Biomarkers for Identifying Individuals at Risk of Alzheimer Disease
Received: 01-Jan-1970 / Accepted Date: 01-Jan-1970 / Published Date: 30-Mar-2018 DOI: 10.4172/1522-4821.1000390
Abstract
Background: Alzheimer Disease (AD), the most common form of dementia, is a progressive and irreversible neurodegenerative disorder. Promising preventative strategies includes identification of potential modifiable risk factors for AD that could help identify individual who are at risk of AD. This study focuses on identifying biochemical factors associated with non- familial AD.
Methods: One hundred and ten individuals which included 55 AD patients and 55 healthy controls were recruited for the study. Patients clinically diagnosed by a neurologist as AD and controls with no clinical or family history of any neurological disease were subjected to Mini-Mental State Examination (MMSE) were evaluated for fourteen relevant biochemical markers using commercial kits. MDR analysis was carried out which is considered a basic machine learning tool for understanding the role of interaction and combination of the factors towards the outcome. PCA is performed to support the MDR interpretation. Through clustering analysis the probably causative factors towards the disease can be identified.
Results: MDR analysis revealed that the overall best fit model included 10 factors which had a maximal testing accuracy of 61%, cross-validation consistency of 8/10. PCA analysis has further reduced the factors to Iron, TSAT, HDL, VitB12, FA, and Hcy which are important in disease initiation/progression. Apart from the cases, 9% of the controls who had lower MMSE also had low Iron, TSAT, HDL, VitB12, FA, and high Hcy.
Conclusion: As per the results obtained, we would suggest a medical practice where, screening individuals above the age of 55 years with both MMSE and selected biochemical parameters (Iron, TSAT, HDL, VitB12, FA, and Hcy) should be carried out to identify those at risk of developing AD. Higher risk individuals can be suggested for modifications in diet/life style, enhancing certain nutritional components which may constitute promising strategies in postponing, slowing, and/or preventing cognitive decline in AD.
Keywords: Alzheimer disease; Folic acid; Vitamin B12; High-density-lipoprotein
Introduction
Alzheimer’s Disease (AD) is the most frequent cause of age related dementia and possibly contributes to 60-70% of cases with dementia above the age of 60 years (Barker et al., 2002). AD is a progressive neurological disease, with high levels of fibrillary Amyloid β (Aβ) deposits in the brain (Esparza et al., 2013), leading to the irreversible loss of neurons and intellectual abilities, including memory and reasoning, which becomes severe enough to impede social and occupational functioning (Alzheimer’s Association, 2016). Apart from this, there are various genetic and non-genetic factors which are considered to contribute to cognitive decline and progression of AD (Holtzman et al., 2011). In the past decade, observational studies have suggested a wide range of probable modifiable risk factors for AD, which could be important for planning preventive strategies (Ritchie et al., 2010; Beydoun et al., 2014; Solomon et al., 2014; Lista et al., 2015). Modifiable risk factors for AD fall into two categories. First category is vascular risk factors which includes midlife hypertension, diabetes mellitus, Dyslipidaemia and cardiovascular disease. Second group includes nutritional and life style factors which include nutritional deficiency (Vitamin B, Iron), Smoking etc.
The essential vitamin B nutrient called Folic Acid (FA) plays an important role in the synthesis of DNA and RNA precursors and for the conversion of Homocysteine (Hcy) to methionine. FA has been found to be low in AD cases when compared to age matched healthy individuals (Hui et al., 2015). Similarly, Vitamin B12 (VitB12), in the form of methylcobalamin also serves as a coenzyme for methionine synthase, during the remethylation of Hcy to methionine. Hcy is a non-protein α-amino acid, increased level of which has been associated with atherosclerosis and neurological disease including AD (Ciaccio & Bellia, 2010; Zhuo, Wang & Pratico, 2011). Contradictory results regarding reduced VitB12 in AD are available (Politis et al., 2010; Komurcu et al., 2016; Morris et al., 2006).
Dysregulation of iron metabolism has been observed in patients of neurodegenerative diseases. Some reports indicate altered iron concentration in the brain of AD patients as well as reduced serum Iron levels (Oshiro et al., 2011; Sastre et al., 2015). Lipids especially cholesterol is an important component of the nervous system and is essential for axonal growth, synaptic formation and remodeling, processes that are crucial for learning, formation of memories and neuronal repair (Mauch et al., 2001)]. High serum/ plasma cholesterol levels have been suggested as a risk factor for AD (Popp et al., 2013)]. According to a study by Cheng et al., (2014), elevated cholesterol and Hcy levels together contribute to reduced cognitive function in the elderly (Chen et al., 2015). Hence to understand these AD risks in an individual, measurement of the metabolites of Vitamin B’s, Lipids and Iron profiles and recording their comorbidities is essential. Our main aim was to identify risk individuals by using biomarkers that are already routinely assessed or which will be cost-effectively introducible in clinical practice.
In the present study, 14 routinely used biochemical factors belonging to three pathways which were reported in literature were evaluated, i.e.,
• One carbon metabolism markers: Folic Acid (FA), Homocysteine (Hcy), Vitamin B12 (VitB12).
• Iron parameters: Iron, Transferrin Saturation (TSAT), Total Iron Binding Capacity (TIBC).
• Lipid parameters: Total cholesterol (TChol), Triglycerides (TAG), High-Density-Lipoprotein cholesterol (HDL-C), Low-Density-Lipoprotein Cholesterol (LDL-C), Very Low- Density-Lipoprotein Cholesterol (VLDL), TChol/HDL Ratio, LDL/HDL Ratio and Non-HDL-C.
Imbalances in these parameters will help us to identify the high risk individuals and intervene at the earliest.
Samples from AD patients and healthy controls were collected and subjected to biochemical factor evaluation (Chen et al., 2015; Clarke et al., 1998; Postiglione et al., 2001; Tao et al., 2014; Wang et al., 2015; Presecki et al., 2011) and the data obtained was subjected to MDR analysis. This to the best of our knowledge is the first time when biomarkers associated with the pathology of AD patients were subjected to MDR to suggest preventative strategies.
Materials and Methods
Patient Selection Criteria
One hundred and ten individuals which included 55 AD patients and 55 healthy controls (>55 years) were recruited for the study between April 2013 and May 2016. Age of AD patients, which were clinically identified by a neurologist ranged from 55 to 91 years, based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorder Association for probable AD (NINCDSADRDA) (McKhann et al., 1984). Healthy controls above the age of 55 years, were examined (Clinically and by Mini-Mental State Examination, MMSE) to exclude neurological disorders and cognitive impairment.
The purpose of the study was explained and a written consent was obtained from patients or caregivers and control subjects. Demographic, socio-economic and lifestyle information was obtained using a predesigned proforma. The study was approved by the “Vasavi Ethics Committee” (Vasavi Medical and Research Centre) and 3 mL of blood was collected. Serum analysis was carried out for evaluating biochemical parameters using commercially kits (Thyrocare, Hyderabad, India).
Methodology
Serum levels of FA, Hcy, VitB12, Iron parameters: Iron, Transferrin Saturation (TSAT), Total Iron Binding Capacity (TIBC) and Lipid parameters: Total Cholesterol (TChol), Triglycerides (TAG), High Density Lipoprotein Cholesterol (HDL-C), Low Density Lipoprotein Cholesterol (LDL-C), Very Low Density Lipoprotein Cholesterol (VLDL), TChol/HDL Ratio, LDL/HDL Ratio and Non-HDL-C were determined in all subjects included in this study (Table 1). Assays were carried out by using wellestablished and sensitive methods for FA, Hcy, and VitB12 by Fully Automated Chemi Luminescent Immuno Assay (Centaur). Serum lipids were measured by commercial colorimetric assays (Olympus AU2700 [Beckman Coulter]); TChol by Enzymatic endpoint CHOD- PAP method, TAG by Enzymatic Glycerol Phosphate Oxidase/Peroxidase method, HDL-C by Enzyme selective protection method, LDL-C by Homogenous Enzymatic Direct Assay, Iron levels were measured using Ferrozine method without deproteinization, TIBC was measured using Spectrophotometric Assay (Advia 2400 [Siemens]), TSAT was calculated from Iron and TIBC value.
Statistical Analysis
Multifactor Dimensionality Reduction (MDR) analysis was carried out for the 14 biochemical factors. MDR is a basic machine learning algorithm which helps in identifying the best interactive combinations of the factors to a single outcome. In this study, interaction between the biochemical factors in the pathogenesis of AD was assessed in 110 samples, in which 50% were cases and 50% were Healthy controls. The biochemical factors each have two levels (0, 1) while the class has two levels (0, 1) that code controls and cases. Each best model was accompanied with a testing accuracy, cross validation consistency, and significance level, which were determined by performing permutation testing. Cross validation consistency reflects the number of times MDR found the same model as it divided up the data into different segments. Balanced accuracy is defined as (sensitivity+specificity)/2 where sensitivity=true positives/(true positives+false negatives) and specificity=true negatives/(false positives+true negatives). This gives an accuracy estimate that is not biased by the larger class.
In order to identify the components that had the highest likelihood of combining to elicit the cascade of events involved in the establishment of the disease, Principal Component Clustering Analysis (PCA) was performed, which helps in reducing the variables studied into manageable components to obtain a screen plot which would help in the identification of possible clusters and interactions which could lead to disease phenotype through relatedness.
Results
The mean age of AD patients was 72 ± 8.3 years while that of healthy controls was 69.5 ± 8.4 years, indicating that the cases and controls were approximately of the same age group. Of the 55 AD patients, 35 were male and 20 were female, while healthy controls had 24 males and 31 females. All the women in the study were post-menopausal. The AD patients had Mini-Mental State Examination (MMSE) of 15 ± 4, whereas healthy controls had MMSE score of 25 ± 3.
Serum marker levels of the selected pathways were determined in all 110 individuals included in this study. They are as follows:
• One carbon metabolism markers: (FA, Hcy, VitB12.
• Iron parameters: Iron, TSAT, TIBC.
• Lipid parameters: TChol, TAG, HDL-C, LDL-C, VLDL, TChol/HDL Ratio, LDL/HDL Ratio and Non-HDL-C.
On correlation of mean and SD of the levels between the patients and controls it was observed that HDL-C, VitB12, LDL/ HDL ratio, TC/HDL ratio, FA, Hcy and VLDL were significantly different between the patients and controls (Table 1). Low HDL was seen in 70.9% AD patients and 38.1% controls (OR: 6.52, 95% CI: 3.4 to 12.3, p<0.0001), Low VitB12 was observed in 50.9% AD patients and 18.1% controls (OR: 4.74, 95% CI: 2.4 to 9.0, p<0.0001), High LDL/HDL ratio was observed in 25.4% AD patients and 5.4% controls (OR: 6.33, 95% CI: 2.3 to 17.3, p=0.0003), High TC/HDL ratio was observed in 30.9% AD patients and 12.7% controls (OR: 3, 95% CI: 1.5 to 6.2, p=0.0028), Low FA was seen in 36.4% AD patients and 21.8% controls (OR: 2.08, 95% CI: 1.1 to 3.8, p=0.021), High Hcy was observed in 60% AD patients and 47.3% controls (OR: 1.69, 95% CI: 0.9 to 2.9, p=0.066), High VLDL was observed in 49.1% AD patients and 32.7% controls (OR: 1.95, 95% CI: 1.1 to 3.4, p=0.022).
| S. No. | Attributes | Cases | Controls | P-value | ||
|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | |||
| 1. | HDL (mg/dl) | 36.6 | 7.3 | 44.4 | 10.3 | < 0.0001* |
| 2. | VitB12 (pg/ml) | 373.6 | 223.4 | 494.2 | 224.4 | 0.0057* |
| 3. | LDL/HDL ratio | 2.7 | 1.2 | 2.3 | 0.8 | 0.0267* |
| 4. | TChol/HDL ratio | 4.5 | 1.2 | 3.9 | 1 | 0.0047* |
| 5. | FA (ng/ml) | 9.3 | 6.5 | 13.7 | 8 | 0.0026* |
| 6. | Hcy (μmol/L) | 18.3 | 7.8 | 14.5 | 6 | 0.0050* |
| 7. | VLDL (mg/dL) | 36.5 | 20.4 | 25.7 | 9 | 0.0014* |
| 8. | TSAT (%) | 21.2 | 10.5 | 22.2 | 9 | 0.5592 |
| 9. | TIBC (µg/dL) | 334 | 55.6 | 335.4 | 52.2 | 0.8879 |
| 10. | LDL (mg/dL) | 101.1 | 45.7 | 98.5 | 35.5 | 0.7431 |
| 11. | TChol (mg/dL) | 161.2 | 44 | 167.2 | 41 | 0.4604 |
| 12. | TAG (mg/dL) | 153.4 | 70.8 | 128.9 | 68.5 | 0.0687 |
| 13. | Iron (µg/dL) | 69.2 | 31.7 | 72 | 24.5 | 0.6039 |
| 14. | Non-HDL (mg/dL) | 124.7 | 41.1 | 122.8 | 38.5 | 0.8005 |
Table 1: Mean and SD of 14 biochemical parameters
Multifactor Dimensionality Reduction (MDR) Analysis
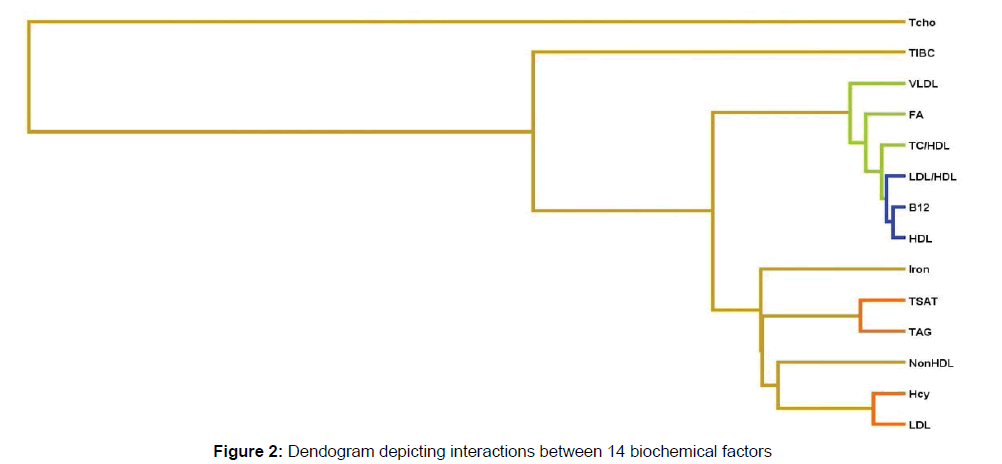
Evaluation of the 14 biomarkers by MDR revealed that 5 of them were significantly different in cases and controls (Figure 1A). These were: VitB12, HDL, LDL/HDL, TC/HDL and LDL (Table 2). Of these 5 factors, VitB12, HDL, LDL/HDL and TC/HDL showed independent interaction with AD pathogenesis indicating that Low VitB12 (8.8%), HDL (7.94%), increased LDL/HDL ratio (5.92%) TC/HDL ratio (3.58%) contributes to AD individually (Figure 1B). Considering the dendogram interaction graph, of these 14 factors, VitB12, HDL, LDL/HDL, TC/HDL, FA and VLDL showed no synergistic interaction with the remaining study factors indicating that it independently contributes to disease whereas, epistatic interaction was seen between TSAT-TAG, Hcy-TC/ HDL and Hcy-LDL indicating that it synergistically contributes to AD (Figure 1C). There appears a moderate epistatic interaction between LDL-Hcy and TAG-TSAT showing the combination which leads to disease, whereas HDL, VitB12, LDL/HDL showed redundant or no interaction. Iron, Non HDL lipids and TIBC showed extremely week interaction with AD in combination with other factors studied. Based on the dendogram (Figure 2) it can be said that the factors which play a role as the initiators of the disease are the TSAT-TAG, LDL-Hcy, Iron and Non HDL lipid.
Figure 1: Interaction Entropy model using MDR software: (A) Circle Graph showing interactions along with percentages, (B) Independent interaction between the factors (Green and blue suggest redundancy or correlation, Yellow suggests independence), (C) Synergistic interaction between the factors (Red and orange suggest there is a synergistic relationship)
| S. No. | Factors | Full form | χ2 | P-value |
|---|---|---|---|---|
| 1. | B12 | Vitamin B12 | 13.026316 | 0.000307* |
| 2. | HDL | High-Density Lipoprotein | 11.88 | 0.000567* |
| 3. | LDL/HDL | Low-Density Lipoprotein/High-Density Lipoprotein | 8.418722 | 0.003714* |
| 4. | TC/HDL | Total Cholesterol/High-Density Lipoprotein | 5.329457 | 0.020968* |
| 5. | LDL | Low-Density Lipoprotein | 3.975904 | 0.046156* |
| 6. | TSAT | Transferrin saturation | 3.117409 | 0.07746 |
| 7. | VLDL | Very Low-Density Lipoprotein | 3.046154 | 0.080929 |
| 8. | FA | Folic Acid | 2.820513 | 0.093067 |
| 9. | Hcy | Homocysteine | 1.791293 | 0.180769 |
| 10. | TIBC | Total Iron Binding capacity | 1.586538 | 0.207821 |
| 11. | TAG | Triacylglyceride | 0.972075 | 0.324163 |
| 12. | Iron | Iron | 0.92874 | 0.33519 |
| 13. | Non-HDL | Non-High-Density Lipoprotein | 0.909091 | 0.340356 |
| 14. | TChol | Total Cholesterol | 0.051765 | 0.82002 |
Table 2: Biochemical factors evaluated in AD
Each best model was accompanied with a testing accuracy, cross validation consistency, and significance level, which were determined by performing permutation testing. The overall best MDR model included all the 10 factors (FA, Hcy, B12, IRON, TSAT, TIBC, TCHO, HDL, VLDL, TC/HDL) and had a maximal testing accuracy of 61%, cross-validation consistency of 8/10 (Table 3). These combinations has maximum number of statistically potential factors which plays role in disease initiation and progression.
| S. No. | Best model | Training balance accuracy | Testing balance accuracy | Cross Validation Consistency (CVC)* |
|---|---|---|---|---|
| 1. | HDL | 0.6737 | 0.5727 | 6/10 |
| 2. | HDL, LDL/HDL | 0.6838 | 0.5364 | 4/10 |
| 3. | B12, TAG, VLDL | 0.7212 | 0.6364 | 7/10 |
| 4. | Hcy, B12, TIBC, HDL | 0.7677 | 0.6727 | 9/10 |
| 5. | Hcy, B12, TIBC, HDL, TC/HDL | 0.8 | 0.5636 | 5/10 |
| 6. | Hcy, B12, TIBC, HDL,VLDL, TC/HDL | 0.8374 | 0.5909 | 4/10 |
| 7. | Hcy, B12, TSAT, TIBC, HDL, VLDL, TC/HDL | 0.8788 | 0.6182 | 4/10 |
| 8. | FA, Hcy, B12, IRON, TIBC, HDL, TAG, TC/HDL | 0.9061 | 0.5636 | 4/10 |
| 9. | FA, Hcy, B12, IRON, TSAT, TIBC, HDL, TAG, TC/HDL | 0.9283 | 0.6091 | 4/10 |
| 10. | FA, Hcy, B12, IRON, TSAT, TIBC, TCHO, HDL, VLDL, TC/HDL | 0.9414 | 0.6182 | 8/10 |
| 11. | FA, Hcy, B12, IRON, TSAT, TIBC, TCHO, HDL, TAG,VLDL, TC/HDL | 0.9424 | 0.6 | 4/10 |
| 12. | FA, Hcy, B12, IRON, TSAT, TIBC, TCHO HDL, TAG, LDL, VLDL, TC/HDL | 0.9424 | 0.6091 | 5/10 |
| 13. | FA, Hcy, B12, IRON, TSAT, TIBC, TCHO HDL, TAG, LDL, VLDL, TC/HDL, LDL/HDL | 0.9424 | 0.6091 | 6/10 |
| 14. | FA, Hcy, B12, IRON, TSAT, TIBC, TCHO, HDL, TAG, LDL, VLDL, TC/HDL, LDL/HDL , NON-HDL | 0.9424 | 0.6091 | 10/10 |
Table 3: Best fit Model
Principal Component Analysis (PCA)
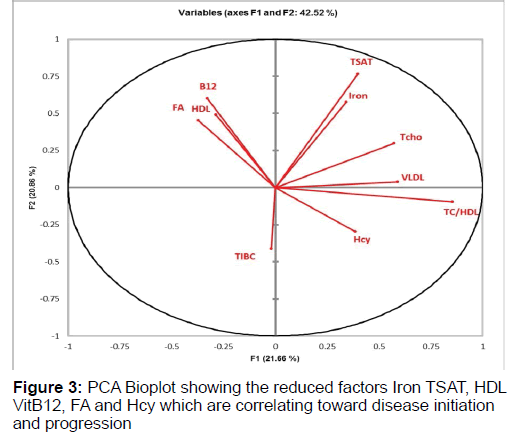
PCA Analysis was performed on the 10 selected factors from the MDR analysis which are FA, Hcy, VitB12, IRON, TSAT, TIBC, TCHO, HDL, VLDL, TC/HDL to understand that best possible combination of factors which interact and correlate in disease stage. This analysis are read in terms of angles. The bioplot shows correlation between the factors and its contribution towards the disease pathogenesis. The bio plot obtained from the analysis showed that the factors which were clustered in same quadrant and have narrow angle were having high correlation among themselves whereas factors having angle more than 90° to each other had negative correlation (Figure 3). The factors in the quadrants are as follows:
• Quadrant I: Iron, TSAT, TCho, VLDL
• Quadrant II: FA, HDL, VitB12
• Quadrant III: TIBC
• Quadrant IV: Hcy, TC/HDL
Iron-TSAT and VitB12-HDL has strong positive correlation indicating that this cluster together pointing towards higher probability of being the combination that may lead to the disease manifestation whereas negative correlation exists between factors FA-HDL-VitB12 and Hcy indicating that low FA (Less than 5.9 ng/ml) or low VitB12 (Less than 300 pg/ml) and low HDL (<40 increases Hcy (more than 15 μmol/ml) hence risk of AD.
Discussion
AD is an irreversible, progressive neurological disease. Although genetic alterations have been associated with familial AD, several biochemical factors are also considered to play a role in the etiology of the disease (Koyama et al., 2013; Delacourte et al., 1999). While there are imaging and cerebrospinal fluid based tests for the early detection of this disease, it is important to identify other biochemical biomarkers that could be used for noninvasive and inexpensive screening.
In addition, although some genetic risk factors have been identified (Tanzi, 2012), aging remains by far the main risk factor for SAD which is a commonly observed disorder after 75 years of age, but rare before age 60 (Mangialasche, Xu & Kivipelto, 2013). In the present study, mean age at onset of AD symptoms was 68.4 ± 8.4 years, indicating that the observable onset of AD symptoms is around 60 years. Early detection of the disease can help in effective management and therapy. Hence screening individuals for risk of AD above the age of 55 can help identify them and help them towards alleviating the intensity of the disease.
In the present study, of the 14 biochemical markers evaluated, Iron, TSAT, HDL, VitB12, FA and Hcy played major role in AD pathogenesis. These factors were all studied individually and never together, this study is first of its kind were markers from three different pathways were studied together. Iron dyshomeostasis is critical in AD pathology, based on observations that patients had elevated iron levels in cortical, subcortical, and white matter areas affected by the disease (Raven et al., 2013; Peters, Connor & Meadowcroft, 2015). In our study, we observed that 47.2% of the AD cases and 38.1% of control had low Iron status. None of the AD cases showed Iron overload, which contradicted the existing belief (Ayton, Lei & Bush, 2013; Lehmann et al., 2006). Iron transport into the brain is accomplished by Transferrin (Tf). TSAT is a commonly used indicator of iron deficiency and iron overload. The transferrin/iron ratio suggests reduced iron mobilization in AD brains, indicating a disturbance of iron metabolism in the AD patients (Loeffler et al., 1995). In our study, MDR and PCA analysis revealed that TSAT contributed to AD, but in synergy with TAG and as the Iron levels decrease the TSAT % also decreases. High TAG, low Iron and low TSAT appear to contribute to AD.
FA and VitB12 are closely connected with the metabolism of Hcy, a sulfur-containing nonessential amino acid. Several investigators have measured plasma levels of FA, Hcy, VitB12 in AD subjects and healthy matched controls (Coppede, 2010), the majority of the studies report that plasma Hcy is increased in AD subjects (Zhuo, Wang & Pratico, 2011; Seshadri, 2006), while FA levels are reduced significantly compared to controls (Clarke et al., 1998; Postiglione et al., 2001). Some authors observed significantly decreased levels of VitB12 in AD subjects compared to controls (Koseoglu & Karaman, 2007). On the contrary there are reports which do not find any association of AD with blood levels of Hcy, FA and VitB12 (Ravaglia et al., 2005; Ulusu et al., 2015). A recent study by Chung et al., (2016) suggests that elevated levels of plasma Hcy/homocysteine thiolactone contribute to AD pathology via the Aβ-fibrinogen interaction (Chung et al., 2016) suggesting the role of homocysteine metabolism in AD pathogenesis. In our study, one carbon metabolism factors FA, VitB12 and Hcy appears to be associated with AD. Oikonomidi et al., (2016), investigated the relationship between markers of amyloid pathology and homocysteine metabolism which revealed that higher Aβ1-42 CSF levels were associated with low VitB12 plasma levels indicating the protective role of VitB12 which is diminishing in AD patients (Oikonomidi et al., 2016). FA and VitB12 has direct impact on the levels of Hcy which was evident by the PCA analysis which revealed that as the FA and VitB12 decreases the levels of Hcy inversely increases hence indicating that FA-VitB12-Hcy can be important biomarkers. Our study supports that reduced HDL contributes to AD which has earlier been reported by others (Anstey, Lipnicki, & Low, 2008; Van Vliet, 2012; Purnell et al., 2009). HDL plays a pivotal role in preserving cognitive function under normal and pathological conditions. Dyslipidemia, and particularly low HDL-C may lead to cognitive impairment through cerebral hypoperfusion, embolism, or disruption of white matter (Reitz et al., 2010). Thus, a low HDL-C level could contribute in the etiology of AD. In vitro and in vivo studies by Adaikalakoteswari et al., (2015) on the role of low or no VitB12 on lipid metabolism revealed that total intracellular cholesterol which are Tcho and TC/ HDL ratio as well as extracellular homocysteine were significantly increased in low or no VitB12. HDL was not significantly altered by the VitB12 status (Adaikalakoteswari et al., 2015). In our study HDL and VitB12 were positively correlated indicating its role in AD disease progression.
Of the 55 controls included in the MDR analysis, it was observed that 9% of individuals (5/55) had lower MMSE, low Iron, TSAT, HDL, VitB12, FA, and High Hcy suggesting that screening individuals above the age of 55 years with both MMSE and selected biochemical parameters (Iron, TSAT, HDL, VitB12, FA and Hcy) is warranted to identify individuals at risk of developing AD. They can be advised dietary and lifestyle modifications along with Vitamin B12 supplement may be a promising strategy to postpone, slow and prevent cognitive decline in the elderly (Canevelli et al., 2016).
Conclusion
Due to the lack of effective treatments for AD and the high morbidity of this disease, primary prevention is of utmost importance. If early detection of the imbalances in the lipid and one carbon parameters is identified, prevention through dietary modification, lifestyle changes and essential VitB12 supplementations can be effective in delaying or reducing the incidence/occurrence or severity of this disabling cognitive disease. Follow up studies with early intervention require to be planned.
Acknowledgement
Funding for this work was granted by the Hyderabad Science Society, Hyderabad (HSS 01/06/2016-BIO). Our thanks are due to Mr. Dinesh for technical assistance in drawing the blood samples.
References
- Alzheimer's Association. (2016). Alzheimer's disease facts and figures. Alzheimers Dement, 12(4), 459-509. Anstey, K.J, Lipnicki, D.M., & Low, L.F. (2008). Cholesterol as a risk factor for dementia and cognitive decline, A systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry, 16(5), 343-354. Adaikalakoteswari, A., Finer, S., Voyias, P.D., McCarthy, C.M., Vatish, M., Moore, J., et al., (2015). Vitamin B(12) insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin Epigenetics, 7(1), 14. Ayton, S., Lei, P., & Bush, A.I. (2013). Metallostasis in Alzheimer's disease. Free Radic Biol Med, 62, 76-89. Barker, W.W., Luis, C.A., Kashuba, A., Luis, M., Harwood, D.G., Loewenstein, D., et al., (2002). Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord, 16(4), 203-212. Beydoun, M.A., Beydoun, H.A., Gamaldo, A.A., Teel, A., Zonderman, A.B., & Wang, Y. (2014). Epidemiologic studies of modifiable factors associated with cognition and dementia, Systematic review and meta-analysis. BMC Public Health, 14, 643. Chen, H., Liu, S., Ji, L., Wu, T., Ma, F., Ji, Y., et al., (2015). Associations between Alzheimer’s Disease and Blood Homocysteine, Vitamin B 12, and Folate, A case-control Study. Curr Alzheimer Res, 12(1), 88-94. Ciaccio, M., & Bellia, C. (2010). Hyperhomocysteinemia and cardiovascular risk, Effect of vitamin supplementation in risk reduction. Curr Clin Pharmacol, 5(1), 30-36. Cheng, Y., Jin, Y., Unverzagt, F.W., Su, L., Yang, L., Ma, F., et al., (2014). The relationship between cholesterol and cognitive function is homocysteine-dependent. Clin Interv Aging, 9, 1823-1829. Chen, H., Liu, S., Ji, L., Wu, T., Ma, F., Ji, Y., et al., (2015). Associations between Alzheimer's disease and blood homocysteine, vitamin B12, and folate, A case-control study. Curr Alzheimer Res, 12(1), 88-94. Clarke, R., Smith, A.D., Jobst, K.A., Refsum, H., Sutton, L., & Ueland, P.M. (1998). Folate, vitamin B12, and serum total homocysteine levels in confirmed alzheimer disease. Arch Neurol, 55(11), 1449-1455. Coppede, F. (2010). One-carbon metabolism and Alzheimer's disease, Focus on epigenetics. Curr Genomics, 11(4), 246-660. Chung, Y.C., Kruyer, A., Yao, Y., Feierman, E., Richards, A., Strickland, S., et al., (2016). Hyperhomocysteinemia exacerbates Alzheimer's disease pathology by way of the beta-amyloid fibrinogen interaction. J Thromb Haemost, 14(7), 1442-1452. Canevelli, M., Lucchini, F., Quarata, F., Bruno, G., & Cesari, M. (2016). Nutrition and dementia, Evidence for preventive approaches? Nutrients, 8(3), 144. Delacourte, A., David, J.P., Sergeant, N., Buee, L., Wattez, A., Vermersch, P., et al., (1999). The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology, 52(6), 1158-1165. Esparza, T.J., Zhao, H., Cirrito, J.R., Cairns, N.J., Bateman, R.J., Holtzman, D.M., et al., (2013). Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol, 73(1), 104-119. Holtzman, D.M., Morris, J.C., & Goate, A.M. (2011). Alzheimer's disease: The challenge of the second century. Sci Transl Med, 3(77), 77sr1. Komurcu, H.F., Kilic, N., Demirbilek, M.E., & Akin, K.O. (2016). Plasma levels of vitamin B12, epidermal growth factor and tumor necrosis factor alpha in patients with alzheimer dementia. International Journal of Research in Medical Sciences, 4(3), 734-738. Koseoglu, E., & Karaman, Y. (2007). Relations between homocysteine, folate and vitamin B12 in vascular dementia and in Alzheimer disease. Clin Biochem, 40(12), 859-863. Koyama, A., O'Brien, J., Weuve, J., Blacker, D., Metti, A.L., & Yaffe, K. (2013). The role of peripheral inflammatory markers in dementia and Alzheimer's disease, A meta-analysis. J Gerontol A Biol Sci Med Sci, 68(4), 433-440. Lista, S., Dubois, B., & Hampel, H. (2015). Paths to Alzheimer's disease prevention, From modifiable risk factors to biomarker enrichment strategies. J Nutr Health Aging, 19(2), 154-163. Lehmann, D.J., Worwood, M., Ellis, R., Wimhurst, V.L.J., Merryweatherâ€Clarke, A.T., Warden, D.R., et al., (2006). Iron genes, iron load and risk of Alzheimer's disease. J Med Genet, 43(10), e52. Loeffler, D.A., Connor, J.R., Juneau, P.L., Snyder, B.S., Kanaley, L., DeMaggio, A.J., et al., (1995). Transferrin and iron in normal, Alzheimer's disease, and Parkinson's disease brain regions. J Neurochem, 65(2), 710-724. Morris, M.C., Evans, D.A., Schneider, J.A., Tangney, C.C., Bienias, J.L., & Aggarwal, N.T. (2006). Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer's disease. J Alzheimers Dis, 9(4), 435-443. Mauch, D.H., Nagler, K., Schumacher, S., Goritz, C., Muller, E.C., Otto, A., et al., (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science, 294(5545), 1354-1357. McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E.M. (1984). Clinical diagnosis of Alzheimer's disease, report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology, 34(7), 939-944. https://www.intechopen.com/books/understanding-alzheimer-s-disease/prevention-of-alzheimer-s-disease-intervention-studies. Oshiro, S., Morioka, M.S., & Kikuchi, M. (2011). Dysregulation of Iron Metabolism in Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Adv Pharmacol Sci, 1-8. Oikonomidi, A., Lewczuk, P., Kornhuber, J., Smulders, Y., Linnebank, M., Semmler, A., et al., (2016). Homocysteine metabolism is associated with cerebrospinal fluid levels of soluble amyloid precursor protein and amyloid beta. J Neurochem, 139(2), 324-332. Politis, A., Olgiati, P., Malitas, P., Albani, D., Signorini, A., Polito, L., et al., (2010). Vitamin B12 levels in Alzheimer's disease, association with clinical features and cytokine production. J Alzheimers Dis, 19(2), 481-488. Popp, J., Meichsner, S., Kolsch, H., Lewczuk, P., Maier, W., Kornhuber, J., et al., (2013). Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer's disease. Biochem Pharmacol, 86(1), 37-42.Postiglione, A., Milan, G., Ruocco, A., Gallotta, G., Guiotto, G., & Di Minno, G. (2001). Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer's dementia. A case-control study. Gerontology, 47(6), 324-329. Presecki, P., Muck-Seler, D., Mimica, N., Pivac, N., Mustapic, M., Stipcevic, T., et al., (2011). Serum lipid levels in patients with Alzheimer's disease. Coll Antropol, 35, 115-120. Peters, D.G., Connor, J.R., & Meadowcroft, M.D. (2015). The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer's disease, Two sides of the same coin. Neurobiol Dis, 81, 49-65. Purnell, C., Gao, S., Callahan, C.M., & Hendrie, H.C. (2009). Cardiovascular risk factors and incident Alzheimer disease, A systematic review of the literature. Alzheimer Dis Assoc Disord, 23(1), 1-10. Ritchie, K., Carriere, I., Ritchie, C.W., Berr, C., Artero, S., & Ancelin, M.L. (2010). Designing prevention programmes to reduce incidence of dementia, Prospective cohort study of modifiable risk factors. BMJ, 341, c3885. Raven, E.P., Lu, P.H., Tishler, T.A., Heydari, P., & Bartzokis, G. (2013). Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer's disease detected in vivo with magnetic resonance imaging. J Alzheimers Dis, 37(1), 127-136. Ravaglia, G., Forti, P., Maioli, F., Martelli, M., Servadei, L., Brunetti N., et al., (2005). Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr, 82(3), 636-643. Reitz, C., Tang, M.X., Schupf, N., Manly, J.J., Mayeux, R., & Luchsinger, J.A. (2010). Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol, 67(12), 1491-1497. Solomon, A., Mangialasche, F., Richard, E., Andrieu, S., Bennett, D.A., Breteler, M., et al., (2014). Advances in the prevention of Alzheimer's disease and dementia. J Intern Med, 275(3), 229-250. Sastre, M., Ritchie, C.W., & Hajji, N. (2015). Metal ions in alzheimer’s disease brain. JSM Alzheimer’s Dis Related Dementia, 2(1), 1014. Seshadri, S. (2006). Elevated plasma homocysteine levels, risk factor or risk marker for the development of dementia and Alzheimer's disease? J Alzheimers Dis, 9(4), 393-398. Tao, Y., Wang, Y., Rogers, J.T., & Wang, F. (2014). Perturbed iron distribution in Alzheimer's disease serum, cerebrospinal fluid, and selected brain regions, A systematic review and meta-analysis. J Alzheimers Dis, 42(2), 679-690. Tanzi, R.E. (2012). The genetics of Alzheimer disease. Cold Spring Harb Perspect Med, 2(10), a006296. Ulusu, N.N., Yilmaz, G., Erbayraktar Z., Evlice A.T., Aras, S., Yener, G., et al., (2015). A Turkish 3-center study evaluation of serum folic acid and vitamin B12 levels in Alzheimer disease. Turk J Med Sci, 45, 1159-1166. Van Vliet, P. (2012). Cholesterol and late-life cognitive decline. J Alzheimers Dis, 30, S147-S162. Wang, Z.X., Tan, L., Wang, H.F., Ma, J., Liu, J., Tan, M.S., et al., (2015). Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer's Disease, A replication study and meta-analyses. J Alzheimers Dis, 47(3), 565-581. Zhuo, J.M., Wang, H., & Pratico, D. (2011). Is hyperhomocysteinemia an Alzheimer's disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol Sci, 32(9), 562-571.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 6361
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 5511
- PDF downloads: 850