Research Article Open Access
Biomarker Response in the Polychaete Nereis succinea as Early Signal for Heavy Metals Pollution in the Red Sea, Egypt
Rashad El Sayed Mohammed Said1*, AbdAllah Tharwat AbdAllah1, Mohsen Abdelhafez Mostafa1 and Nasser Abdellatif El-Shimy21Department of Zoology, Al Azhar University, Assiut Branch, Egypt
2Department of Zoology, Assiut University, Assiut, Egypt
- *Corresponding Author:
- Said REM
Department of Zoology, Al Azhar University, Egypt
Tel: +201027227142
E-mail: banjawy@yahoo.com
Received date: April 06, 2017; Accepted date: September 04, 2017; Published date: September 11, 2017
Citation: Said REM, AbdAllah AT, Mostafa MA, El-Shimy NA (2017) Biomarker Response in the Polychaete Nereis succinea as Early Signal for Heavy Metals Pollution in the Red Sea, Egypt. J Ecol Toxicol 1:104.
Copyright: © 2017 Said REM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecology and Toxicology
Abstract
The polychaete annelids (family nereidae) are abundant in intertidal zone. These animals have potential to accumulate and tolerate some toxic metals in their tissues. In the present study, the four heavy metals; Mn, Pb, Cd and Cu in the whole body of the polychaete worm Nereis succinea sampled from two areas were measured. The concentration of heavy metals determined for individuals inhabited both studied sites has confirmed the potential of the selected worm to accumulate heavy metals from ambient habitat. On the other hand, histopathological investigations have clarified that the granular deposition of heavy metals within tissues of N. succinea was related to the degree of heavy metals loads from site to another.
Keywords
Red sea; Nereis succinea ; Heavy metals; Biomarker; Histopathology
Introduction
Aquatic pollution is one of the issues that have gained a worldwide attention. Environmental pollutants impact not only threats the biotopes, but also deteriorates the human health [1]. Regarding aquatic pollution, both marine and fresh water eco systems are subjected to different types and sources of pollution. Among the most hazardous pollutants are heavy metals. The risk of heavy metals is being in the fact that these substances cannot be degraded or removed via biological processes and magnify in the food web [2-6].
The determination of metal concentration within tissues is the appropriate method to evaluate the bioavailability and the effective metal concentration in water. Therefore, it's easy to measure the heavy metal concentrations regardless of its amount in the natural environment [7,8]. The levels of biomagnification of heavy metals in food web containing fish for example as consumer were higher several folds than those in the surrounding habitat [9-11].
The polychaete Nereis succinea is benthic and thereby will expose to high concentrations of heavy metals from sediments and interstitial water. Such benthic organisms are intermediate links in the marine foodweb The polychaetes are capable of accumulation of heavy metals from their environment via different routes including ingestion of sediment and subsequent accumulation over gut epithelia as well as from pore water and overlying water via cutaneous uptake i.e. diffusion over the body surface [12]. The process of metal uptake is governed by many factors such as bio-ecological and physiological activities of organisms and species [13,14]. A good biomonitor organism is that can provide data and information about its ecosystem [7,10,11,15-17].
The selected organism selected for biomonitoring must accumulate contaminants several folds of their persistence in habitat. In addition, these accumulators should detoxify or convert the toxic substances into non-harmful stored compounds. Previous works have indicated the use of polychaetes for biomonitoring, since some polychaetes such as Nereis succinea possess the criteria of sentinel organisms that enable them to be the appropriate biomonitors [8,10,11,18].
The present study aims to compare the concentration of heavy metals; Mn, Cd, Cu and Pb at soft tissues of Nereis succinea inhabited marine biological station MBS and fish port station FBS. Histopathological studies will be conducted for individuals collected from two sites to evaluate the effect of accumulated heavy metals on gut tissues of studied individuals.
Materials and Methods
Study area and sampling protocol
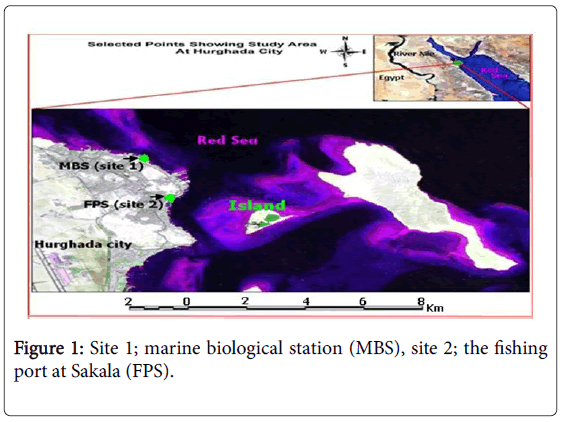
Two sites were chosen for the present study. The first one was the Marine Biological Station (MBS), and the second site was the Fishing Port at Sakala (FPS). Both sites (Figure 1) are located on the Red Sea coast at Hurghada city (Red Sea governorate).
Sampling
Worms were picked from sediments by hand. Worms were transferred into a container filled with sea water. To keep the body shape straight and somewhat to remain more uniform, worms were anaesthetized by adding drops of ethanol till specimens are relaxed, these should be added gradually (a few drops at a time). Then worms were transferred into a container and fixed with 10% normal formalin. After 24 hours, animals were ready to be transferred to 70% ethyl alcohol for identification and histopathological studies [19].
For metal analysis, worms were kept in freezer after transported to lab in ice bags. Heavy metals in tissues were determined according to [7,8,10,11].
For light microscopy, ethanol preserved worms were dehydrated, cleared, then embedded in paraffin and processed routinely for light microscopy. Tissues were sectioned at 5 μ and stained with haematoxylin and eosin (H&E) [20].
Sections were examined by light microscope and photographed. Head (with appendages) and parapodia were stained with acetocarmine stain prepared according to the method of [18].
Identification of polychaete species
For identification, all measurements were carried out using a scaled ruler on a binocular microscope according to [21,22]. Adult worms were chosen, “Adult females” are defined as individuals possessing microscopically visible coelomic gametes. Measurements of body width are performed without parapodia according to [23].
The common morphometric characters as width or length of setiger (segments bearing parapodia) width or length of the prostomium and the anterior part of the body or size used in identification according to [24-26].
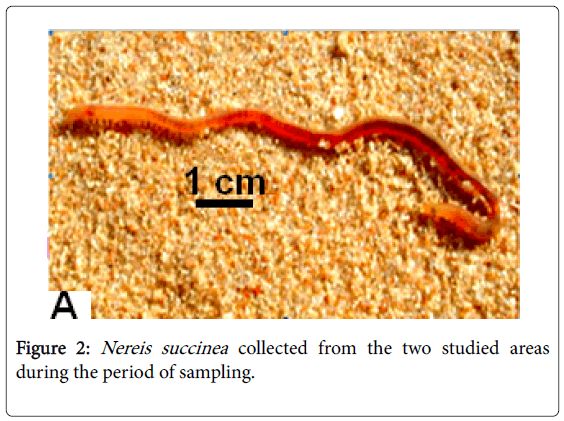
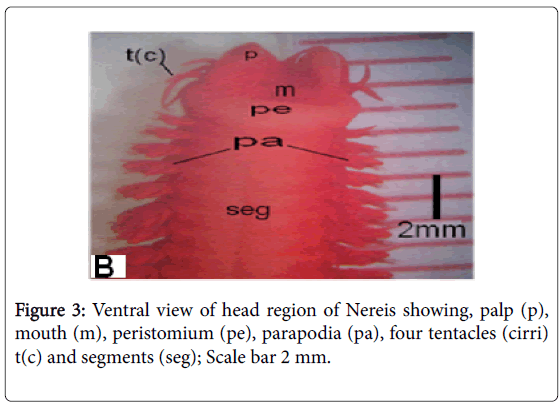
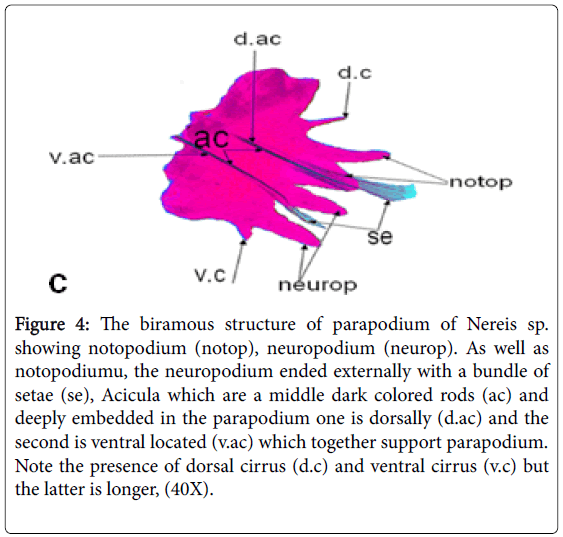
Because doubtable differences in morphological characters in the collected polychaetes were present, other groups of the same samples were sent to the "Educational Museum of the Egyptian Fauna in Zoology Department Faculty of Science Assiut University, for accurate identification for each site collected samples (Figures 2-4).
Figure 4: The biramous structure of parapodium of Nereis sp. showing notopodium (notop), neuropodium (neurop). As well as notopodiumu, the neuropodium ended externally with a bundle of setae (se), Acicula which are a middle dark colored rods (ac) and deeply embedded in the parapodium one is dorsally (d.ac) and the second is ventral located (v.ac) which together support parapodium. Note the presence of dorsal cirrus (d.c) and ventral cirrus (v.c) but the latter is longer, (40X).
Results and Discussion
Annelid worms were previously used as bioindicators for their inhabitant aquatic ecosystem. Bervoets et al. [27] used Tubificid worms as predictors for ecological changes in aquatic ecosystem. The present survey had revealed that two studied sites are different in their ecology. MBS is a protected area away from human activities and sources of pollutions. In contrast the 2nd site (FPS) is impacted by several activities of fishing. The annelid polychaetes belong to family nereidae and represented the same specie Nereis succinea . It has been noted that the second site is richer in nutrients in the term of eutrophication.
Consequently, animals of the second site are healthier than those of the first one. Surprisingly, the annelids from the second site are slightly shorter than the opposite population. To explain that, in previous work conducted by [19] on Nereis divirsicolor from two different sites in pollution degree, they found that Nereis divirsicolor was differed significantly from clean to polluted habitats.
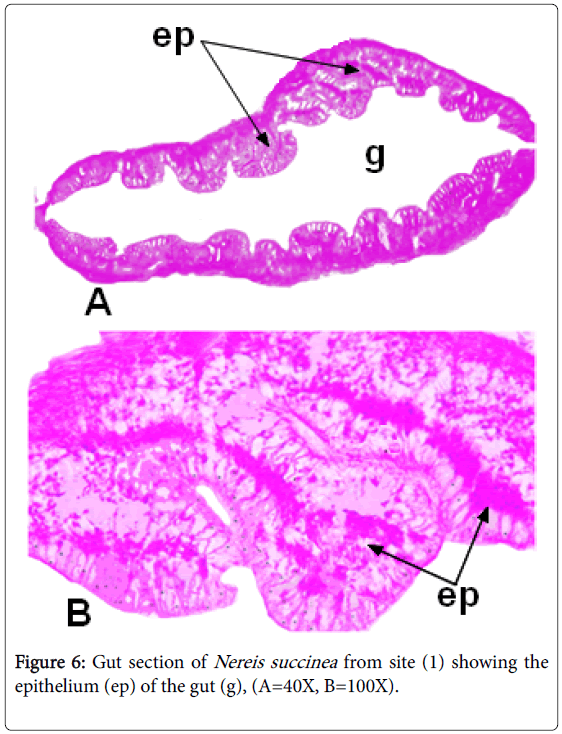
Authors [19] attributed these morphological differentiations to the nature of the two areas. We must take in consideration that disturbed ecosystems tend to be characterized by small reselected species [26]. In the current work, the polychaete Nereis succinea could take up and accumulate higher heavy metals concentrations (Table 1 and Figures 4 and 6) from the 2nd site than the 1st one.
| Site | Mn | Pb | Cd | Cu |
|---|---|---|---|---|
| Site 1 | 0.18 ± 0.02 | 0.59 ± 0.46 | 0.26 ± 0.23 | 0.31 ± 0.34 |
| Site 2 | 0.87 ± 0.56 | 0.78 ± 0.67 | 0.85 ± 0.33 | 0.95 ± 0.13 |
Table 1: Heavy metal concentrations (μg/g) in tissue of Nereis at the two sites.
The mean values of manganese (0.87 ± 0.56), cadmium (0.85 ± 0.33) and copper (0.95 ± 0.13) were more than three folds in the 2nd site when compared with those of the 1st site; (0.18 ± 0.02), cadmium (0.26 ± 0.23) and copper (0.31 ± 0.34). In fact sediments of FPS are muddy and finer than those of MBS. Sediments can be considered as a sink for many pollutants, thus the accumulation of heavy metals there represent the past and present accumulations. Selck and Forbes [12] stated that More than 90% of the Cd body burden in the polychaete Capitella sp. was resulted from sediment-associated pool of Cd.
It is easy for aquatic organisms to take up heavy metals from their habitat via the active transport though their permeable bodies [15]. Metal ions have affinity for proteins and other cellular constituents. The active transport pump through carrier protein has an active role as route of entry of some metals. Free cadmium ion for example, has a similar radius to that of calcium and will be taken up through the calcium [28,29].
The observation of granular depositions in epithelial layer lining the gut of Nereis collected from FPS might be attributed to metal storage, this finding agrees with [8,10]. Many phyla could store such granules in their bodies [30].
This work is in agreement with [26,31] who revealed the presence of accumulated copper in tissue of Nereis diversicolor appeared as greenblack deposited granules in the epidermis of the body wall and parapodia. Mild ulceration in the lining of gut has been observed in animals collected from the second site.
The deterioration of guts is a result of compartmentalization of granular metals in gut epithelium. Author observed the accumulation of zinc in barnacle Elminius modestus as detoxified pyrophosphate granules. In the fresh water Hyridella depressa, the microscopic examinations have revealed the presence of granular aggregations in different tissues.
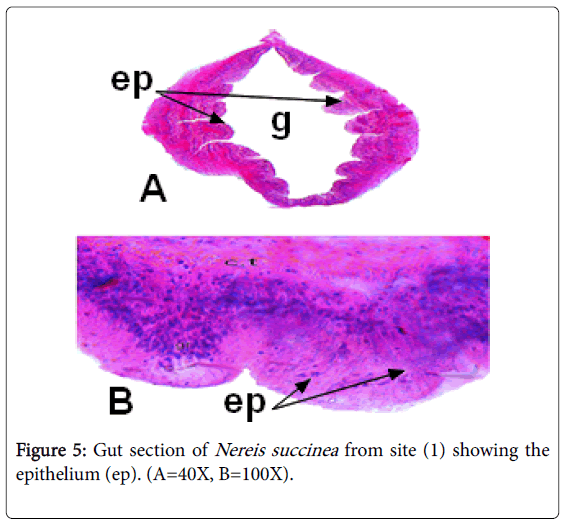
The presence of granules at the gut epithelial cells (Figure 5) may be attributed to the intake sediments adsorbed heavy metals on their surfaces. At the time of digestion, the adsorbed metals are collected at the epithelial cells as granular structures [28,29,32,33,]. In fact, the granular aggregations were previously thought to play roles in removal of toxic metals [15].
Future studies are needed to further investigate ecological biotic and abiotic factors that control bioaccumulation of heavy metals within soft tissues of polychaete worms.
Conclusion
The light microscope examinations of Nereis gut has demonstrated a high accumulation capability of the worms collected from polluted site (2) compared with that of worms collected from relatively clean site (1).
References
- Awheda I, Ahmed AY, Fahej MAS, Elwahaishi SS, Smida FA (2015) Fish as bioindicators of heavy metals pollution in marine environments: A review. IJAR.
- Ravera O (2001) Monitoring of the aquatic environment by species accumulator of pollutants: A review. Jlimnol 60: 63-78.
- Shukor Y, Baharom NA, Rahman FA, Abdullah MP, Shamaan NA et al (2006) Development of heavy metals enzymatic–based assay using papain. Anal Chim Acta 566: 283-289.
- Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2: 112-118.
- Torres MA, Barros MP, Sara CG, Campos EP, Satish R et al (2008) Biochemical Biomarkers in algae and marine pollution: A review. Ecotox Environ Safe .71
- Stanciu G, Mititelu M and Gutaga S (2005) Pesticides and heavy metals determination in marine organisms from Black Sea. Chemical Bulletin 50: 1-2.
- AbdAllah AT (2006) Investigations on bioconcentration and toxicity of lead and copper to the freshwater prosobranch Lanistes carinatus, Malacologia 48: 27-34.
- Said REM (2009) Ecological and biological studies on some marine annelids inhabiting the Red Sea, Egypt Al Azhar Univ. F.Sci. Assuit-Egypt.
- Stanchevai M, Makedonski L and Petrova E (2013) Determination of heavy metals (Pb, Cd, As and Hg) in Black Sea Grey Mullet (Mugil cephalus). Bulg J Agric Sci 19.
- AbdAllah AT (2014) Light structure as biomarker for heavy metal bioaccumulation and toxicity in molluscan gastropod. Formatex Publishers pp: 330-334.
- AbdAllah AT (2017) Efficiency of invertebrate animals for risk assessment and biomonitoring of hazardous contaminants in aquatic ecosystem, a review and status report. Environ risk assess remediat 1: 13-
- Selck H and Forbes VE (2004) The relative importance of water and diet for uptake and subcellular distribution of cadmium in the deposit–feeding polychaete, Capitella sp. Mar Environ Res 57: 261-279.
- Luoma SN (2002) Processes affecting trophic transfer and resultant effects of metals: Implications for monitoring metal pollution in the sea. Ciesm Workshop Monographs 75-78.
- Luoma SN (1983) Bioavailability of trace metals to aquatic organisms - A review. Sci total environ 28: 1-22.
- Simkiss K, Taylor M, and Mason AZ (1982) Metal detoxification and bioaccumulation in molluscs. Marine Biological Letters 3: 187-201.
- Philips DJH and Rainbow PS (1993) Biomonitors of Trace Aquatic Contaminants. Elsevier Applied Science. 371p.
- Rainbow PS and Philips DJH (1993) Cosmopolitan bio-monitors of trace metals. Marine Pollution Bulletin 26: 593-601.
- Said REM, AbdAllah AT, Mostafa MA & Elshimy NA (2017) Efficiency of polychaete Nereis (neanthes) succinea as biomonitor for heavy metals pollution in the Red Sea, Egypt. Atmos. Terr. Ocean. Sci 2: 18-23.
- Durou C, Smith BD, Romeo M, Rainbow PS, Mouneyrac C et al. (2007) From biomarkers to population responses in Nereis diversicolor: Assessment of stress in estuarine ecosystems. Ecotox Environ Safe 66: 402-411.
- Bancroft JD and Stevens A (1996) Theory and practice of histological techniques (3rd edn.) Churchill Livingstone, Edinburgh &New York.766p.
- Klemm DJ (1985) A guide to the fresh water Annelida (Polychaeta, Naidid, Tubificid Oligochaeta and Hirudinea) of North America.
- Omena EP, Amaral ACZ (2001) Morphometric study of the nereidid Laeonereis acuta (Annelida: Polychaeta). J Mar Biol Assoc U.K 81: 423-426.
- Gatenby JB, Beams HW (1950) The microtomistis Vade-Mecum (11th edn.) Bolles, Lee, London.
- Bakken T (2002) A new species of Neanthes (Polychaeta, Nereididae) from southern Australia. Memoirs of the Museum of Victoria 59: 327-331.
- Caron A, Boucher L, Desrosiers G, Retie`re C (1995) Population dynamic of the polychaetes Nephtys caeca in an intertidal estuarine environment (Qubec, Canada). J Mar Biol Assoc U.K 75: 871-884.
- Attrill MJ, & Depledge MH (1997) Community and population indicators of ecosystem health: Targeting links between levels of biological organization. Aquat Toxicol 38: 183-197.
- Bervoets L, De Jonge M, Blust R (2016) Identification of threshold body burdens of metals for the protection of the aquatic ecological status using two benthic invertebrates. Environ Pollut 210: 76-84.
- Pullen JSH, Rainbow PS (1991) The composition of pyrophosphate heavy metal detoxification granules in barnacles. J Exp Mar Biol Ecolo 150: 249-266.
- Wang W, Stupakoffl I, Fisher NS (1999) Bioavailability of dissolved and sediment-bound metals to a marine deposit-feeding polychaete. Mar Ecol Prog Ser 178: 281-293.
- Leno EN, Matrin PJ, Bastida R (2000) Estimation of size 23 classes in Laeonereis acuta (Polychaeta: Nererdidae) based on jaw length and body width useable in trophic studies. Bulltin of Marine Science 76: 39-43.
- Bryan GW, Hummerstone LG (1971) Adaptation of the polychaete Nereis diversicolor to estuarine sediments containing high concentrations of heavy metals 1-General observations and adaptation to copper. J Mar Biol Assoc U.K 51: 845- 863.
- Brown BE (1982) The form and function of metal containing ‘granules’ in invertebrate tissues. Biol Rev 57: 662-667.
- Byrne M, Vesk PA (2000) Elemental composition of mantle tissue granules in Hyridella depressa (Unionida) from the Hawkesbury-Nepean River system, Ausralia: Inferences from catchment chemistry. Mar Freshwater Res 51: 183-92.
Relevant Topics
- Biogeochemistry and climate
- Biomagnification
- Bioremediation
- Climate Change
- Cyanobacteria
- Ecological niche
- Ecological pyramids
- Ecological succession
- Endophytic bacteria
- Environmental monitoring
- Eutrophication
- Food pyramid
- Fresh water algal blooms
- Global warming
- Human ecology
- Hydrocarbons
- Microcystins
- Microenvironment
- Molecular ecology
- Persistent organic pollutants
- Polychlorinated biphenyls
- Radioactive wastes
- Soil Contamination
- Volatile organic compounds
- Water Quality
- Wind and turbulence
Recommended Journals
Article Tools
Article Usage
- Total views: 4026
- [From(publication date):
September-2017 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 3135
- PDF downloads : 891