminated with tannery wastes and particularly chromium, because of insufficient research. Microorganisms like Pseudomonas aeruginosa, Penicilium chrysogenum and Aspergillus niger isolated from tannery effluent or contaminated sites have been shown to be resistant to Cr (VI)-a highly toxic pollutant, and are able to reduce the concentration in tannery effluents and aqueous solutions [14-17]. These organisms are therefore likely to remove chromium or degrade other tannery effluent pollutants in contaminated soil especially when applied in mixed culture as a consortium. A mixed population can cause a higher level of degradation than a single organism. This can be viewed as a simulation of a natural ecological niche that usually consists of mixed populations. Animal wastes or compost can also be useful for bioremediation, because some reports indicated that cow dung and composted municipal wastes enhanced biodegradation of hydrocarbon in polluted soils [18,19]. This necessitated a laboratory study to test the hypothesis that degradation of tannery wastes in contaminated soil by a consortium of Pseudomonas aeruginosa, Penicillium chrysogenum, Aspergillus niger isolated from tannery effluent can be enhanced in the presence of animal wastes.
Materials and Methods
Collection of samples
Polythene bags previously sterilised by overnight immersion in 75% alcohol were used to collect soil samples up to a depth of 10 cm from 6 randomly selected areas in Kano, Northern Nigeria where tannery effluents were frequently discharged. For the purpose of comparison, soil samples were also collected from 6 randomly selected areas in Kano that were not exposed to tannery wastes.
Physical and chemical analyses of effluent and soil contaminated with tannery effluent
The pH of the soil samples was determined in aqueous solution with a pH meter while soil nitrogen was determined by the Kjedahl method. The methods of Saxena et al. [20] and Kobayashi et al. [21] were used to determine concentrations of tannin and phenol, respectively. Sulphide was determined by phosphoric acid steam distillation and spectrophotometry with N-N-diphenyl-p-phenylenediamine (DPD) [22]. Cr (VI) was determined in the soil by the spectrophotometric method using 1.5-diphenyl carbazide and 0.5M NaOH-0.28M Na2CO3 as leaching reagent [23].
Treatment of soil contaminated with tannery waste
The soil samples were dispensed in 100 g quantities into 6 1 L flasks and sterilized at 121°C for 30 minutes. On cooling the flasks were each inoculated with combination (consortium) of the treatment organisms (20 mL peptone water suspension of 106 cells of Pseudomonas aeruginosa and 20 mL potato broth suspension of macerated 5 cm diameter mycelia of Penicillium chrysogenum and Aspergillus niger). The inoculum size as stated above was determined by plate count for P. aeruginosa while the 5 cm diameter mycelium was cut from the edge of the mycelium of P. chysogenum or A. niger freshly propagated on Potato Dextrose Agar plates. Thereafter the flasks were set aside on the laboratory bench for 42 days during which they were manually turned over with oven-sterilised glass rods at weekly intervals for aeration. A shaker was not used, because the agitation could not turn over the soil medium as desired. The soil was moistened with 20 mL sterile tap water each time it was turned over. The pH was measured at weekly intervals while phenol, sulphide, nitrogen, tannin and Cr (VI) concentrations were determined as before at the end of the experiment (42 days).
Another set of flasks was mixed with cow dung (80 g contaminated soil+20 g cow dung/flask) and inoculated with the consortium as before and set aside for 42 days. The control set was not inoculated with the consortium. Moistening, aeration and measurement of pH was repeated as before at similar intervals. Analyses of the chemical constituents were also conducted after the treatment period. All the experiments were repeated trice for the purpose of ensuring reliability of the results. The results were analysed by one-way ANOVA and Tukey post hoc tests.
Microbial population changes
After manual mixture, 1 g soil samples were withdrawn from each flask on commencement and subsequently at fortnightly intervals for determining bacterial and fungal population changes. The populations were determined by plate count using Nutrient Agar and Malt Extract Agar incorporated with streptomycin and chloramphenicol antibiotics for bacteria and fungi, respectively.
Oxidation of sulphide and utilisation of ammonium nitrogen by treatment organisms
Tubes containing 50 mL Thiosulphate Broth (g/L: 5.0 glucose, 5.0 Na2S2O3, 0.1 K2HPO4, 0.2 NaHCO3, 0.1 NH4Cl, 0.0025 bromophenol blue, pH 8.0 [24,25] were inoculated with I mL normal saline suspension of 105 P. aeruginosa cells or macerated 5 cm diameter mycelia of P. chrysogenum or A. niger. The tubes were incubated at room temperature (30 ± 2°C) for 10 days after which the pH and indicator colour were recorded. Reduction of pH and colour intensity was taken as ability to oxidize sulphide.
For testing the ability of the treatment organisms to use ammonium nitrogen, a medium consisting of (g/L): glucose 2.5, (NH4)2SO4 1.0, K2HPO4-3H2O 0.5, NaCl 1.0, MgSO4·7H2O 0.25, FeSO4·7H2O 0.2, agar 20 pH 7.2-7.4 [26] was used. The medium was inoculated with a loopful of P. aeruginosa, from fresh Nutrient Agar plates and 5 cm diameter mycelia of P. chrysogenum and A. niger from fresh Potato Dextrose Agar plates. Appearance of growth on the medium indicated the ability to use ammonium nitrogen.
Growth of maize in treated tannery waste-contaminated soil
Germination and growth of maize seeds were used to test the efficacy of the treatment that best reduced the level of the tannery waste constituents in the contaminated soil. Plastic pots containing 200 g of uncontaminated, untreated contaminated and treated (microbial consortium; cow dung; cow dung+microbial consortium) contaminated soils were used for the tests. Sterile tap water was added at 50 mL/pot at intervals of 3 days for the 21 days duration of the experiment. The stem height and leaf size were used as indices of growth. The results were analysed by ANOVA and Tukey post hoc statistical tests. In addition the maize plant (roots, stem, and leaves) from the treated soil that best promoted growth and untreated contaminated soil were analysed for Cr (VI) using the nitric acid and hydrogen peroxide digestion method and atomic absorption spectrometer procedure of EPA 200.3.
Results and Discussion
The results showing the concentrations of tannery effluent pollutants in soil are presented in Table 1. The pH of the normal soil was near neutral while that of the effluent contaminated soil was slightly acidic. The chemical pollutants’ concentrations in the soil were markedly higher than in normal soil and also exceeded permissible limits. This can be attributed to the frequency of discharge of the effluents into the soil. It was also reported that soils exposed to tannery wastes in Sokoto, Northern Nigeria had concentrations of tannery pollutants that exceeded permissible limits [5]. Although there are other constituents of tannery effluent that can impact the environment, the main toxic constituents are chromium, hydrogen sulphide and phenol which could exist as chlorinated phenols [27]. It was based on this knowledge that Cr (VI), phenol, tannin, and sulphide were selected as the pollution indicators to be analysed. Ammonium nitrogen was added to the test list, because run-offs containing high concentrations of the compound can cause eutrophication in nearby water bodies.
| Pollutants | *Contaminated soil | *Normal soil |
|---|---|---|
| pH | 6.3 ± 0.0.7 | 7.1 ± 0.2 |
| Nitrogen (mg/kg) | 164.7 ± 11.6 | 0.9 ± 0.02 |
| Sulphide (mg/kg) | 29.7 ± 5.4 | 0.9 ± 0.01 |
| Phenol (mg/kg) | 1191.3 ± 35.8 | 7.6 ± 0.5 |
| Tannin (mg/kg) | 142.4 ± 9.8 | 87.1 ± 4.8 |
| Cr (VI) (mg/kg) | 139.2 ± 6.5 | 0.02 ± 00.001 |
*Values are presented as M ± SD.
Table 1: Concentration of some pollutants in soil contaminated with tannery effluent.
Table 2 presents the effect of the three treatment measures on the concentration of the chemical pollutants investigated in the contaminated soil. Comparison by ANOVA tests showed that the three treatment protocols significantly reduced the level of pollutants (Table 2). Multiple comparison by Tukey test indicated that treatment with combination of cow dung and microbial consortium was the most effective because it reduced the pollutants significantly better (P=0.015) than other treatments. The reduction in the concentration of phenol and tannins was expected because both compounds are widespread in the environment as intermediates in natural decomposition of wood and forest litters by microorganism. It is known that a variety of microorganisms including species of Pseudomonas, Aspergillus and Penicillium are associated with degradation of phenolic compounds in soils [28-30].
| Chemical parameter | Soil treatment | *F(P) | |||
|---|---|---|---|---|---|
| Untreated | Microbial consortium | Cow dung | Cow dung + microbial consortium | ||
| Nitrogen (mg/kg) | 164.7 ± 11.6 | 40.0 ± 4.2 | 14.1 ± 1.4 | 3.4 ± 0.5 | 55.6(0.000) |
| Sulphide (mg/kg) | 29.7 ± 5.4 | 1.0 ± 0.06 | 6.1 ± 0.6 | 0.2 ± 0.02 | 7.8(0.002) |
| Phenol (mg/kg) | 1191.3 ± 35.8 | 25.4 ± 2.9 | 52.2 ± 4.8 | 6.0 ± 0.5 | 265.0(0.000) |
| Tannin (mg/kg) | 142.4 ± 9.8 | 5.3 ± 0.6 | 26.4 ± 2.6 | 2.3 ± 0.1 | 19.5(0.000) |
| Cr (VI) (mg/kg) | 139.2 ± 6.5 | 93.0 ± 5 | 5.5 ± 0.3 | 0.07 ± 0.01 | 167.2(0.000) |
*ANOVA: values in parenthesis indicate level of significance. Concentrations of the chemical parameters are presented as Mean ± SD.
Table 2: Effect of microbial consortium and cow dung treatments on the concentration of chemical pollutants in soil contaminated with tannery effluent.
The significant reductions in sulphide and ammonium nitrogen can be attributed to the activity of P. aeruginosa, because the results in Table 3 showed that it was the only organism in the consortium that oxidized sulphide and utilised ammonium nitrogen. Sulphide is part of the natural biogeochemical cycle of sulphur and it is becoming recognized that some heterotrophs e.g., Pseudomonas, Bacillus and Micrococcus are capable of sulphur oxidation [25,31,32].
| Microorganisms | Sulphide oxidation* | Use of ammonium nitrogen medium |
|---|---|---|
| Pseudomonas aeruginosa | Yes | + |
| Penicillium chrysogenum | No | - |
| Aspergillus niger | No | - |
*Oxidation=lowering pH and colour change of medium; +, Growth; -, No growth (See Materials and Methods)
Table 3: Oxidation of sulphide and utilization of ammonium nitrogen.
The removal of Cr (VI) from the contaminated soil can be attributed to the combined action of the test organisms especially A. niger and P. chrysogenum, and electron donors in the cow dung. Biological wastes including cow dung have been reported to be reservoirs of electron donors that can reduce Cr (VI) to the less available Cr (III) in the soil [10,11,33]. For example organic compounds like citric acid that can transform metals by acting as chelating ligands are formed in cow dung [11]. The role of the cow dung is buttressed by the results in Table 2 and the post hoc analyses which showed that Cr (VI) was significantly (P=0.000) better removed from the soil by cow dung treatment than by the microbial consortium. Filamentous fungi including species of Penicillium and Aspergillus are known to be capable of immobilizing Cr (VI) and other heavy metals by cell wall binding, secretion of metalbinding metabolites and intracellular uptake [34,35].
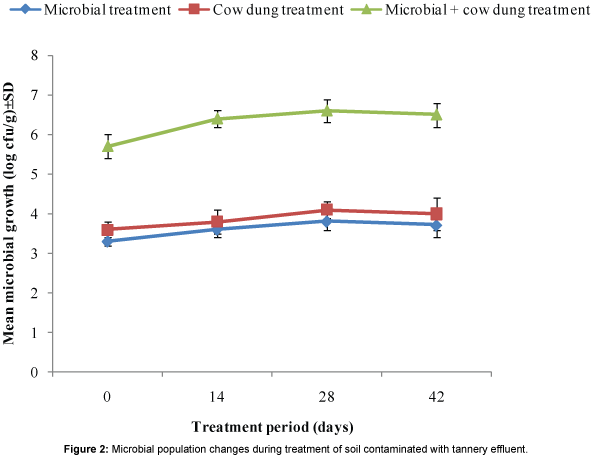
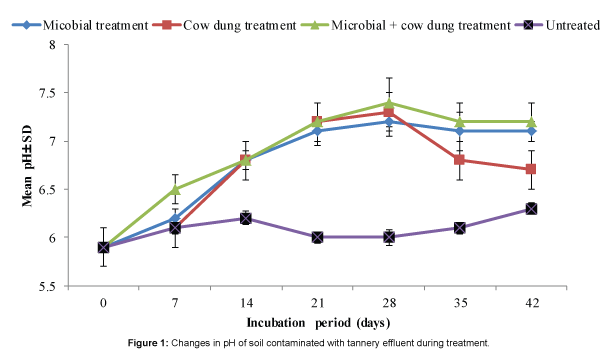
The pH of treated contaminated soils increased and peaked by the 28th day and the overall trend of the changes in pH was identical for the three treatments (Figure 1). It can be inferred that the changes in pH of treated soils when compared to untreated soil indicated metabolic activity of the microorganisms. This inference is corroborated by the pattern of changes of microbial population in treated soils (Figure 2) which tended to be identical for the three treatments and also with that of pH as shown in Figure 1. Despite the identical pattern of population changes, the microbial population in soil treated with combination of cow dung and microbial consortium was markedly higher than in soils exposed to the other treatments (Figure 2). This was expected because the starting microbial population was higher being a combination of microorganisms from the cow dung and the consortium.
The growth of maize in treated contaminated soil was significantly higher than in untreated contaminated soil (Table 4). Growth of maize in soil treated with combined cow dung/microbial consortium was the best, because it was not significantly different from growth in normal soil (Table 4). This observation is supported by the results presented in Table 5, which showed that Cr (VI) concentration in maize propagated in soil treated with cow dung/microbial consortium was significantly lower than in maize planted in untreated soil. This finding is evidence of the success of the treatment stratagem.
| Soil treatment | Plant growth index (Mean ± SD) | |
|---|---|---|
| Leaf area (cm2) | Plant height (cm) | |
| None (uncontaminated soil) | a,c80.0 ± 1.0 | a42.1 ± 1.0 |
| Microbial consortium | b,c40.2 ± 1.4 | b23.9 ± 1.0 |
| Cow dung | b,c48.1 ± 1.1 | b28.9 ± 0.8 |
| Cow dung+Microbial consortium | a78.7 ± 1.4 | a40.6 ± 0.6 |
| None (contaminated soil) | a,b,c10.5 ± 1.8 | a,b,c4.8 ± 0.5 |
| F statistics | 1342.7 (P=0.000) | 516.3 (P=0.000) |
Significant difference: from growth in uncontaminated soil, aP>0.05; bP<0.05; from growth in untreated contaminated soil, cP<0.05. NA, not applicable.
Table 4: Growth of maize in treated soil contaminated with tannery effluent.
| Tissue | Cr (VI) (mg/g) M ± SD | |
|---|---|---|
| Treated soil | Untreated contaminated soil | |
| Roots Stem Leaves | *0.006 ± 0.00 *0.004 ± 0.00 *0.002 ± 0.00 |
*2.24 ± 0.05 *2.02 ± 0.04 *1.34 ± 0.02 |
*Significant difference [(t test) P=0.000]
Table 5: Cr (VI) in plant tissue following growth in soil contaminated with tannery effluent and treated with combination of cow dung and microorganisms.
Conclusion
The results of the study showed that soil contaminated with tannery effluents can be remedied by treatment with P. aeruginosa, A. niger and P. chrysogenum, and cow dung. Cow dung is plentiful in Northern Nigeria, because it is the base of pastoralists as well as the location of the tannery industries. Farmers would therefore have access to cow dung which they can use for bioremediation of farmlands contaminated with tannery effluents. A bioremediation strategy involving application of cow dung and tested microbial consortium would need to be developed and made available to the mostly illiterate peasant farmers. A field assessment would be required to achieve this, because this study was limited to the laboratory.
References
- Hasegawa MC, Barbosa AM, Takashima K (2011) Biotreatment of industrial tannery wastewater using Biotryoshpharia rhodiria. Journal of Serbia Chemical Society 76: 439–446.
- Li M, Kai Y, Qiang H, Dongying J (2006) Biodeterioration of gallotannins and ellagitannum. J Basic Microbiol 46: 68-84.
- Prabhavathi C, Sirshendu D (2010) Treatment of Fat liquoring Effluent from a Tannery using Membrane Separation Process. Journal of Hazardous Material 176: 343-443
- UNIDO (2011) Introduction to treatment of tannery effluents: What every tanner should know about effluenttreatment. United Nations Industrial Development Organization, Vienna International Centre, Vienna, Austria.
- Rabah AB, Ibrahim ML (2010) Physico-chemical and microbiological characterization of soils laden with tannery effluents in Sokoto, Nigeria. Nigerian Journal of Basic and Applied Science 18: 65-71.
- Nachiyar CV, Rajkumas GS (2003) Degradation of Tannery and Textile Dye by Pseudomonas aeruginosa. World Journal of Microbiology and Biotechnology 19: 609-614.
- Srivastava T, Chandra H, Triphati K, Naraian R, Sahu KR (2008) Removal of Chromium (VI) through biosorption by Pseudomonas spp. Isolated from tannery effluent. J Basic Microbiol 48: 135-139
- Durai G, Rajaismman M (2011) Biological treatment of tannery wastewater: A Review. Journal of Environmental Science and Technology 4: 1-17.
- Murugan K, Al-Sohaibani A (2011) Biocompatible Removal of Tannin and Associated Color from Tannery Effluent Using the Biomass and Tannin Hydrolase Enzymes of Mango Industry Solid Waste Isolate Aspergillus candidus. Research Journal of Microbiology 5: 262-271.
- Krishna KR, Philip L (2005) Bioremediation of Cr(VI) in contaminated soils. Journal of Hazardous Materials 121: 109-117.
- Santiago M, Santhamani S (2010) Remediation of chromium contaminated soils: Potential for phyto and Bioremediation. 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia pp. 211-214.
- Zhang P, Jin C, Zhao Z, Tian G (2010) 2D crossed electric field for electrokinetic remediation of chromium contaminated soil. J Hazard Mater 177: 1126-1133.
- Dhal B, Thatoi HN, Das BD, Pandey BD (2013) Chemical and microbial remediation of hexavalentchromium from contaminated soil and mining/metallurgical solid waste: A review. Journal of Hazardous Materials 250: 272-291.
- Srivastava S, Thakur IS (2006) Biosorption potency of Aspergillus niger for removal of chromium (VI). Current Microbiology 53: 232-237.
- Jayanthi M, Kanchana D, Saranraj P, Sujitha D (2014) Bioadsorption of Chromium by Penicillium chrysogenum and Aspergillus niger Isolated from Tannery Effluent. International Journal of Microbiological Research 5: 40-47.
- Awasthi G, Chester A, Chaturvedi R, Prakash J (2015) Study on Role of Pseudomonas aeruginosa on Heavy Metal Bioremediation. International Journal of Pure and Applied Bioscience 3: 92-100.
- Balaji R, David E (2016) Isolation and Characterization of Cr (VI) Reducing Bacteria and Fungi Their Potential Use in Bioremediation of Chromium Containing Tannery Effluent (Ambur and Ranipet, Vellore dist, Tamilnadu). Advanced research Journal of Life Sciences 2: 1-4.
- Okolo JC, Amadi EN (2004) Optimising crude oil biodegradation in a sandy loam soil using a mixture of cow dung and poultry manure. International Journal of Agriculture and Rural Development 5: 69-76.
- Adekunle IM (2011) Bioremediation of soils contaminated with Nigerian petroleum products using composted municipal wastes. Bioremediation Journal 15: 230-241.
- Saxena V, Mishra G, Saxena A, Vishwakarma KK (2013) A comparative study on quantitative estimation of tannins in Terminalia chebula, Terminalia belerica, Terminalia arjuna and Saraca indica using spectrophotometer. Asian Journal of Pharmaceutical and Clinical Research 6: 148-149.
- Kobayashi F, Maki T, Nakamura Y (2012) Biodegradation of phenol in seawater using bacteria isolated from the intestinal contents of marine creatures. International Biodeterioration and Biodegradation 69: 113-118.
- Environmental Agency (2010) The determination of easily liberated sulphide in soil and similar matrices. Environment Agency Head Office, Rio House, Waterside Drive, Aztec West, Almondsbury, Bristol BS32 4UD.
- Lazo P (2009) Determination of Cr(VI) in environmental samples evaluating Cr(VI) impact in a contaminated area. Environmental Application & Science 4: 207-213.
- Vidyalakshmi R, Sridar R (2007) Isolation and Characterization of Sulphur Oxidizing Bacteria. Journal of Culture Collections 5: 73-77.
- Behera BCM, Patra S, Dutta SK, Thatoi HN (2014) Isolation and characterisation of sulphur oxidising bacteria from mangrove soil of Mahanadi River Delta and their sulphur oxidising ability. Journal of Applied & Environmental Microbiology 2: 1-5.
- Yu C, Wang Y, Guo T, Shen W, Gu M (2012) Isolation and identification of ammonia nitrogen degradationstrains from industrial wastewater. Engineering 4: 790-793.
- Mwinyihija M (2010) Ecotoxicological Diagnosis in the Tanning Industry. Springer pp. 17-35.
- Basha KM, Rajendran A, Thangavelu V (2010) Recent advances in the biodegradation of phenol: A review. Asian Journal of Experimental Biological Science 1: 219-234.
- Kotresha D, Vidyasagar GM (2008) Isolation and characterization of phenol-degrading Pseudomonas aeruginosa MTCC 4996. World Journal of Microbiology and Biotechnology 24: 541-547.
- Kraus TEC, Randy A, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems- a review. Plant and Soil 256: 41-66.
- Cho K, Hirai M, Shoda M (1992) Degradation of hydrogen sulfide by Xanthomonas sp. strain DY44 isolated from peat. Applied and Environmental Microbiology 58: 1183-1189.
- El-Bestawy E, Al-Fassi F, Amer R, Aburokba R (2013) Biological treatment of leather-tanning industrial wastewater using free living bacteria. Advances in Life Science and Technology 12: 46-65.
- James BR, Bartlett RJ (1983) Behaviour of chromium in soils. VII. Adsorption and reduction of hexavalent forms. Journal of Environmental Quality 12: 177-181.
- Dias MA, Lacerda ICA, Pimentel PF, de Castro HF, Rosa CA (2002) Removal of heavy metals by an Aspergillus terreus strain immobilized in a polyurethane matrix. Lett Appl Microbiol 34: 46-50.
- Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnology Progress 11: 235-250.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14419
- [From(publication date):
July-2016 - Apr 16, 2025] - Breakdown by view type
- HTML page views : 13127
- PDF downloads : 1292