Research Letter Open Access
Biological Treatment of Meat Processing Wastewater Using Lab-Scale Anaerobic-Aerobic/Anoxic Sequencing Batch Reactors Operated in Series
David Nzioka Mutua1*, Eliud Nyaga Mwaniki Njagi1, George Orinda1, Geoffry Obondi1, Frank Kansiime2, Joseph Kyambadde3, John Omara3, Robinson Odong3 and Hellen Butungi3
1Department of Biochemistry and Biotechnology, School of Pure and Applied Science, Kenyatta University, PO Box 43844, 00100, Nairobi, Kenya
2Department of Biochemistry, Institute of Environment and Natural Resources, Makerere University, PO Box 7062, Kampala, Uganda
3Department of Biochemistry, Faculty of Science, Makerere University, PO Box 7062, Kampala, Uganda
- *Corresponding Author:
- David Nzioka Mutua
Department of Biochemistry and Biotechnology
School of Pure and Applied Sciences
Kenyatta University, PO Box 43844-00100
Nairobi, Kenya
Tel: +254720261585
E-mail: mutua.david@ku.ac.ke
Received date: June 24, 2016; Accepted date: July 20, 2016; Published date: July 21, 2016
Citation: Mutua DN, Njagi ENM, Orinda G, Obondi G, Kansiime F, et al. (2016) Biological Treatment of Meat Processing Wastewater Using Lab-Scale Anaerobic- Aerobic/Anoxic Sequencing Batch Reactors Operated in Series. J Bioremed Biodeg 7: 362. doi:10.4172/2155-6199.1000362
Copyright: © 2016 Mutua DN, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In the eastern Africa sub-region, many industries discharge untreated effluents to nearby water resources, thereby polluting the environment. This is because the technologies applicable for wastewater treatment are expensive for these small-medium sized companies with low profit margins. Slaughterhouses belong to this category of industrial setup. The objective of this study was to investigate treatment of meat processing wastewater using anaerobic– aerobic/anoxic Sequencing Batch Reactors (SBRs) operated in series. Reactors were operated for one year using meat processing wastewater. Hydraulic retention time was 2 days for the anaerobic SBR, and 1 day for the aerobic/ anoxic SBR while the organic loading was 12.8 kg COD/m3/day. In the anaerobic SBR, removal efficiencies for total and soluble chemical oxygen demand (TCOD and SCOD), total suspended solids (TSS) and turbidity were 79, 76, 79, and 70%, respectively, with effluent mean concentrations of 3554 ± 58 mg/L, 762 ± 3 mg/L, 2307 ± 21, and 2800 ± 9 FAU. Conductivity, ammonia-nitrogen, ortho-phosphates and total phosphorus concentrations increased by 38, 80, 81 and 71%. Pollutant removal efficiencies in the aerobic/anoxic SBR were 98, 96, 97, 89, 74, 97, 91, 90, and 86% for TCOD, SCOD, BOD, TSS, turbidity, ammonium nitrogen (NH4 +–N), total nitrogen (TN), orthophosphorus (o-PO4 3-–P), and total phosphorus (TP), respectively. Except TKN (35 ± 4 mg/L) and o-PO4 3-–P (8 ± 1 mg/L), all other parameters in the aerobically treated effluent met national discharge standards. Thus, abattoir effluent can be treated using anaerobic–aerobic/anoxic SBR system.
Keywords
Biological treatment; City Abattoir; Meat processing; Sequencing batch reactors (SBR); Wastewater
Introduction
In the eastern Africa sub-region, facilities for the treatment of domestic and industrial effluents are either inefficient or non-existent thus leading to discharge of high contents of organic matter and nutrients (nitrogen and phosphorus) into nearby surface waters [1,2]. A typical example is City Abattoir (Kampala, Uganda), which on average discharges 400 m3/day of highly recalcitrant untreated effluent into Inner Murchison Bay of Lake Victoria causing oxygen depletion [3,4], eutrophication [5], health complications [1,6] and global warming [7]. As a result, there is pressure to meet environmental discharge standards in line with Sustainable Development Goal (SDG) number 6.
Activated sludge-based sequencing batch reactor (SBR) is an efficient system for treating organic-rich effluents [8]. The SBR technology is well documented in laboratory [5,9], pilot-scale [10] and full-scale studies [11,12]. The bioreactor can be anaerobic, aerobic or anoxic [3]. Each phase has four sequential steps: feed, react, settle and draw [9]. Transitioning between anaerobic, aerobic and anoxic conditions triggers the use of different electron donors and acceptors, thus promoting the transformation and thereby removal of carbon, nitrogen and phosphorus [8].
Biological nitrogen removal involves nitrification: a two-step aerobic oxidation of ammonia to nitrate [12]. Ammonia oxidizing bacteria such as Nitrosospira, Nitrosomonas, Nitrosolobus and Nitrosococcus oxidizes ammonia to nitrite and then, nitrite oxidizing bacteria such as Nitrobacter, Nitrospina and Nitrococcus oxidizes nitrite to nitrate [3,4]. This process is usually combined with denitrification, during which nitrate/nitrite is reduced to nitrogen-N2 by anoxic heterotrophic bacteria like Bacillus and some fungi [12]. During denitrification, electrons from organic carbon are transferred to nitrate instead of oxygen to create a proton motive force for ATP generation [8]. This process is inhibited by dissolved oxygen which represses nitrate reduction enzyme [13].
Simultaneous nitrification and denitrification can occur in a single reactor under low dissolved oxygen concentration [14]. This is due to the occurrence of aerobic/anoxic micro zones in the floc or within the bioreactor and also due to the presence of new types of microorganisms [12]. The environmental factors affecting nitrification include: pH, un-ionized ammonia, un-ionized nitrous acid, reduced sulphur components and metals [13].
Biological phosphorus removal (BPR) depends is dependent on excessive uptake of phosphorus by phosphorus-accumulating organisms (PAOs). This is achieved by alternating anaerobic, aerobic and anoxic conditions (those containing nitrate, not oxygen, as an electron acceptor) [8]. Under anaerobic conditions, organic matter is mineralized into volatile fatty acids (VFAs) which are taken up by PAOs and stored as poly-ß-hydroxyalkaonates (PHAs) [14]. The energy to assimilate the VFAs is derived from breakdown of polyphospates (PPs) bonds, releasing phosphorus into the solution [10]. During the aerobic/anoxic phase, the stored PHA is oxidized to release energy for the PAOs to take up phosphorus from solution and form intracellular PPs for cellular growth [3]. Oxygen acts as an electron acceptor [10]. Since phosphorus release in the anaerobic phase is less than its uptake under aerobic/anoxic conditions, net phosphorus removal from mixed liquor is achieved. Phosphorus is removed from the bioreactor by wasting excess phosphorus-rich sludge [15]. BPR is affected by pH, sludge retention time, excessive aeration, nitrate, nitrite, temperature and carbon source [13].
In combined denitrification and BPR systems, carbon availability is the limiting factor. Denitrifiers and PAOs are in competition for the available carbon. Both processes are disturbed by this competition hence a fine balance should be struck on the length of the aerobic and anoxic phase [10].
The configuration and operation of SBR depends on the type of the wastewater and the treatment objectives [3]. SBR can be anaerobic, aerobic or anoxic [8]. Most of the SBRs have one or two of these phases with only a few three-phase combination [3,16]. Besides, the bio-system performance efficiency depends on the sequential arrangement of these phases, the duration of each phase, hydraulic retention time, sludge retention time, organic loading and environmental factors [8,17,18]. The objective of this study was to investigate biological treatment of City Abattoir meat processing wastewater using anaerobic–aerobic/ anoxic SBRs operated in series. To evaluate the treatment performance of the system, removal performances for carbon, nitrogen and phosphorus were analyzed.
Materials and Methods
Model reactors
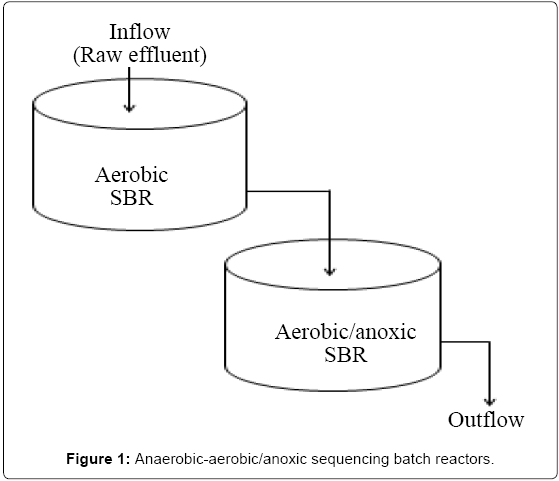
Two reactors made of plastic, each with a total volume of 250 L and a working liquid volume of 200 L were set up at Makerere University and operated at room temperature. The anaerobic and aerobic SBR were seeded with anaerobic and aerobic sludge obtained from a brewery wastewater treatment Plant at Port bell, Luzira, Uganda. The initial concentration of mixed liquor volatile suspended solids (MLVSS) was approximately 10,000 mg/L. The reactors were batch-fed periodically with raw wastewater collected from City Abattoir, and the organic loading rate progressively increased by augmenting the volume of wastewater fed to the systems. After steady-state conditions were obtained (3 months), the reactors were operated sequentially (Figure 1), with each reactor operational cycle consisting of the feed, reaction, settling and, draw phases. The 24 hr operating cycle consisted of the following periods: (a) filling, 0.30 hours; (b) reaction, 41 hours; and (c) decanting, 0.30 hours for the anaerobic reactor, and (a) filling, 0.25 hours; (b) reaction, 17 hr; (c) settling, 6.5 hours and (d) decanting, 0.25 hours for the aerobic reactor. At the end of each cycle, 100 litres of the supernatant was decanted, followed by feeding of an equal amount of wastewater. The system operated at a nominal Sludge Retention Time (SRT) of 5 days and a total Hydraulic Retention Time (HRT) of 2 days and 1 day for the anaerobic and aerobic/anoxic SBR, respectively. The organic loading was 12.8 kg COD/m3/day, during the study period. The properties of wastewater used during experimental studies are shown in Table 1.
| Parameter | Anaerobic SBR | ||
|---|---|---|---|
| Inflow conc. | Outflow conc. | Removal efficiency (%) | |
| TCOD | 15812 ± 241 | 3554 ± 58 | -79 |
| SCOD | 3176 ± 100 | 762 ± 3 | -76 |
| BOD5 | 13659 ± 67 | 1869 ± 27 | -86 |
| TKN | 1022 ± 139 | 400 ± 30 | -61 |
| NH4–N | 58 ± 9 | 288 ± 7 | +80 |
| NO2–N | NIL | NIL | - |
| NO3–N | NIL | NIL | - |
| TP | 61 ± 8 | 129 ±1 | +71 |
| o-PO43-–P | 16 ± 1 | 82 ±1 | +81 |
| Turbidity | 9335 ± 130 | 2800 ±9 | -70 |
| TS | 10760 ± 300 | 2307 ±21 | -79 |
| pH | 6.57 ± 0.12 | 6.56 ± 0.03 | -0.2 |
| EC | 1.86 ± 0.2 | 3.02 ± 0.01 | +38 |
| Temperature | 23.53 ± 0.1 | 25.7 ± 0.2 | +9 |
| Concentrations of TCOD, BOD5, TKN, NO2-N, NO3-N, TP, o-PO43-, and TS are expressed in mg/L; Turbidity, EC, and temperature are expressed in (FAU), (ms/ cm3) and (°C), respectively. - signifies reduction, + signifies increment. | |||
Table 1: Mean ± standard error values of the physiochemical parameters determined for the raw wastewater, and anaerobic SBR effluent (n=8).
Analytical procedure
Physical water quality variables (pH, electrical conductivity and temperature) were measured in situ twice a week using portable WTW (Wissenchaftlich Technishe Werkstatten) microprocessor probes and meters. Chemical parameters such as ammonium-nitrogen (NH4–N), nitrite-nitrogen (NO2–N), nitrate-nitrogen (NO3–N), total kjeldahl nitrogen (TKN), ortho-phosphate (o-PO43-–P), total phosphorus (TP), biochemical oxygen demand (BOD5), soluble chemical oxygen demand (SCOD), total chemical oxygen demand (TCOD), turbidity, and solids content (TSS) were analyzed according to standard methods.
Statistical analysis
Data analysis was done using ANOVA to compare the treatment performance of the SBRs based on parameter removal efficiency determined from influent and treated effluent mean ± standard error values.
Results
Treatment of abattoir effluent in anaerobic sequencing batch reactor
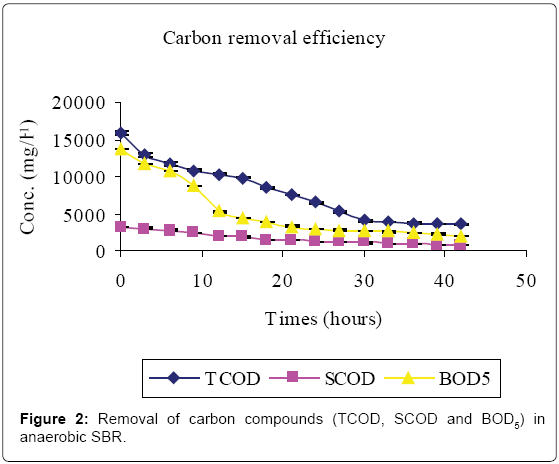
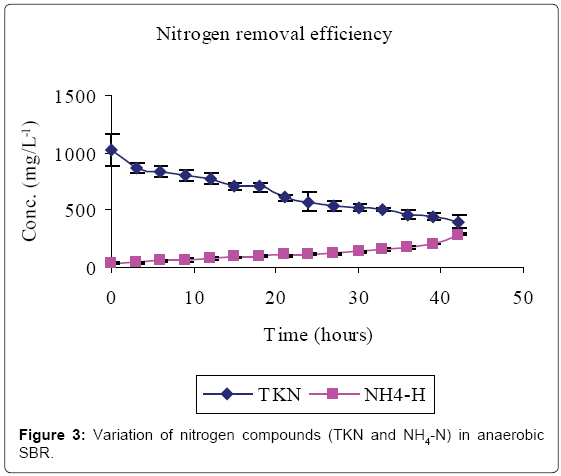
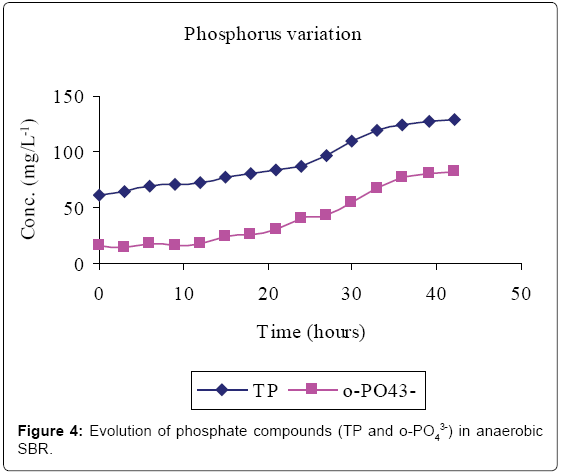
Figures 2-4 and Table 1 show the characteristics of inflow and outflow wastewater, together with pollutant removal efficiencies attained during anaerobic sequencing batch reactor treatment. The influent concentrations of TCOD, SCOD, BOD5, TKN, NH4-N, TP, o-PO43-, turbidity, TS, pH, EC and temperature were 15812 ± 241 mg/L, 3176 ± 100 mg/L, 13659 ± 67 mg/L, 1022 ± 139 mg/L, 58 ± 9 mg/L, 61 ± 8 mg/L, 16 ± 1 mg/L, 9335 ± 130 FAU, 10760 ± 300 mg/L, 6.57 ± 0.12, 1.86 ± 0.2 ms/cm3 and 23.53 ± 0.1°C, respectively.
The removal efficiencies for TCOD, SCOD, BOD5, TKN, turbidity and TSS were 79, 76, 86, 61, 70 and 79% respectively, with effluent mean concentrations of 3554 ± 58 mg/L, 762 ± 3 mg/L, 1869 ± 27 mg/L, 400 ± 30 mg/L, 2800 ± 9 FAU and 2307 ± 21 mg/L, respectively. Comparably, NH4-N, TP, o-PO43-, pH, EC and temperature increased by 80, 71, 81, 0.2, and 38% registering an effluent concentration of 288 ± 7 mg/L, 129 ± 1 mg/L, 82 ± 1 mg/L, 6.56 ± 0.03, 3.02 ± 0.01 ms/cm and 25.7 ± 0.2°C, respectively.
Treatment of abattoir effluent in aerobic/anoxic sequencing batch reactor
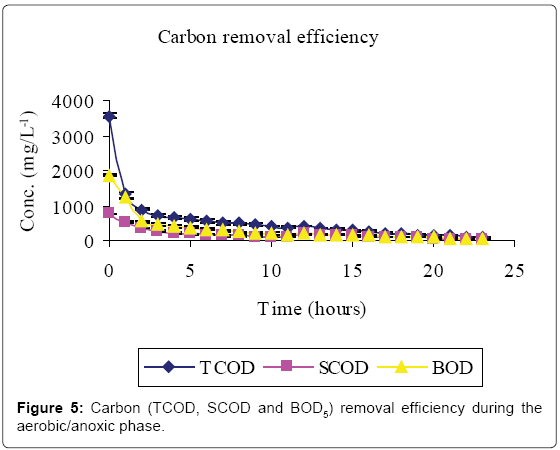
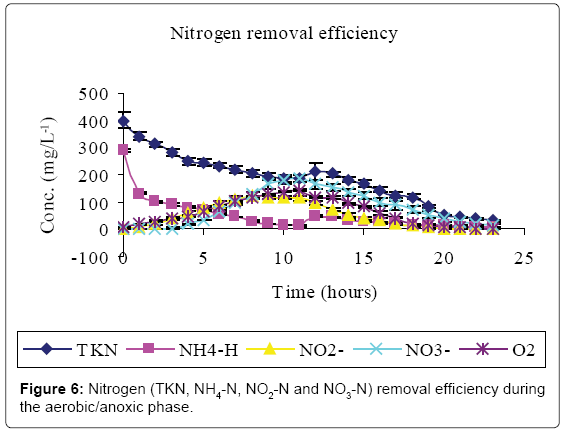
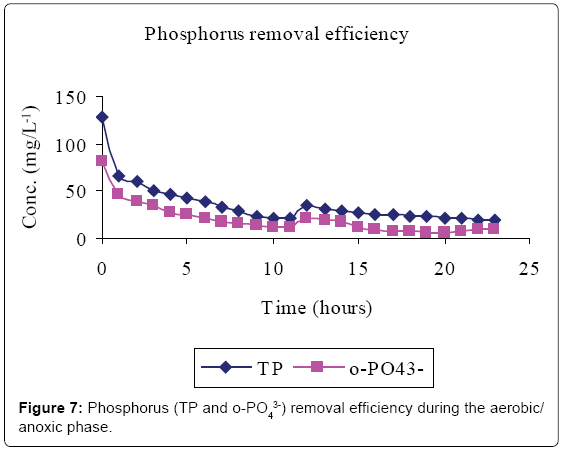
Figures 5-7 and Table 2 show the inflow and outflow parameters of wastewater, together with pollutant removal efficiencies attained during aerobic/anoxic sequencing batch reactor treatment. The influent concentrations of TCOD, SCOD, BOD5, TKN, NH4-N, NO2-N, NO3-N, TP, o-PO43-, turbidity, TSS, pH, EC and temperature were 321 ± 75 mg/L, 923 ± 12 mg/L, 1210 ± 32 mg/L, 383 ± 20 mg/L, 233 ± 7 mg/L, 0 mg/L, 0 mg/L, 81 ± 1 mg/L, 67 ± 5 mg/L, 2762 ± 50 FAU, 1350 ± 47 mg/L, 0.91 ± 0.1 mg/L 6.98 ± 0.04, 2.91 ± 0.17 mS/cm and 23.84 ± 0.11°C, respectively.
| Parameter | Aerobic/anoxic SBR | ||
|---|---|---|---|
| Inflow conc. | Outflow conc. | Percentage change | |
| TCOD | 3554 ± 58 | 80 ± 5 | -98 |
| SCOD | 762 ± 3 | 31 ± 10 | -96 |
| BOD5 | 1869 ± 27 | 54 ± 12 | -97 |
| TKN | 400 ± 30 | 35 ± 4 | -91 |
| NH4–N | 288 ± 7 | 8 ± 1 | -97 |
| NO2–N | NIL | .00 ± 0 | -100 |
| NO3–N | NIL | 16 ± 8 | +16 |
| TP | 129 ±1 | 18 ± 1 | -86 |
| o-PO43-–P | 82 ±1 | 8 ± 1 | -90 |
| Turbidity | 2800 ±9 | 738 ± 9 | -74 |
| TS | 2307 ±21 | 254 ± 12 | -89 |
| pH | 6.56 ± 0.03 | 7.00 ± 0.0 | +6 |
| EC | 3.02 ± 0.01 | 1.64 ± 0.01 | -46 |
| Temperature | 25.7 ± 0.2 | 22.04 ± 0.02 | -14 |
| DO | 0.91 ± 0.1 | 1 ± 3 | +9 |
| Concentrations of TCOD, BOD5, TKN, NO2-N, NO3-N, TP, o-PO43-, and TS are expressed in mg/L; Turbidity, EC, and temperature are expressed in (FAU), (ms/ cm3) and (°C), respectively. - signifies reduction, + signifies increment. | |||
Table 2: Mean ± standard error values of the different parameters determined for the raw wastewater, and aerobic/anoxic SBR effluent (n=8).
In the aerobic-anoxic phase, TCOD, SCOD, BOD5, TKN, NH4-N, TP, o-PO43-, turbidity, TSS and temperature removal efficiencies were 98, 96, 97, 91, 97, 86, 90, 74, 89 and 14% respectively, with effluent mean concentrations of 80 ± 5 mg/L, 31 ± 10 mg/L, 54 ± 12 mg/L, 35 ± 4 mg/L, 8 ± 1 mg/L, 18 ± 1 mg/L, 8 ± 1 mg/L, 738 ± 9 FAU, 254 ± 12 mg/L and 22.04 ± 0.02 ± 0.1°C, respectively. Comparably, NO2-, NO3- and DO which had increased in aerobic phase by 115, 184 and 94% decreased in anoxic phase by 100, 98 and 93% to register an effluent concentration of 0.00 ± 0, 16 ± 8 and 1 ± 3 mg/L, respectively. During this phase pH, EC and temperature varied from 6.71, 1.64 ms/cm3, and 22.04°C to 7.64, 7.71 ms/cm3 and 25.32°C to register an effluent concentration of 7.00 ± 0.0, 1.64 ± 0.01 ms/cm3 and 22.04 ± 0.02°C, respectively.
Discussion
This study has shown that meat processing effluent can be treated biologically using anaerobic–aerobic/anoxic SBRs operated in sequence. The high Carbon (TCOD, SCOD and BOD5) levels in the influent (Table 1, Figure 2) were mainly due to the fact that the abattoir effluent employed in this study was of high organic strength [2]. Cellulose, which mainly originates from animal feed residues, is a major component of abattoir effluents which contributes significantly to COD and suspended solids [19]. Slaughterhouse wastewater is also rich in proteins originating from blood which has a TCOD of 375, 000 mg/L-1 [1].
A reduction in SCOD in Figure 2 was due to microbial activity while total COD, TSS and turbidity reductions were due to microbial activity, solids settlement and floatation [10,14]. Anaerobic fill phase was marked by high TKN reduction (Figure 3) due to settling of the blood [20]. Heterotrophic ammonification further decreased organic nitrogen in a process that also produces CO2 and HCO3- [14]. The HCO3- production increased the system alkalinity, and thus pH, while ammonification raised the pH and EC [21].
Lipids in the wastewater were anaerobically hydrolysed to long chain fatty acids (LCFA) and glycerol which are subsequently acidified to volatile fatty acids (VFAs) [14]. Phosphate accumulating organisms (PAOs; mainly Candidatus Accumulibacter phosphatis) preferentially assimilate VFA across their cell membranes for the synthesis of intracellular carbon/energy reserves of poly-ß-hydroxyalkanoate (PHA) [3]. The energy required for VFA assimilation is provided by the hydrolysis of high-energy intracellular polyphosphate bonds [10]. This resulted in the release of orthophosphorus (Figure 4) and increased temperature. The early stages of anaerobic phase were characterised by low phosphorus release because only VFA in the influent was available. However, as more VFA was made available by bacterial fermentation, more polyphosphate bonds were cleaved to supply the additional energy demands for the VFA uptake hence more phosphorus was released.
Total phosphorus content was found to be higher than that of orthophosphate in the study carried out. This was mainly due to the fact that orthophosphate represents the reactive fraction of phosphorus which is biologically available, while dissolved organic and inorganic phosphorus are generally not biologically available [15].
Compared to the influent raw wastewater, the treatment efficiencies obtained for this reactor were high but still do not meet national discharge standards (COD, 100 mg/L; TSS, 100 mg/L; Turbidity, 300 NTU/FAU; NH4–N, 10 mg/L, TN, 10 mg/L; ortho-P, 5 mg/L and total-P, 10 mg/L; [22]). Thus, the anaerobic effluent necessitated further processing in the aerobic SBR to reduce pollutant concentrations.
At the initial stages of aeration, there was a conspicuous delay in the first occurrence of both NO2- and NO3- when the DO was below 0.91 ± 0.1 mg/L-1. Although effective nitrification has been reported in systems with residual oxygen as low as 0.5 mg/L-1, DO concentrations below 1.5 ml/L limit the nitrification process [4,5]. Moreover, the nitrification is inhibited at pH below 6.8 [23] which existed at this point. Autrotrophic nitrifiers first use NH4–N for cell synthesis, the NH4–N that is left after cell synthesis is then removed via nitrification [24].
Aeration caused loss of protons as a result of CO2 stripping creating a localized high pH. The high pH caused re-distribution of ammonium ions to the more volatile ammonia form [25,26]. Heterotrophic assimilation and volatilization can thus account for the high carbon and nitrogen treatment efficiency observed in Figures 5 and 6, respectively.
Nitrification started once the DO and pH were in the applicable range of 6.9. Ammonia is oxidized to NO2- which is subsequently oxidized to NO3- concentration [3]. This explains the higher concentrations of NO3- than NO2- at the end of aerobic phase (Figure 6).
In the aerobic phase, the organic matter serves as the electron donor while the oxygen serves as the electron acceptor [8]. Heterotrophs have a higher affinity for NH4 –N and oxygen than autrotrophs [12]. PAOs, being heterotrophs, preferentially take up oxygen and hydrolyse the high-energy PHA phosphoanhydride bonds for energy to grow and assimilate phosphate (released in the anaerobic zone and additional phosphate present in the influent wastewater) in amounts that are much greater than biosynthetic needs. The phosphates are converted into intracellular polyphosphate stores [10]. This caused a decrease in phosphate levels in the bulk liquid (Figure 7). Since PHA oxidation releases 24 to 36 times more energy in the aerobic zone than is used to store polyhydroxybutyrate (PHB) in anaerobic zone, phosphorus uptake is significantly more than the phosphorus release [15].
In the anoxic phase, oxidized nitrogen compounds were reduced to dinitrogen gas [12]. Denitrifiers use organic carbon as energy source and NO2--NO3- as electron sink [8]. However, the preceding nitrification phase is known to consume reducing power [3] hence sufficient organic carbon must be provided for proper denitrification [10]. Obaja et al. [27] and Pedros et al. [28] suggest the use of wastewater as a source of carbon.
Anaerobic effluent (equivalent to 10% of the aerobic reactor operational volume) was used to supply organic carbon required for denitrification during the settling phase. This volume was experimentally found to be sufficient for complete denitrification of oxidized forms of nitrogen within the system. The introduction of NH4 –N rich anaerobic effluent accounted for the spike in nitrogen content at the onset of denitrification (Figure 6). Denitrification results in a rise in alkalinity of the system [13], with corresponding increase in pH. According to Azhdarpoor et al. [3] assimilative and dissimilative carbon utilization by denitrifying and other bacteria is responsible for further carbon reduction in Figure 5.
Phosphorus removal in the aerobic/anoxic SBR was above the recommended discharge limits, with outflow concentration of 8 ± 1 and 18 ± 1 mg/L registered for o-PO43-–P and TP, respectively possibly due to its release during the anoxic settlement phase of the SBR system [10,13], and hence minimal phosphorus removal through sludge wasting [29].
Conclusions
Meat processing wastewater can be efficiently treated using anaerobic–aerobic/anoxic SBRs operated in series. Except TKN (35 ± 4 mg/L) and o-PO43-–P (8 ± 1 mg/L), all other parameters (TCOD, SCOD, BOD, TSS, turbidity, NH4 +–N, and TP) in the treated effluent met national discharge standards.
Acknowledgements
This study received financial support from the Swedish International Development Co-operation Agency (Sida)/Department of Research Co-operation (SAREC) under the East African Regional Programme and Research Network for Biotechnology Policy Development (BIO-EARN).
Conflict of Interest
The authors declare no conflict of interest.
References
- Baskar M, Sukumaran B (2015) Effective Method of Treating Wastewater from Meat Processing Industry Using Sequencing Batch Reactor. International Research Journal of Engineering and Technology 2: 27
- Kundu P, Debsarkar A, Mukherjee S (2013) Treatment of Slaughter House Wastewater in a Sequencing Batch Reactor: Performance Evaluation and Biodegradation Kinetics. BioMed Research International 2013: 1-11.
- Azhdarpoor A, Mohammadi P, Dehghani M (2016) Simultaneous removal of nutrients in a novel anaerobic–anoxic/aerobic sequencing reactor: removal of nutrients in a novel reactor. International Journal of Environmental Science and Technology 13: 543-550
- Kyambadde J (2005) Optimizing Processes for Biological Nitrogen Removal in Nakivubo Wetland, Uganda. PhD Thesis. Department of Biotechnology, Royal Institute of Technology, Stockholm, Sweden, pp: 1-62.
- Abubakar S, Latiff AA, Lawal IM, Jagaba AH (2016) Aerobic treatment of kitchen wastewater using sequence batch reactor (SBR) and reuse for irrigation landscape purposes. American Journal of Engineering Research 5: 23-31
- Dhanalakshmi D, Maleeka BSF, Rajesh G (2016) Biodegradation and bioremediation of food industry waste effluents. International Journal of Advanced Research in Science, Engineering and Technology 3: 1195-1197
- Yang X, Wu X, Hao H, He Z (2008) Mechanisms and assessment of water eutrophication. Journal of Zhejiang University Science 9: 197-209.
- Singhal N, Perez-Garcia O (2016) Degrading Organic Micropollutants: The Next Challenge in the Evolution of Biological Wastewater Treatment Processes. Frontiers in Environmental Science 4: 1-5
- Lam KY, Zytner RG, Chang S (2016) Treatment of High Strength Vegetable Processing Wastewater with a Sequencing Batch Reactor. Journal on Agricultural Engineering 2: 30-33
- Lochmatter S, Maillard J, Holliger C (2014) Nitrogen Removal over Nitrite by Aeration Control in Aerobic Granular Sludge Sequencing Batch Reactors. International Journal of Environmental Research and Public Health 11: 6955-6978
- Eslami H, Hematabadi PT, Ghelmani SV, Vaziri AS, Derakhshan Z (2015) The Performance of Advanced Sequencing Batch Reactor in Wastewater Treatment Plant to Remove Organic Materials and Linear Alkyl Benzene Sulfonates. Health Science 7: 33-34
- Fernandes H, Jungles MK, Hoffmann H, Antonio RV, Costa RHR (2013) Full-scale sequencing batch reactor (SBR) for domestic wastewater: Performance and diversity of microbial communities. Bioresource Technology 132: 262-268
- Puig S, Corominas LI, Balaguer MD, and Colprim J (2007) Biological nutrient removal by applying SBR technology in small wastewater treatment plants: carbon source and C/N/P ratio effects. Water sci technol. 55: 135-141
- Aponte-Morales VE, Tong S, Ergas SJ (2016) Nitrogen Removal from Anaerobically Digested Swine Waste Centrate Using a Laboratory-Scale Chabazite-Sequencing Batch Reactor. Environmental Engineering Science 33: 324-332
- Gebremariam SY, Beutel MW, Christian D, Hess TF (2011) Research Advances and Challenges in the Microbiology of Enhanced Biological Phosphorus Removal - A Critical Review. Water Environment Research 83: 195-219
- KuÅÂ?mierczak J, Anielak P, Rajski L (2012) Long-term cultivation of an aerobic granular activated sludge. Electronic Journal of Polish Agricultural Universities 15: 6.
- Danial O, Salim MR, Salmiati (2016) Nutrient removal of grey water from wet market using sequencing batch reactor.Malaysian Journal of Analytical Sciences 20: 142-148
- Milia S, Malloci E, Carucci A (2016) Aerobic granulation with petrochemical wastewater in a sequencing batch reactor under different operating conditions. Desalination and Water Treatment, pp: 1-10.
- Mittal G (2007) Regulations Related to Land-application of Abattoir Wastewater and Residues. Agricultural Engineering International: the CIGR E-journal, Invited Overview 10: 1-37
- Mane SS, Munavalli GR (2012) Sequential Batch Reactor- Application to Wastewater –A Review Proceeding of International Conference SWRDM-2012, Maharastra, India, pp: 1-8
- Merzouki M, Bernet N, Delgenes J, Benlemlih M (2005) Effect of pre-fermentation on denitrifying phosphorus removal in slaughterhouse wastewater. Bio-resource Technology 96: 1317-1322
- http://www.gov.ug/content/national-environment-management-authority-nema
- Hayatsu M, Tago K, Saito M (2008) Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Science and Plant Nutrition 54: 33-45.
- Szatkowska B (2007) Performance and control of biofilm systems with partial nitritation and anammox for supernatant treatment. PhD Thesis, p: 1035. ISBN 978-91-7178-729-3.
- Szatkowska B (2007) Performance and control of biofilm systems with partial nitritation and anammox for supernatant treatment. PhD Thesis, p: 1035. ISBN 978-91-7178-729-3.
- Paredes D, Kuschk P, Mbwette T, Stange F, Müller R, et al. (2007) New Aspects of Microbial Nitrogen Transformations in the Context of Wastewater Treatment – A Review. Engineering in Life Sciences 7: 13-25.
- Kim J, Zuo Y, Regan J, Logan B (2008) Analysis of Ammonia Loss Mechanisms in Microbial Fuel Cells Treating Animal Wastewater. Biotechnology and Bioengineering 99: 112-1127.
- Obaja D, Macé S, Mata-Alvarez J (2005) Biological nutrient removal by a sequencing batch reactor (SBR) using an internal organic carbon source in digested piggery wastewater. Bioresource Technology 96: 7-14
- Pedros PB, Onnis-Hayden A, Tyler C (2008) Investigation of nitrification and nitrogen removal from centrate in a submerged attached growth bioreactor. Water Environment Research 80: 222-228
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13172
- [From(publication date):
July-2016 - Jan 29, 2025] - Breakdown by view type
- HTML page views : 12166
- PDF downloads : 1006