Biomarkers for HER2-positive Metastatic Breast Cancer that Go Beyond Hormone Receptors
Received: 01-Oct-2023 / Manuscript No. bccr-23-117067 / Editor assigned: 03-Oct-2023 / PreQC No. bccr-23-117067 / Reviewed: 17-Oct-2023 / QC No. bccr-23-117067 / Revised: 23-Oct-2023 / Manuscript No. bccr-23-117067 / Published Date: 30-Oct-2023 DOI: 10.4172/2572-4118.1000211 QI No. / bccr-23-117067
Abstract
HER2-positive metastatic breast cancer represents a distinct subtype of breast cancer with aggressive clinical behavior. While the overexpression of human epidermal growth factor receptor 2 (HER2) is a hallmark of this subtype, its heterogeneity necessitates a more nuanced understanding to guide treatment decisions. Beyond traditional hormone receptor status, identifying comprehensive biomarkers is crucial for optimizing the management of HER2-positive metastatic breast cancer. This abstract explores the evolving landscape of biomarkers in this context. In recent years, research has highlighted the significance of several biomarkers that offer a deeper insight into the intricacies of HER2-positive metastatic breast cancer. Notably, beyond the status of estrogen receptor (ER) and progesterone receptor (PR), additional markers such as PIK3CA mutations, PTEN loss, and the tumor-infiltrating lymphocytes (TILs) density have emerged as pivotal factors in tailoring therapeutic strategies. PIK3CA mutations have been found to modulate the response to HER2-targeted therapies and endocrine therapies, shedding light on the crosstalk between signaling pathways. Concurrently, PTEN loss has been associated with resistance to HER2- targeted agents, offering a crucial avenue for patient stratification. Furthermore, TILs, as indicators of the immune microenvironment, play a role in predicting response to immunotherapies in HER2-positive metastatic breast cancer. In addition to genomic and immune markers, liquid biopsies have gained prominence as a non-invasive approach to detect HER2 alterations and monitor treatment response. Circulating tumor DNA (ctDNA) and exosomes markers can provide real-time information about disease progression and emerging resistance mechanisms. This abstract emphasizes the evolving significance of biomarkers that extend beyond hormone receptor status in HER2-positive metastatic breast cancer. Understanding the molecular and immunological landscape of this subtype is essential for individualizing treatment and optimizing patient outcomes. In an era of precision oncology, these comprehensive biomarkers offer a promising path towards improving the management of HER2-positive metastatic breast cancer patients, ultimately enhancing their quality of life and overall survival.
Keywords
HER2-positive breast cancer; Metastatic breast cancer; Biomarkers; HER2 amplification; Hormone receptor status; PIK3CA mutations
Introduction
Breast cancer is a heterogeneous disease with distinct molecular subtypes, and human epidermal growth factor receptor 2 (HER2)- positive metastatic breast cancer represents a subset characterized by the overexpression or amplification of the HER2 receptor. This subtype accounts for approximately 15-20% of all breast cancers and is associated with aggressive clinical behavior. Historically, treatment decisions for HER2-positive metastatic breast cancer have often focused on HER2 status and hormone receptor status, categorizing patients as hormone receptor-positive (HR+) or hormone receptornegative (HR-) [1]. However, recent advancements in molecular and immune profiling have revealed a more complex landscape, highlighting the need to move beyond traditional hormone receptor status and explore comprehensive biomarkers that can better inform therapeutic strategies. The identification of these comprehensive biomarkers is imperative because HER2-positive metastatic breast cancer is not a monolithic entity; it exhibits significant molecular and clinical heterogeneity [2 ]. Beyond the HER2 receptor itself, a deeper understanding of the underlying biology is essential to guide the selection of treatment options that offer the best chances of durable responses and improved patient outcomes. In this context, this review aims to explore the evolving role of biomarkers in HER2-positive metastatic breast cancer, with a particular focus on markers that extend beyond hormone receptor status. We will delve into the significance of genomic alterations, immune microenvironment, and the emerging role of liquid biopsies in characterizing this disease [3 ]. By elucidating the intricate landscape of these biomarkers, we aspire to contribute to the ongoing efforts to personalize treatment strategies and enhance the quality of life and survival of patients with HER2-positive metastatic breast cancer. Human epidermal increase thing receptor-2 (HER2) is overexpressed in 15–20% of breast most cancers (BC) cases, ensuing in an aggressive scientific behavior. The introduction of trastuzumab has contributed to revert the negative prognosis of HER2-positive metastatic BC patients. In Europe, at present, the pool of accredited capsules for the therapy of HER2-positive metastatic BC consists of trastuzumab, lapatinib, pertuzumab and T-DM1. Thanks to these therapeutic advances the standard survival of HER2-positive metastatic BC sufferers now exceeds 50 months from the analysis of superior disease, with statistics from the real-world putting matching the effects of scientific trials [4].
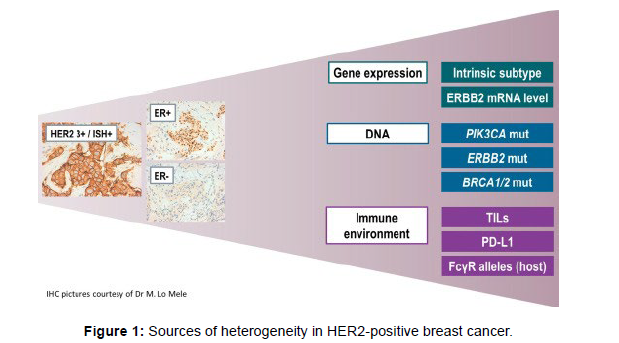
The modern therapeutic algorithms can be similarly customized in accordance to hormone receptors (HR) co-expression. The solely therapy particularly authorised for HR−/HER2+ ailment is the mixture of lapatinib and trastuzumab. More picks are handy for the subset of HR+/HER2+ patients. Combinations of endocrine remedy with single agent anti-HER2 drug signify an alternative for chosen HR+/HER2+ sufferers in accordance to the effects of randomized trials displaying advantage from the addition of trastuzumab or lapatinib to an aromatase inhibitor. More recently, two randomized research evaluated the function of twin blockade blended with endocrine remedy for HR+/ HER2+. Although for therapeutic decisions, at present, we dichotomize HER2-positive BC in HR+ and HR−, there are many other sources of biologic heterogeneity including: gene expression, DNA mutations and the immune microenvironment (Figure 1). None of these new potential biomarkers is ready for clinical application, however research in the field is moving rapidly also fostered by the development of new anti- HER2 treatments. This review summarizes the updated evidence on biomarkers that hold the greatest promise to become potentially useful tools for optimizing HER2-positive metastatic BC patients’ prognostic stratification and treatment in the next future [5,6].
Immune biomarker DNA mutations in her2-positive metastatic breast cancer beyond hormone receptors
The emergence of immune biomarker DNA mutations as critical components in the evaluation of HER2-positive metastatic breast cancer is shaping the landscape of personalized therapy for this aggressive disease. While hormone receptor status has long been the primary focus of breast cancer classification and treatment decisions, our understanding of the complex interplay between immune biomarkers and genomic alterations is shedding new light on the management of HER2-positive breast cancer. This article explores the burgeoning realm of immune biomarker DNA mutations, encompassing factors such as tumor-infiltrating lymphocytes (TILs) and programmed deathligand 1 (PD-L1) expression, in the context of HER2-positive metastatic breast cancer. We delve into how these immune biomarkers, working in concert with genomic alterations, are revolutionizing our approach to patient stratification, therapy selection, and the development of cuttingedge immunotherapeutic strategies. As we navigate the complexities of HER2-positive metastatic breast cancer, this exploration of immune biomarker DNA mutations serves as a beacon guiding us toward more effective, tailored, and personalized approaches to treatment. It highlights the potential to harness the power of the immune system to augment the response to HER2-targeted therapies, ultimately improving outcomes and quality of life for patients facing this formidable challenge [7-9].
Future Directions
Advancements in technology and our understanding of the molecular landscape of HER2-positive MBC open doors to an era of precision medicine in breast cancer. The integration of diverse biomarkers, including HER2 status, hormone receptor status, PIK3CA mutations, PTEN loss, immune-related markers, and comprehensive genomic profiling, promises to refine patient stratification, guide personalized therapy, and improve outcomes.
Conclusion
In the era of precision medicine, the assessment of comprehensive biomarkers for HER2-positive MBC is crucial. Beyond HER2 and hormone receptor status, the evaluation of molecular and genomic markers, as well as immune-related parameters, has the potential to transform the management of HER2-positive MBC. The incorporation of these biomarkers into clinical practice, along with the continued development of targeted and immunotherapeutic strategies, will lead to more tailored and effective treatments for this challenging disease, ultimately improving the prognosis and quality of life for patients with HER2-positive metastatic breast cancer. As we stand at the intersection of immunology and genomics, it is becoming increasingly evident that patient stratification and treatment decisions should not rely solely on HER2 amplification or hormone receptor status. Immune biomarkers add another layer of complexity and opportunity, offering the potential to augment the therapeutic impact of HER2-targeted agents and, in certain cases, open doors to innovative immunotherapeutic approaches. This evolving landscape provides hope and optimism for patients with HER2-positive metastatic breast cancer, as it underscores the potential for precision medicine to tailor treatments to the individual, enhancing therapeutic efficacy while minimizing toxicity. As we move forward, the continued exploration of immune biomarker DNA mutations will be integral in shaping the next generation of treatment strategies, with the ultimate goal of improving prognosis, enhancing the quality of life, and advancing the prospects for patients facing this formidable challenge.
Conflict of Interest
None
Acknowledgment
None
References
- Kim KS, Shin KH, Choi N, Lee SW (2016) Hypofractionated whole breast irradiation: new standard in early breast cancer after breast-conserving surgery. Radiat Oncol J 34: 81-87.
- Meng QY, Cong HL, Hu H, Xu FJ (2020) Rational design and latest advances of co delivery systems for cancer therapy. Materials Today Bio 7: 100056.
- Mamounas EP, Bandos H, Lembersky BC, Jeong JH, Geyer Jr CE, et al. (2019) Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20: 88-99.
- Coles CE, Aristei C, Bliss J, Boersma L, Brunt AM, et al. (2020) International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 32: 279-281.
- Bianchini G, De Angelis C, Licata L, Gianni L (2022) Treatment landscape of triple-negative breast cancer-Expanded options, evolving needs. Nat Rev Clin Oncol 19: 91-113.
- Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T (2018) Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res 8: 1483-1507.
- Sjöström M, Chang SL, Fishbane N, Davicioni E, Zhao SG, et al. (2019) Clinicogenomic radiotherapy classifier predicting the need for intensified locoregional treatment after breast-conserving surgery for early-stage breast cancer. J Clin Oncol 37: 3340.
- Azria D, Riou O, Castan F, Nguyen TD, Peignaux K, et al. (2015) Radiation-induced CD8 T-lymphocyte apoptosis as a predictor of breast fibrosis after radiotherapy: results of the prospective multicenter french trial. EBioMedicine 2: 1965-1973.
- Chaudhary LN (2020) Early stage triple negative breast cancer: management and future directions. Semin Oncol 47: 201-208.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Griguolo F (2023) Biomarkers for HER2-positive Metastatic BreastCancer that Go Beyond Hormone Receptors. Breast Can Curr Res 8: 211. DOI: 10.4172/2572-4118.1000211
Copyright: © 2023 Griguolo F. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1296
- [From(publication date): 0-2023 - Oct 19, 2025]
- Breakdown by view type
- HTML page views: 989
- PDF downloads: 307