Review Article Open Access
Biomimetic Single and Multi-Lumen Bilayer Design of Electroconductive Nerve Grafts for Neuroengineering
Cameron H Menzies1, Antonio Lauto2, Lloyd Mirto2, Ben Van Gogh1, Andrew Ruys1, Sri Bandyopadhyay3, Paul Carter4 and Philip Boughton1*
1Biomedical Engineering, AMME School, The University of Sydney, Sydney, Australia
2Medical Science SoSH, University of Western Sydney, Sydney, Australia
3School of Materials Science & Engineering, University of New South Wales, Sydney, Australia
4Cochlear Pty Ltd, Sydney Australia
- Corresponding Author:
- Cameron H Menzies
The University of Sydney, Australia
E-mail: cmen7987@uni.sydney.edu.au
Received date June 03, 2013; Accepted date July 14, 2013; Published date July 20, 2013
Citation: Menzies CH, Boughton P, Lauto A, Mirto L, Bandyopadhyay S (2013) Biomimetic Single and Multi-Lumen Bilayer Design of Electroconductive Nerve Grafts for Neuroengineering. J Biomim Biomater Tissue Eng 18:105. doi: 10.4172/1662-100X.1000105
Copyright: © 2013 Menzies CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
A versatile nerve graft was developed for use in peripheral nerve regeneration. Existing techniques such as autography require additional surgery, while current synthetic nerve grafts are only capable of facilitating neuroregeneration across small lesions (<5 mm). Electroconductive polymers, hydrogel and composite systems were reviewed, to help develop a biomaterials basis for the design. A novel configuration for an electroconductive nerve scaffold has been proposed, incorporating an insulated multi-lumen design for re-connecting larger nerve gaps. A single open-lumen conduit and a more advanced multi-channel design with insulating sheaths were fabricated in order to mimic the fascicular architecture seen in peripheral nerves. The scaffold employed a synthetic biomaterial composite of polycaprolactone matrix filled with functionalised Multiwalled Carbon Nanotubes (f-MWCNTs). The composite suspension was electrospun under the influence of 5kV electric field. 80mm single and multi-lumen scaffolds were formed that were then capable of being securely sutured to kangaroo tail spinal cord without tearing. The scaffolds were tension tested and found to have a Young’s Modulus of 15.7 MPa ± 2.98 (p=0.95), and a tensile strength of 1.172 MPa ± 0.16 (p=0.95) and 1.375 MPa ± 0.08 (p=0.95) for the single and multi-channel grafts respectively. A preliminary neurotoxicity study using N2A cell-line showed strong cell-scaffold adhesion and viability.
Keywords
Peripheral nerve regeneration; Nerve repair; Grafting; Nerve guide conduit; Electrospinning; Neuroengineering; Poly(caprolactone); Carbon nanotubes; Multi-channel nerve guide
Introduction
Thousands of people sustain peripheral nerve injuries (PNI) and spinal cord injuries (SCI) each year. The nervous system is damaged primarily by traumatic injury, surgery, and cumulative trauma disorder, e.g. carpal tunnel or tumour. In the United States, 360,000 people suffer from upper extremity paralytic syndromes on an annual basis, whereas in Europe more than 300,000 cases of peripheral nerve injuries occur annually [1]. Traumatic injuries occur due to compression, crush, stretch and laceration of the nervous tissue, resulting from an injury such as a motor incident, sporting injury, or traumatic fall. Trauma accounts for about 87% of all peripheral nerve injuries, while surgery accounts for roughly 12% (often as a result of tumour resection). Peripheral nerve injuries occur with surprisingly high frequency, reported in as many as 5% of all trauma patients [2].
Damage to the peripheral nervous system or spinal cord has severe consequences, including paralysis or major loss of sensory/motor function, as well as the stress on the mental health and financial situation of the patient and family. Less than 1% of people with an SCI fully recover, and the lifetime cost of a quadriplegic is estimated at greater than $1 million [3]. PNI is often less severe, but in many cases can result in significant loss of function or neuropathic pain, and depending on the severity may require extensive surgery. Autograft surgery is hardly an ideal treatment, due to nerve misalignment, loss of function at the donor site, and increased risk of infection. Furthermore, despite modern surgical techniques and equipment, functional restoration of the lesion is often inadequate, usually only 50% of cases exhibit good restoration of function [4].
At the present time, clinically available nerve grafts each have fundamental limitations, and usually produce substandard results when compared to autografts. Hence, they are typically used in a clinical setting only if the patient cannot undergo autograft surgery [5]. (Detailed descriptions of the mechanisms behind nervous repair can be seen for the spinal cord [6] and peripheral nerves [7]).
Clearly, there is a considerable need for research in the field of biomedical engineering to develop novel grafting strategies to improve the function of patients suffering debilitating neural injuries. Modern strategies employ biocompatible and biodegradable materials as scaffolds for tissue adhesion and growth, as well as for vehicles of molecules and stem cells that stimulate neuron growth and communication. This recent development in neuroengineering offers a promising alternative to conventional treatments, which removes the sacrifice of a healthy nerve (in cases of autograft surgery) and supports and guides the axons during their growth, while avoiding scar tissue infiltration into the site of injury.
Electroconductive scaffolds have recently been considered. The use of electric current to stimulate neural cell differentiation, proliferation, adhesion and neural networking has the potential to facilitate nerve regeneration at an exceptional rate. Direct-current electric fields are present in all developing and regenerating animal tissues. It has been shown that endogenous electric fields (EF’s) are present at the neurogenesis stages of embryonic development [8], and are involved with setting up a 3D structure of the nervous system [9]. However, their existence and potential impact on tissue repair and development are largely ignored [10].
Electrospun nanofibrous scaffolds have recently received much attention in tissue engineering due to the resemblance with collagen fibres in extracellular matrix, high surface area to volume ratio, their highly porous yet tightly woven structure, and the ability to be manipulated in terms of mechanical and degradable properties [11]. Poly (caprolactone) and carbon nanotubes were electrospun and used in this research for their micro scale architecture, in a conductive polymer composite.
Conductive polymer composites (CPCs) are a blend composed of an insulating matrix and electrical conductive fillers. PCL was chosen to be used as the insulating matrix, due to its properties described earlier, such as its ability to facilitate more infiltration of neural tissue ingrowth, higher rate of myelination of fibres, and less infiltration of inflammation molecules and cells [12]. PCL has also been shown to have great biocompatibility properties, slow degradation rates that could match the rate of nerve regeneration, and the ability to be electrospun into nanowires without being damaged [13]. Furthermore, PCL is a component of the FDA-approved nerve guide, ‘Neurolac’, hence its peripheral nerve regenerative capacity is well documented [14].
Carbon nanotubes (CNTs) were chosen as the electroconductive filler, due to their excellent electrical conductivity, strong mechanical properties, and similar nanoscale dimensions to neurites. CNTs have demonstrated that they are not neurotoxic, can be modified to improve their neurite growth ability [15], have enhanced Neural Stem cell (NSC) differentiation and excitation in vitro [16]. They have also shown no toxicity to mice injected with multi-walled cabon nanotubes (MWCNTs) in vivo [17], and can be modified such that they are entirely cleared from the body of a mouse within 3 hrs [18]. Furthermore, Zeng et al. showed that PCL maintains its well defined biodegradability profile when mixed with CNTs, meaning the PCL/CNT composite is feasible in this regard [19]. COOH-MWCNTs were chosen because they have shown significantly lower cell production levels of IL-6 (an inflammatory cytokine) production when compared to pristine- MWCNTs and OH-MWCNTs [20], and have the best effect on cell biocompatibility, proliferation, or differentiation when compared to OH-MWCNTs, SWCNTs, and MWCNTs [21].
Materials and Methods
Materials
The PCL was a high molecular weight linear polyester derived from caprolactone monomer, having a molecular weight of 80,000 (Capa® 6800), purchased from Perstorp UK Ltd.
The CNTs were purchased from Cheap Tubes Inc. (VT, USA), in collaboration with Dr Sri Bandyopadhyay, associate Professor of Nanotechnology; Polymer/ Composites and Fly Ash Specialist at UNSW. The COOH-MWCNTs were <8 nm in diameter, and 10-30 μm in length and for the rest of this report will be referred to as functionalised- MWCNTs, or ‘f-MWCNTs’.
Methods
Conductive polymer composite production: The CPC was created firstly by creating a PCL solution in a 2 L jar. It came in 3mm pellets and was stirred and heated (50°) with acetone to create the solution in the weight ratio of 1:4 (PCL : Acetone). Once the solution was consistent, f-MWCNTs were added.
The solution was made so the composite would be 20% wt. f-MWCNT (80% wt. PCL), and then diluted down into 10% and 5% wt. f-MWCNT composite. All 3 concentrations were kept in separate jars so experiments could be performed on the different composites. The jars were maintained at 20%wt. composite with respect to the acetone solvent maintaining 80% wt.
In accordance with the CNT Material Data Safety Sheet (MSDS) [22], several safety issues were addressed while adding CNT to the solution. Lab coat, protective gloves, safety goggles, particulate respirator and full face mask were worn to protect hands, eyes, and respiratory tract from particulate CNT matter. A local exhaust system kept the room ventilated, and free from airborne particles.
Microscopic analysis: The solution was then examined using a Leica DMRXE optical microscope, and digital image analysis with software ‘Leica QWin Plus’ to study the solution. The software allows point-to-point calculation on a microscopic scale, which was used to obtain size data for the f-MWCNT particles in solution.
Dispersion of f-MWCNTs: Initial microscopy of the composite showed agglomerated f-MWCNT in small clusters and bundles, which meant they didn’t possess a fibrous morphology in the composite solid. This is a significant problem because the fundamental properties that are trying to be incorporated into the composite (i.e., high electrical conductivity and strong mechanical properties), become severely reduced. To counteract this, f-MWNT must be individually arranged in an electrically conductive network within the composite, and hence released from the agglomerates whilst in solution. In order to achieve this, shear force must be applied to the agglomerates (which can be approximated as spherical particles in a Newtonian fluid) [23].
In order to supply this force, 3 methods were tried:
Stirring/heating: firstly by hand vigorously using a stirring rod, and secondly by magnetic stirrer built into the hotplate, used at a rotation of ˜50 rpm for 12 hours, at 60°.
Ultrasonication: Ultrasonication is the action of applying ultrasound energy to a sample to cause agitation in particles, and was employed in this case to break up agglomerated f-MWCNTs. It is commonly used for this exact purpose [20,24-26], and is probably the most widely used and most effective mechanical technique [27]. The ultrasound was supplied by a Vibra cell 501 model, at 20 kHz and 500 W (Sonics and Materials Inc.), for 3s on, 1s off, total duration 40 mins. The ultrasound was delivered to the solution via a titanium tipped probe of 10mm diameter.
Electrospinning: can be used to disperse CNTs in electrospun fibres [28]. Due to the high elongation of the polymer jet during electrospinning, the CNTs tend to orient along the fibre axis and are embedded in the fibre core [29].
As described earlier, electrospun non-woven materials have small pore size, yet high overall porosity, and high surface area; therefore, they can be used in a wide variety of biomedical applications, especially in scaffolds in tissue engineering. Because there was no access to a commercial electrospinning device on campus, a prototype electrospinning machine was designed and built with the aid of Ben van Gogh (Honours student, Bachelor of Biomedical Engineering, University of Sydney). It was built using a Van de Graaff generator as the power source, connected to a needle used to charge the polymer or composite. An empty aluminium red bull can acted as the collector, which was earthed via an alligator clip, and the whole design was housed in a polycarbonate frame.
The voltage generated was sent through a high quality, thick gauge wire to the syringe tip, where it was taped on. The needle used was a 20 gauge (0.9 mm outer diameter, 0.6 mm inner diameter) for the initial PCL electrospinning, and afterwards a copper tube (2 mm outer diameter, 1.1 mm inner diameter) replaced the needle for electrospinning of the composite.
Electrospinning of PCL solution: The electrospinning of PCL solution was initially tried to quickly calibrate and optimise the machine for use with the composite material; however it took multiple trials before this was achieved. It was initially set up so the syringe was filled with PCL 20%wt solution. After turning on the motor and Van de Graaff generator, the syringe was squeezed by hand at roughly 250 μL/ min. The room was at standard temperature and pressure (STP). The needle tip was placed 18 cm from the can (which rotated at 1 or 5 rpm), and a voltage of approximately 5 KV was generated across this gap by the Van de Graaff generator.
Electrospinning of f-MWCNT/PCL composite: Electrospinning of the composite followed much the same procedure as the straight PCL solution, but was only possible at closer distances to the collector, namely around 8 cm. Other experimental parameters had to be modified quite significantly as well, explained in the results/discussion with more detail.
Electrical conductivity testing of the f-MWCNT/PCL composite: The three composites (5,10, and 20% wt. f-MWCNT) were all tested for electrical conductivity using a multimeter with a range of resistance from 20 Ω to 2 MΩ, similarly to an earlier process used in an experiment of comparable nature [30].
The conductivity of the composite was then calculated using the following formula:
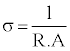
σ - Material conductivity in Siemens per meter (S/m - or more commonly S/cm for this application). R - resistance in Ohms, A - cross sectional area, and l - distance between the 2 aluminium plates.
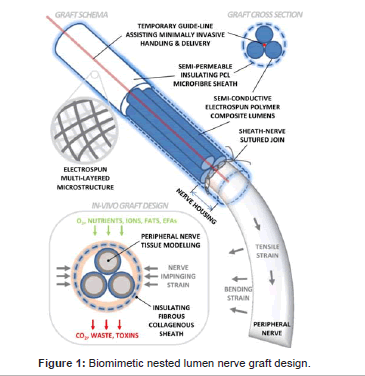
Design of novel electroconductive scaffold prototype: 2 designs were manufactured, a large, single lumen design, and a multi-channel approach enclosing 4 smaller sized lumens bound together with an external layer. The prototypes were made using the electrospun material, and were 6-8 mm thick. The designs employed a novel idea regarding nerve grafts, an outer insulating layer and an inner electroconductive layer: a structure yet to be considered in neuroengineering.
Tesile testing of the scaffolds: Tensile properties of the 2 different scaffold types were investigated using a 6-axis Bose spine simulator with a 250 N load cell. Three scaffolds of each type were super-glued at each end between 2 small pieces of cardboard (2 cm × 3 cm) before placing in position. This functioned not only to protect the scaffolds from possible damage caused by the serrated grippers, but also increased the material’s yield strength at the grips (a result of the super-glue), ensuring the scaffolds would exhibit mid-substance failure instead of at the grips. The dimensions of each sample were then accurately measured using vernier callipers for calculations for stress and strain. The samples were then tested to failure under tension, at a rate of 1 mm/sec. Multiple sensors of the machine were linked to a computer to interpret the data using Win-Test v.4 software.
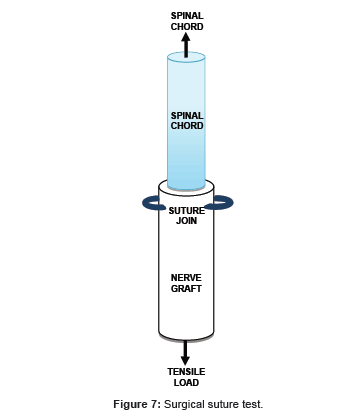
Surgical suture test
A kangaroo tail was dissected to allow access to the spine. A subsection of 10 cm was isolated containing 2 vertebrae, and then further stripped to reveal the spinal cord. The spinal cord was then sutured to one end of a scaffold sample to simulate a surgical procedure, and weighted to test its structural integrity.
Neurotoxicity study: In this study, the electrospun nanofibre mats were tested for neurotoxicity using Neuro-2A (N2A) cells. N2A cells are a mouse neuroblastoma cell line widely used to study neuronal differentiation, neurite growth, synaptogenesis and signalling pathways [31]. The in-vitro experiment was developed to analyse the innate neurotoxicity of the electrospun f-MWCNT composite and PCL nanofibres, and the ability of the scaffold morphology to promote cell adhesion. The experiments were performed at the UWS Campbelltown School of Biomedical and Health Sciences, with the aid and collaboration of Dr Antonio Lauto and Ph.D. candidate Lloyd Mirto.
Cell culture and sterilisation: The N2A cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), with high glucose, 10% fetal calf serum, penicillin-streptomycin, and L-glutamate, and kept in an incubator for the duration of the experiment at 37° and 5% CO2. Nanofibre samples were cut into 1 cm × 1 cm squares, and then placed in a heat sealed packet, then sterilised by soaking in 70% ethanol for 24 hours. The packet used was semi-permeable, allowing the ethanol to evaporate, and hence maintaining a sterile environment for the samples for transportation.
The nanofibre samples were then prepared into triplicates (3 × f-MWCNT composite, 3 × pure PCL), and placed individually into 24-well plates. They were sealed to the bottom of the well using the same PCL/acetone adhesive utilised for the inter-layer sealant of the prototype scaffolds of Section 4.5. Control wells consisted of unaltered wells, and wells containing the PCL/acetone adhesive. The samples/ controls were then seeded with the N2A cells at a cell density of 16.9 ×104, and allowed to grow a full 24 hours before analysis.
Neurotoxicity and cell adhesion analysis: Initially, 10 μL of the media (supernatant) in each well was collected, and mixed with trypan blue in the ratio 1:1. Using a haemocytometer, cells are counted according to whether they are stained by the trypan blue, allowing the ratio of viable: non-viable cells in the supernatant to be calculated; a good indicator of the viable cells to adhere to the test sample.
The in-well contents were then collected, using trypsin - an enzymatic protein which destroys the cells binding proteins, releasing viable cells from their site of adhesion. The trypan blue staining/ haemocytometer counting technique was again used to calculate the viable: non-viable cell ratio, however in this context is used to calculate cell viability of the sample - a good indicator of neurotoxicity.
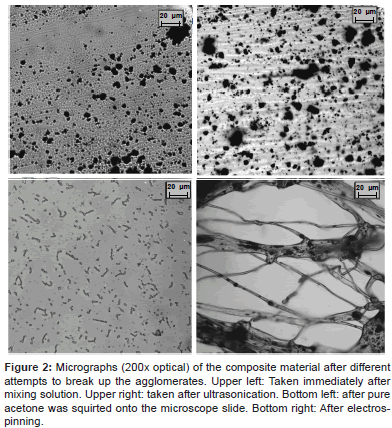
Figure 1: Micrographs (200× optical) of the composite material after different attempts to break up the agglomerates. Upper left: Taken immediately after mixing solution. Upper right: taken after ultrasonication. Bottom left: after pure acetone was squirted onto the microscope slide. Bottom right: After electrospinning.
Results and Discussion
Dispersion of CNT agglomerates
All methods of dispersing agglomerated f-MWCNT proved unsuccessful. This is a problematic for the successful formation of conductive scaffolds (as described before), because the f-MWCNTs lose their fibrous morphology, and accordingly, the fundamental characteristics and purpose of their incorporation (excellent electrical conductivity, strong mechanical properties, and nanoscale dimensions similar to neurites). Figure 2 displays 4 microscope shots (all at 200×) taken at various points in the composite’s production. They show the small agglomerates of f-MWCNT, and the unsuccessful results after multiple attempts at dispersion. A comparison between the pictures allows firstly an understanding of what exactly is meant by ‘agglomerate’ in the material, and secondly an appreciation of the difficulty in breaking them apart.
The ‘agglomeration’ is caused by strong inter-molecular forces that originate from entanglements, electrostatic attraction, and high van der Waals forces of the f-MWCNTs [23]. Due to the nature of van de Waals forces, they are dependent on the distance between 2 particles, becoming much more intense when closer. This has a very important implication: once the initial f-MWCNT agglomerates have formed, they become very difficult to disrupt because the forces are harder to overcome at shorter distances. For the dispersion method, the external forces have to be strong enough to break or disrupt the agglomerates. As seen, the stress requirement needed to break agglomerations was not met by all 4 methods implemented. Even the combination of tensile, gravitational, aerodynamic, rheological, and inertial forces generated by electrospinning [32] were not enough to achieve a properly dispersed composite.
The image corresponding to Figure 2: bottom left was taken after a relatively large volume of pure acetone was squirted onto the microscope slide, and covered with a cover slip. This was performed purely out of curiosity, but had a highly interesting result. It shows the f-MWCNTs evenly dispersed - the desired effect of the dispersion methods. The large addition of acetone lowered the viscosity significantly, hence lowering the shear needed to disperse the agglomerates. Accordingly, the even dispersion of the f-MWCNTs seen here presented a good representation of what the composite should look like after a successful dispersion. The diameters of the structures seen are ≈1 μm, while the diameters of individual fibres are <8 nm, thus it is likely that in the most singular state, the f-MWCNTs form coil-like structures via intramolecular forces.
Interestingly, after this solution was left to dry, the solution re-agglomerated. This secondary agglomeration must have formed immediately after the viscosity of the fluid reached an innate critical value, and has also been observed by others [33]. This observation is important because it draws the conclusion that using dispersion techniques for an extended amount of time, or at an increased intensity cannot inhibit the formation of secondary agglomerates to the point of complete dispersion. This is an important factor to consider while preparing CNT nanocomposites. It also supports the concept of an ‘inherent agglomeration factor’ that can differ significantly based on the specific characteristics of the CNTs themselves, such as a functional group addition.
It has been previously shown that COOH modified CNTs do not show even dispersion readily [34], supporting the findings in this report. For future studies, functionalised CNTs that have little or no ‘inherent agglomeration factor’ should be used to avoid this phenomenon.
Electrospinning
The initial tests involving PCL went very well, considering the makeshift nature of the apparatus. The electric field generated by the Van de Graaff generator was sufficient in producing the Taylor cone, and attracted the nanofibres to the rotating drum properly. After some configuration, the optimum specifics were found to be 23 cm above the can, and 5 rpm, allowing the yield to easily peel off the collecting drum.
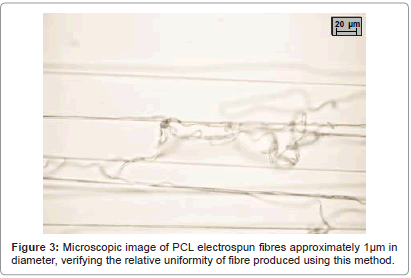
Microscope analysis showed that the fibres created were fairly consistent (Figure 3). However, we were unable to generate the uniformity seen in many another study [35]; >90% of the fibres were ranging from 1-3 μm in diameter, concluding that this method was viable.
After the supposed optimisation of the electrospinning machine, nanofibre mats were attempted to be created from the composite solution. This proved much more difficult than initially anticipated after the rapid success of the pure PCL electrospinning. Upon the initial trial using the 20 gauge needle (inner diameter=0.6 mm) with 20%wt f-MWCNT, it became instantly clogged; preventing any electrospinning from happening. Similarly results were found when tested with 10% and 5% wt. solutions. To solve the problem, the needle was replaced with a 1.1 mm inner diameter copper tube purchased from Hobbyco.
The new inner diameter allowed the solution to run through it easily enough, but the small droplet didn’t make a fully formed Taylor cone. Instead, small, thick droplets (0.5-1 mm in size) built up and when heavy enough could drop from the surface and land on the collector (seen in Figure 1 - left). They had a ‘beaded’ formation with some resemblance to a fibre at one end. When a small amount of acetone was added to slightly dilute the solution, the surface tension was not great enough to hold the solution at the end of the needle tip, causing it to splatter all over the machine.
Eventually, after much trial and error, it was found that only 5% wt. f-MWCNT composite solution could be electrospun, and even then was highly sensitive to viscosity. The 10% and 20% solutions were not able to be electrospun using this apparatus, even after painstakingly varying all parameters many times. The space between the copper tube and the collecting drum was also decreased to just 5 cm, to allow an increase in electric field concentration, which was the crucial factor that allowed only the 5% composite to form a proper Taylor cone and hence, electrospun fibres.
Electrical conductivity testing of f-MWCNT/PCL composite
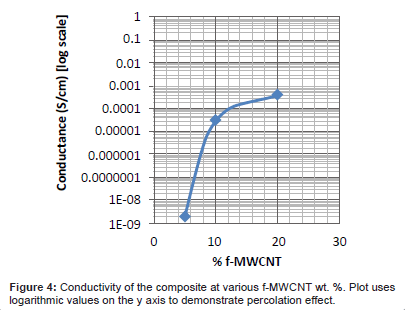
The material conducted electricity at the 10%, and 20% f-MWCNT concentrations, but not at 5%. This was most likely a result of the agglomeration of the f-MWCNT particles, as explained earlier. The material reached percolation at some point between 5%-10%wt. This was particularly irritating because the composite was only able to be electrospun at 5% wt. Hence, the electrospun nanofilm created with f-MWCNT was not able to conduct electricity. Conversely, the 10% and 20% wt. batches were able to conduct electricity (see Figure 4 for values).
Because the conductivity of the composite has a substantial descent somewhere between 5 and 10%, it is evident that electroconductivity breaks down somewhere between these values. This can be explained by percolation theory, whereby at lower concentrations, the f-MWCNTs are rarely touching each other, and therefore cannot form conductive pathways through the material, due to the majority of the composite being insulating PCL matrix. Once a certain value of concentration is met, there are enough f-MWCNTs in the composite for electrons to follow certain conducting paths in the matrix, and percolation occurs, leading to a sudden rise in conductivity. The values of conductivity found in the experiment are notably in the semiconductor range of 10-8 - 103 S/cm [36].
Neurotoxicity study
In order to evaluate the ability of the two types of electrospun nanofibre film’s ability to facilitate growth and adhesion of a neural cell line, Neuro-2A (N2A) cells were cultured on electrospun samples of both f-MWCNT composite and PCL, and are a cell line widely used in studies relating to neuronal development [31]. The results obtained from the neurotoxicity experiments were highly encouraging, showing minimal neurotoxicity and strong binding of cells.
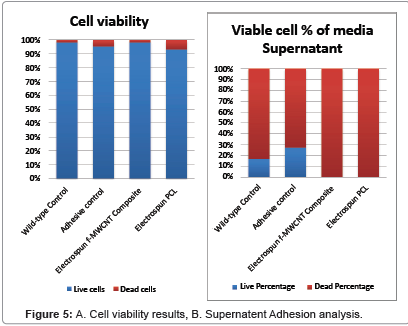
From the experiments we were able to tell that the cells were healthy in all controls (Wild type and PCL adhesive), and were able to adhere to both electrospun materials well. The supernatant adhesion analysis involves counting of viable and non-viable cells floating around in the media. Healthy cells have adhesive proteins on their membrane which allow cell binding. To find healthy cells in this particular test would indicate that cells were not able to bind convincingly to the electrospun materials. However, as seen in Figure 5, effectively no viable cells were found in the supernatant. Therefore both electrospun materials facilitated strong adhesion of the cells.
The viability count displays the high percentage of viable cells recovered after the in-well contents were collected. A high cell viability is usually considered as >90% [37], meaning the cells were able to grow and proliferate readily in the environment. Comparing the f-MWCNT (98%) to the PCL (93%), it can be seen that the cells were able to grow slightly better on the f-MWCNT material, indicating it is a better environment in order to maintain healthy cells. In comparison to the Wild type control, it is clear that both electrospun materials are not neurotoxic.
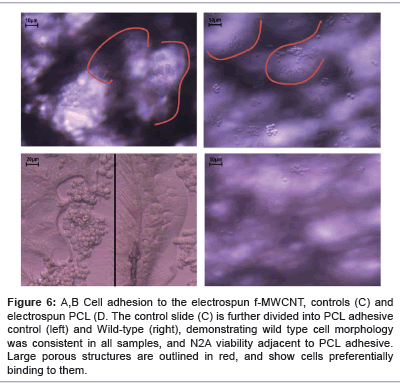
Figure 6 shows microscopic images taken of the 2 electrospun materials, and the controls, and summarises the relevant information for this particular study. Shown in red are large porous structures created by the electrospun nanofibres of f-MWCNT composite. Evidently, the N2A cells preferentially bind in these pores. The electrospun PCL however, does not display the same results. As seen (Figure 6 bottom right), the cells are significantly lower in numbers, and have not shown the same adhesion to that of the f-MWCNT composite. This indicates that the electrospun f-MWCNT composite had no neurotoxic effects, and significantly increases the adhesion and viability of cells that it harbours.
Hence, this experiment has verified the biocompatibility criterion regarding a major aspect of the peripheral nerve’s local environment, and shown cells adhering deep within the interconnected pores throughout the sample. This is a significantly encouraging outcome in terms of this novel design of the nerve scaffold.
Design of novel electroconductive scaffold prototype
The prototypes featured 2 separate variations on scaffolds for use in neuroengineering, single lumen and multi-lumen variants. Both designs incorporated an inner layer comprised of f-MWCNT composite to allow electrical conductivity and enhance neuron regeneration, while the outer layer utilised pure PCL for its elastic and insulation properties.
Figure 5: Cell adhesion to the electrospun f-MWCNT (top left and right), electrospun PCL (bottom right), and controls (bottom left). The control slide (bottom left) is further divided into PCL adhesive control (left) and Wild-type (right), which simply demonstrates that wild type cell morphology was consistent in all samples, and that the N2A cells were able to grow in the presence of the PCL adhesive. Large porous structures are outlined in red, and show the cells preferentially binding to them. Scaling is slightly different for each picture, but cell size can be used as an approximate scale (Cells are roughly 10μm in diameter).
Inner conductive layer: The inner surface contained f-MWCNTs and was specifically tailored for the purpose of creating a niche surface for the neurite to grow and extend on. For further research this may involve the use of longitudinally aligned fibres. The electroconductive polymer surface was designed to enhance the recovery and extension of the neuron, improve the chances of re-innervating the neuron-specific target, as well as inducing the formation of complex neural networks [38].
The electroconductivity of the inner layer may also enable the use of an applied electrical current that runs from an electrode in one end to the other across the site of injury.
Outer insulating layer: The outer layer of the design is comprised of pure electrospun PCL, incorporated as the outer surface for a number of reasons:
Firstly, it is used simply to limit the amount of f-MWCNTs entering the body, as the long term effects of CNTs are not fully understood.
Functionally, the PCL layer was incorporated to the design because of its highly elastic nature when compared to the f-MWCNT composite. Hence it should help maintain the lumen/(s) hollow by counteracting any compressive force with an elastic restoration force.
The PCL should also act as an insulator for electrical activity in the internal conductive zone. This would not only act to restrict potentially harmful electric current leaking into the body when an external stimulus is applied, but would also enhance the effect of endogenously generated micro-currents by containing them to the localised site of neuroregeneration.
Single lumen scaffold: Single lumen nerve conduits are designed to influence axon growth to the distal stump, while preventing neuroma formation and infiltration of fibrous tissue. Single lumen devices are also thought to act as a distinct vessel, effective at localising Schwann cells and macrophages, and allowing trophic factors to accumulate. Generally speaking, it maintains the injured site inside a chamber, acting as a separated environment from the rest of the body [39]. The scaffolds produced were relatively strong, and maintained their shape adequately. When pinched between 2 fingers the scaffolds would restore to their original formation. When looking down into the lumen, it was clear that all lumens were fully expanded, and did not collapse after initial manufacture. The multi-lumen scaffolds were equally as robust.
Multi-lumen scaffold: The multi lumen scaffold is designed with 4 micro-channels that run the length of the structure. Several key reasons are behind the multi-lumen device:
1. The micro-channels through the entirety of the graft serve as a biomimetic system of the multi-channel fascicular architecture and fibrous extracellular matrix found in native nerve. It is therefore designed to influence directed growth of neurons in a longitudinal manner.
2. Because they are created entirely with electrospun materials, the highly porous nature of the channels can facilitate transfer of important endogenous neurotrophins and neurotropins between each other.
3. Furthermore, the multi-lumen design can significantly decrease the chance of luminal collapse that is possible in an open lumen conduit [40].
Tensile testing of scaffold prototypes: As expected, the two scaffold designs exhibited similar results when the values for stress and strain were compared however the multi-lumen scaffolds had a slightly higher tensile strength, and showed a slightly different profile after the ultimate stress was reached. This was presumable a result of the individual lumens in the multi-channel device failing independently (possibly represented by the ‘stepping’ effect seen in both blue graphs), causing a long, drawn out strain curve. While the test was being carried out, a definitive sound made it clear when each of the thin channels failed. In every sample, the f-MWCNT composite failed before the PCL. This was expected, as the f-MWCNT addition is known to increase strength, but decrease elasticity.
Tensile strength: The average tensile strength was expectedly slightly larger in the multi-lumen design compared to the single lumen (refer to Figure 6), simply because slightly more electrospun f-MWCNT composite was used to manufacture the 4 central lumens (1.375 MPa and 1.172 MPa respectively). These values are slightly less than ideal, but still coincide very nicely with the theoretical values of peripheral nerves obtained in previous animal studies: ≈ 2-10 MPa [41,42].
Young’s modulus: It was found that both designs had the same elastic region. The Young’s Modulus of a typical nerve has been shown as 0.4-0.7 MPa in an animal model [41]. The value for the nerve grafts designed were 15.7 MPa, hence substantially larger than values found in previous animal studies.
In practise, this means that the synthetic nerve graft can tolerate significantly more stress through its structure than a physiological nerve. For example, if either scaffold design were implanted in vivo, physiological tension applied across the nerve would be distributed across the synthetic graft, possibly having a protective effect on the delicate nerve fibres in its centre that are undergoing regeneration.
Porosity: The electrospinning machine produced ultra-thin fibre meshes that had a high surface area, and a high porosity with interconnected pores. The rough size of the pores could be measured using images taken during optical microscopy. It was found that the pore size had a large range in size, anywhere from 20-300 μm. Limitations of the electrospinning process played a significant role in this, specifically the insufficient control over the fibre diameter and the morphology of the film, usually causing non-uniform fibre meshes.
It is well understood that the porosity should approach a value sufficient for nutrient and gas exchange, retaining growth factors, and prevention of scarring. A value of 10-38 μm pore size has been proposed [43]. The lower limit should go no lower than 5-10 μm, as capillaries need to infiltrate and provide nutrients to the regenerating tissue. Likewise, it must not be too porous, as fibroblasts (possible responsible for scar tissue formation) can grow and proliferate in pores 15-100 μm [44]. Thus, an ideal size exists.
The maximum limit of pore sizes observed in material created using the electrospinning machine was extremely large, and most likely permissive to scarification, however, the high porosity facilitated the seeding and strong adhesion of N2A cells, validating the high surface area morphology caused by electrospinning. In the final design of the conduit, the process of rolling multiple layers tightly onto each other was devised as mitigation. In vivo tests should be performed to test the results.
Surgical suture test: The trial was designed as a test to show the scaffold could meet some basic surgical requirements. Firstly, suturing of the scaffold to the spinal cord showed that the scaffold could be sutured to a damaged nerve with relative ease, and maintains structural integrity when used for its intended purpose. Secondly, a weight of 1.2 N was hung from the bottom of the scaffold to prove that the sutures and scaffold would remain stable under some tensile load similar to what one might find during manipulation in an actual surgery.
Conclusion
The scaffold designs fabricated here have shown some promising characteristics, including a novel feature that employs an insulating layer around the scaffold. It also satisfies some surgical, tensile and neurotoxic requirements. The scaffold is recommended for further research including in vivo testing, an optimised separation technique of agglomerations, and the use of CNTs that allow for rapid biodegradability and minimal agglomeration.
References
- Chiono V, Tonda-Turo C, Ciardelli G (2009) Chapter 9: Artificial scaffolds for peripheral nerve reconstruction. Int Rev Neurobiol 87: 173-198.
- Brushart TME (1991) The Mechanical & Humoral Control of Specificity In Nerve Repair. Operative Nerve Repair and Reconstruction.
- Girardi FP, Khan SN, Cammisa FP Jr, Blanck TJ (2000) Advances and strategies for spinal cord regeneration. Orthop Clin North Am 31: 465-472.
- Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, et al. (2011) Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng 39: 81-124.
- Kehoe S, Zhang XF, Boyd D (2012) FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 43: 553-572.
- Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7: 617-627.
- Deumens R, Bozkurt A, Meek MF, Marcus MA, Joosten EA, et al. (2010) Repairing injured peripheral nerves: Bridging the gap. Prog Neurobiol 92: 245-276.
- Hotary KB, Robinson KR (1992) Evidence of a role for endogenous electrical fields in chick embryo development. Development 114: 985-996.
- Shi R, Borgens RB (1995) Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn 202: 101-114.
- Huang YC, Huang YY (2006) Biomaterials and strategies for nerve regeneration. Artif Organs 30: 514-522.
- Subramanian A, Krishnan UM, Sethuraman S (2011) Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed Mater 6: 025004.
- Wong DY, Leveque JC, Brumblay H, Krebsbach PH, Hollister SJ, et al. (2008) Macro-architectures in spinal cord scaffold implants influence regeneration. J Neurotrauma 25: 1027-1037.
- Bölgen N, Mencelo√?¬?lu YZ, Acatay K, Vargel I, Pi√?¬?kin E (2005) In vitro and in vivo degradation of non-woven materials made of poly(epsilon-caprolactone) nanofibers prepared by electrospinning under different conditions. J Biomater Sci Polym Ed 16: 1537-1555.
- Bertleff MJ, Meek MF, Nicolai JP (2005) A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg Am 30: 513-518.
- Mattson MP, Haddon RC, Rao AM (2000) Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci 14: 175-182.
- Kam NW, Jan E, Kotov NA (2009) Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett 9: 273-278.
- Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, et al. (2008) A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol 3: 216-221.
- Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, et al. (2006) Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci U S A 103: 3357-62.
- Zeng HL, Gao C, Yan DY (2006) Poly(?-caprolactone)-Functionalized Carbon Nanotubes and Their Biodegradation Properties. Adv Funct Mater 16: 812-818.
- Baktur R (2011) A study on the applications and toxicity assessments of carbon nanotubes in tissue engineering, in All Graduate Theses and Dissertations. Paper 928.2011, Utah State University: United States -- Utah. p. 100.
- Mooney E, Dockery P, Greiser U, Murphy M, Barron V (2008) Carbon nanotubes and mesenchymal stem cells: biocompatibility, proliferation and differentiation. Nano Lett 8: 2137-2143.
- Cheap-Tubes-Inc. (2009) Material Safety Data Sheet. Company Website.
- Pegel, S, Potschke P, Petzold G, Alig I, Dudkin SM, et al. (2008) Dispersion, agglomeration, and network formation of multiwalled carbon nanotubes in polycarbonate melts. Polymer 49: 974-984.
- Thurnherr T, Brandenberger C, Fischer K, Diener L, Manser P, et al. (2011) A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol Lett 200: 176-186.
- Liu HL, Zhang YL, Yang N, Zhang YX, Liu XQ, et al. (2011) A functionalized single-walled carbon nanotube-induced autophagic cell death in human lung cells through Akt-TSC2-mTOR signaling. Cell Death Dis 2: e159.
- Montes-Fonseca SL, Orrantia-Borunda E, Aguilar-Elguezabal A, González Horta C, Talamás-Rohana P, et al. (2012) Cytotoxicity of functionalized carbon nanotubes in J774A macrophages. Nanomedicine 8: 853-859.
- Ruan B, Jacobi AM (2012) Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Res Lett 7: 127.
- Salalha W, Dror Y, Khalfin RL, Cohen Y, Yarin AL, et al. (2004) Single-walled carbon nanotubes embedded in oriented polymeric nanofibers by electrospinning. Langmuir 20: 9852-9855.
- Baji A, Mai YW, Wong SC, Abtahi M, Chen P (2010) Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos Sci Technol 70: 703-718.
- Wang Q, Dai J, Li W, Wei Z, Jiang J (2008) The effects of CNT alignment on electrical conductivity and mechanical properties of SWNT/epoxy nanocomposites. Compos Sci Technol 68: 1644-1648.
- Tremblay RG, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, et al. (2010) Differentiation of mouse Neuro 2A cells into dopamine neurons. J Neurosci Methods 186: 60-67.
- Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS (2005) Electrospinning of nanofibers. J Appl Polym Sci 96: 557-569.
- Nadler M, Werner J, Mahrholz T, Riedel U, Hufenbach W, et al. (2009) Effect of CNT surface functionalisation on the mechanical properties of multi-walled carbon nanotube/epoxy-composites. Compos Part A Appl Sci Manuf 40: 932-937.
- Zhao B, Wang J, Li Z, Liu P, Chen D, et al. (2008) Mechanical strength improvement of polypropylene threads modified by PVA/CNT composite coatings. Materials Letters 62: 4380-4382.
- Rana S, Cho J (2011) Core-sheath polyurethane-carbon nanotube nanofibers prepared by electrospinning. Fibers and Polymers 12: 721-726.
- Guimard NK, Gomez N, Schmidt CE (2007) Conducting polymers in biomedical engineering. Progress in Polymer Science 32: 876-921.
- Wu Z, Hjort K, Wicher G, Fex Svenningsen A (2008) Microfluidic high viability neural cell separation using viscoelastically tuned hydrodynamic spreading. Biomed Microdevices 10: 631-638.
- Bechara S, Wadman L, Popat KC (2011) Electroconductive polymeric nanowire templates facilitates in vitro C17.2 neural stem cell line adhesion, proliferation and differentiation. Acta Biomater 7: 2892-2901.
- Belkas JS, Shoichet MS, Midha R (2004) Axonal guidance channels in peripheral nerve regeneration. Operative Techniques in Orthopaedics 14: 190-198.
- Yao L, de Ruiter GC, Wang H, Knight AM, Spinner RJ, et al. (2010) Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 31: 5789-5797.
- Borschel GH, Kia KF, Kuzon WM Jr, Dennis RG (2003) Mechanical properties of acellular peripheral nerve. J Surg Res 114: 133-139.
- Rydevik BL, Kwan MK, Myers RR, Brown RA, Triggs KJ, et al. (1990) An in vitro mechanical and histological study of acute stretching on rabbit tibial nerve. J Orthop Res 8: 694-701.
- Kokai LE, Lin YC, Oyster NM, Marra KG (2009) Diffusion of soluble factors through degradable polymer nerve guides: Controlling manufacturing parameters. Acta Biomater 5: 2540-2550.
- Ma J, Wang H, He B, Chen J (2001) A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan bilayer material as a scaffold of human neofetal dermal fibroblasts. Biomaterials 22: 331-336.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22691
- [From(publication date):
July-2013 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 17897
- PDF downloads : 4794