Research Article Open Access
Biological Effects of the Plasticizer Tris (2-Ethylhexyl) Trimellitate
| Harunobu Iwase1, Shigeru Oiso2, Hiroko Kariyazono2 and Kazuo Nakamura1* | |
| 1 Department of Biopharmaceutics, Nihon Pharmaceutical University, Japan | |
| 2 Division of Pharmaceutical Health Care and Sciences, Department of Pharmacy, Faculty of Pharmaceutical Sciences, Nagasaki International University, Japan | |
| Corresponding Author : | K Nakamura Department of Biopharmaceutics Nihon Pharmaceutical University 10281 Komuro, Ina-Cho, Kitaadachi-gun Saitama 362-0806, Japan Tel: +81-48-721-1155 Fax: +81-48-721-7267 E-mail: vkazunaka@nichiyaku.ac.jp |
| Received March 05, 2014; Accepted March 20, 2014; Published March 22, 2014 | |
| Citation: Iwase H, Oiso S, Kariyazono H, Nakamura K (2014) Biological Effects of the Plasticizer Tris (2-Ethylhexyl) Trimellitate. Clin Pharmacol Biopharm S2:004. doi:10.4172/2167-065X.S2-004 | |
| Copyright: © 2014 Iwase H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Objectives: An alternative plasticizer, tris (2-ethylhexyl) trimellitate (TOTM), was developed from di-(2-ethylhexyl) phthalate (DEHP) for use in medical tubing. However, little is known about the biological effects of TOTM and thus its safety is not yet well-established. We investigated the leachability of TOTM from TOTM-plasticized Polyvinylchloride (PVC) feeding tubes. Furthermore, we studied whether TOTM influences the cell toxicity of human leukemia (HL)-60 cells, the cell proliferation of human breast cancer MCF-7 cells, and the binding affinity for human estrogen receptor α.
Methods: TOTM or DEHP-plasticized PVC feeding tubes were incubated with liquid nutriment containing soybean based salad oil. Thereafter, these solutions were mixed with extract solution and centrifuged, respectively. Obtained supernatant was served as extract sample. TOTM and DEHP were confirmed by high performance liquid chromatography (HPLC); molecular ion peaks corresponding to TOTM were detected by LC/mass spectrometry. The effects of these plasticizers on human leukemia (HL)-60 viability and breast cancer cell MCF-7 proliferations were investigated. Furthermore, the affinity of these plasticizers for binding human estrogen receptor (ER)-α was measured using an enzyme-linked immunosorbent assay (ELISA) kit. Results: An existence of TOTM was confirmed in an extract sample by HPLC and LC/MS. Furthermore, TOTM decreased viability HL-60 cells while enhancing MCF-7 cell proliferation. However, these effects-as well as the activation of ER-α- were weaker by approximately 10-fold weaker for TOTM than DEHP. Conclusion: The leaching of TOTM from TOTM-plasticized PVC feeding tubes is less toxic than DEHP, adverse effects may be associated with the production of ovarian estradiol via activation of a receptor-mediated signaling pathway. These findings nonetheless indicate that TOTM is a viable and safer alternative to DEHP for plasticizing medical devices for human use.
| Keywords | |
| TOTM; DEHP; Feeding tube; Soybean; LC; LC/mass spectrometry; MCF-7 cells; Human estrogen receptor α; Cytotoxicity | |
| Introduction | |
| Polyvinylchloride (PVC) is used in a wide range of medical devices because of its versatility. PVC is a relatively rigid and brittle polymer. Consequently, plasticizers, which are added to facilitate processing, make PVC flexible, resilient, and easier to handle. Many phthalates including di (2-ethylhexyl) phthalate (DEHP) are commonly used as plasticizers. Many plasticizer-containing PVC medical devices are used as intravenous fluid bags and tubing, blood and plasma bags, enteral feeding and dialysis equipment, catheters, and gloves. DEHP is not chemically bound to the PVC polymer and may leach when PVC medical devices are exposed to blood and drugs. The U.S. FDA Center for Devices and Radiological Health has published a review of the potential patient health risks associated with DEHP leaching from PVC medical devices [1]. Risk assessment of DEHP released from PVC medical devices is an important issue for medical patients. Several reports have demonstrated that plasticizers are easily released when such devices are used with lipophilic substances [2-5]. Furthermore, it has been reported that several pharmaceuticals, such as miconazole, cyclosporin, etoposide, chlordiazepoxide hydrochloride and tacrolimus, are adsorbed into the tubing of an intravenous administration set concurrent with the release of DEHP [6-10]. Some reports have demonstrated that the release of DEHP from PVC tubing is facilitated by surfactants, such as polysorbate 80 (Tween 80) and polyethylene castor oil (HCO60) that are used as solubilizers for water-insoluble drugs [4,11,12]. Subotic et al. [13] reported that DEHP leached from PVC tubes when DEHPcontaining PVC nasogastric tubes were incubated with gastric juice or a feeding solution. Furthermore, Tanaka et al. [14] reported that the concentration of DEHP in the liquid nutriment, namely Ensure Liquid® solution, increased linearly over time and subsequently reached a plateau when this enteral nutrition passed through the PVC tube during enteral tube feeding in a temperature-dependent manner. In contrast, there have been no reports concerning the leachability of tris (2-ethylhexyl) trimellitate (TOTM) from TOTM- plasticized PVC feeding tubes during the incubation of the liquid nutriment. | |
| Several reports have demonstrated that some phthalate esters including DEHP exhibit reproductive, developmental, and testicular toxicities [15-19], and the antiproliferative and adverse effects of DEHP on human keratinocytes [20]. With general levels of intake, reproductive risk appears to be minimal, whereas specific uses, such as long-term medical treatment, have presented potential problems [21]. The effect of DEHP on humans is not well understood, although DEHP intake from intravenous treatment or enteral tubing should be avoided. The Ministry of Health, Labour, and Welfare of Japan has classified DEHP as a chemical agent suspected of causing endocrine disruption and restricted oral tolerable daily intake (TDI) to 0.14 mg/(kg day) [22]. Moreover, data from AdvaMed and other significant studies have prompted the Center for Devices and Radiological Health (CDRH) and the U.S. Food and Drug Administration (FDA) to propose a parenteral tolerable intake of DEHP of 0.6 mg/kg/day [1]. Recently, PVC tubing that contains TOTM as an alternative plasticizer to DEHP has been developed. In 2006, the Japan Medical Devices Manufacturers Association reported a list of PVC-free and DEHP-free medical devices available in Japan [23]. Therefore, the number of medical devices using TOTM-plasticized PVC as an alternative to DEHP-plasticized PVC has increased compared with previous years. There are several reports concerning the leachability of TOTM compared with that of DEHP. Therefore, examining the possibility of TOTM leaching from PVC medical devices under various conditions is necessary. | |
| The methyl thiazolyl tetrazolium (MTT) assay is a colorimetric assay that measures the activity of cellular enzymes by reducing tetrazolium dye to insoluble formazan [24,25]. The MTT assay is generally used to assess cytotoxicity (loss of viable cells) or cytostatic activity (shift from proliferative to resting status) of potential medicinal agents and toxic materials. The human leukemia HL-60 cell line has been used as a model system for studying leukemic cell differentiation [26,27]. This cell line can be induced to differentiate into granulocyte-like or monocyte-like cells by various agents, and differentiated cells lose their proliferative ability. Lee et al. [28] examined the pharmacological activity of DEHP by MTT assay using HL-60 cells. | |
| It has been reported that at least two different estrogen receptors (ER) exist, namely, ERα and ERβ [29], and ERβ might modulate the estrogenic effects of ERα by forming heterodimers with ERα, thereby reducing the ERα-mediated response, resulting in the inhibition of ERα-mediated proliferation of breast cancer cells in vitro [30-32]. Endocrine disruptors (EDs), which are exogenous compounds such as xenoestrogens, including bisphenols and alkylphenols, some polychlorinated biphenyls, and phthalates, can induce estrogenmediated signaling in an abnormal manner by binding to ERs [33,34]. Therefore, xenoestrogens, i.e., EDs, have been assessed as risk factors for human health problems including the immature reproduction system and adverse biological mechanisms in the environment [35-37]. Several reports have demonstrated that xenoestrogens bind to estrogen receptors and mimic estrogenic actions [38-42]. An estrogenic activity of some phthalate plasticizers was investigated using several in vitro tests, such as MCF-7 cell proliferation, ER binding in the rat uterus, and yeasts transfected with human ER gene construct [40-43]. The MCF-7 breast cancer cell line was derived from a pleural effusion taken from a metastatic breast cancer patient [44] and is an ideal model to study the mechanism of estrogenic action because these cells express functional wild-type estrogen receptors [45]. This cell line was used to develop an in vitro screening assay to detect xenoestrogens, such as polychlorinated biphenyls, bisphenol A, and phthalates [42,46,47]. Presently, TOTM has been used as a plasticizer in Japan instead of DEHP because of its suspected relevance as an ED as well as its obstruction of the function of testis and reproduction. In Japan, TOTM-plasticized PVC is commonly used in a wide range of medical-grade materials, such as feeding tubes. However, few studies have reported the possible patient risk of exposure to TOTM-containing medical devices. | |
| In the present study, we investigated whether liquid nutriment that included soybean-containing salad oil can leach TOTM from TOTMplasticized PVC feeding tubes. In addition, we measured HL-60 cell viability and MCF-7 cell proliferation to compare the cell toxicity of TOTM to that of DEHP as well as the endocrine-disrupting risks of these two plasticizers. | |
| Materials and Methods | |
| Chemicals and reagents | |
| DEHP (Purity: >98%) and TOTM (Purity: >95%) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Dimethyl sulfoxide (DMSO), isopropanol, ethyl acetate, methanol, and acetonitrile were obtained from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Roswell Park Memorial Institute (RPMI) 1640 medium, Dulbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were obtained from Life Technologies Corporation (Carlsbad, CA, USA). Charcoal was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Ensure Liquid was obtained from Abbott Laboratories (Abbott Park, IL, USA). Nisshin sarada oil was obtained from The Nisshin OilliO Group, Ltd. (Tokyo, Japan). TOTM-plasticized PVC feeding tubes (content of TOTM, approximately 30%) were obtained from Atomed Cebu, Inc. (Lapu-lapu, Philippines). Penicillin A and streptomycin were obtained from Meiji Seika Pharma Co., Ltd. (Tokyo, Japan). Tissue culture test plates were obtained from TPP AG (CH- 8219, Trasadingen, Switzerland) | |
| Cells | |
| Human leukemia HL-60 cells were obtained from DS Pharma Biomedical Co., Ltd. (Osaka, Japan). The HL-60 cells were cultured in RPMI medium supplemented with antibiotics (100 U/mL of penicillin A and 100 U/mL of streptomycin) and 10% heat-inactivated FBS and maintained in a 37°C humidified incubator containing 5% CO2. Human breast cancer MCF-7 cells were obtained from National Institute of Biomedical Innovation JCRB Cell Bank (Osaka, Japan). The MCF-7 cells were cultured in DMEM with 5% heat-inactivated FBS and maintained in a 37ºC humidified incubator containing 5% CO2. | |
| Methodology Evaluation of TOTM release from TOTMplasticized PVC feeding tubes | |
| Nutrient samples were produced as follows: 240 mL of liquid nutriment (Ensure Liquid) was added to 10 mL of the soybeancontaining salad oil, and this nutrient served as the test nutriment. In contrast, the liquid nutriment without salad oil served as the control nutrient. Ten centimeters of TOTM-plasticized PVC feeding tube (inside diameter ; 3 mm and outside diameter ; 4mm) were soaked in 30 mL of the test nutrient or the control nutrient, and incubated at 40°C for 24 h. Subsequently, 1 mL exudate was added to 5 mL extract solution (methanol : acetone = 1 : 1), and was mixed by vortex-mixer. Thereafter, this solution was centrifuged for 10 min at 2190 × g, and collected supernatant were served as extract sample. | |
| Measurement of TOTM and DEHP | |
| The existence of TOTM in extract sample was confirmed in high performance liquid chromatography (HPLC) and liquid chromatography/mass spectrometry (LC/MS) [46]. HPLC conditions of TOTM analysis were as follows: column, Waters XTerra Phenyl, 5 μm (4.6 mm i.d. × 250 mm length, Waters Co., Milford, MA, USA); mobile phase, 3:2:1 (v/v) acetonitrile:methanol : distilled water ; flow rate, 1 mL/min (HPLC), 0.5 mL/min (LC/MS); internal standard, TOTM or DEHP; detector, SPD-20AVP; column oven, CTO-20AVP; calculator, LC solution (Shimadzu Co., Kyoto, Japan). The LC/MS was performed using an LC/MS-2010 EV (Shimadzu Co.) equipped with an electrospray ionization source (ESI). | |
| Cell viability assay | |
| The MTT assay was performed to measure the mitochondrialdependent reduction of tetrazolium dye to insoluble formazan as an indicator of cell viability. HL-60 cells were seeded in 96-well plates at a density of 2×104/mL (0.2 ml/well) and incubated for 24 h. Different concentrations of TOTM and DEHP were added to the plates. After incubation for 48 h, cells were washed with PBS twice, and 10 μL of MTT solution (5 mg/mL in PBS) were added to the plates that were incubated for an additional 4 h. Subsequently, 100 μL of acidic isopropanol was added to each well that was then mixed by vigorous pipetting to dissolve the precipitated formazan. UV-visible absorption was measured at 595 nm using an ELISA plate reader (ImmunoMini NJ-2300, Cosmo Bio Co., Ltd., Tokyo, Japan). The addition of ethanol instead of plasticizers to the HL-60 cells served as the control (plasticizer included 0 mM). Cell viability was examined in the absence and presence of various concentrations of TOTM and DEHP. Cell numbers were expressed as percentages, where the control with 0.1% ethanol alone was designated as 100%. | |
| Cell proliferation assay | |
| MCF-7 cells were grown and maintained in DMEM supplemented with 5% FBS. The MCF-7 cells were plated into 12-well tissue culture test plates at an initial concentration of 1,000 cells per well, and the medium was replaced by experimental medium (5% charcoal-dextran human serum supplemented with phenol red-free DMEM) after a 24-h incubation in the growth medium. The plasticizers were dissolved in ethanol, diluted with phenol-red free DMEM, and added to each well. The culture was continued for 72 h, and the absorbance was measured at 540 nm using an ELISA plate reader (Immuno Mini NJ-2300). Cell proliferation was examined in the absence (at 0 mM) and presence of various concentrations of TOTM and DEHP. The concentration of plasticizers at 0 mM served as the control. Cell numbers were expressed as percentages, where the control with 0.1% ethanol alone was designated as 100%. | |
| Screening of TOTM and DEHP for ERα ligands | |
| The formation of an ERα-TOTM or -DEHP complex to the coactivator peptide on the plate was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (EnBio RCAS for ERα, Tokyo, Japan) [48,49]. After immobilization of the coactivator (SRC1 peptide) to the wells of the plate, the plate was incubated at 25°C for 1 h with gentle shaking; ERα dilution standards and samples were added to the plate. After five washes, horseradish peroxidase (HRP)-conjugated anti-human ER antibody was added into each well and incubated at 37°C for 30 min. After five washes, 50 μL of tetramethylbenzene (TMB) substrate was added into each well, and the plate was incubated in the dark at 37°C for 15 min. After the addition of the stop solution, each well was read at an absorbance of 450 nm using an ELISA plate reader (ImmunoMini NJ-2300). The absorbance of the plate was measured in the absence (at 0 mM) and presence of various concentrations of TOTM and DEHP. The concentration of plasticizers at 0 mM served as the control. Ligand intensity was expressed as a percentage, where the control with 5% DMSO alone was designated as 100%. | |
| Statistical analysis | |
| All experiments were performed five times. Results are presented as the mean ± standard deviation (SD). Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Fisher’s protected least-significant difference test. Parametric data were analyzed by two-way repeated measures ANOVA followed by Scheffe’s post hoc test. Proportional differences were analyzed using the Fisher’s exact chi-square analysis. Differences were considered to be significant at P<0.05. Stat View J-4.5 software (Abacus Concepts Inc., CA, USA) was employed for all calculations. | |
| Results | |
| HPLC analysis of plasticizers (DEHP and TOTM) standard products and Peak-1 | |
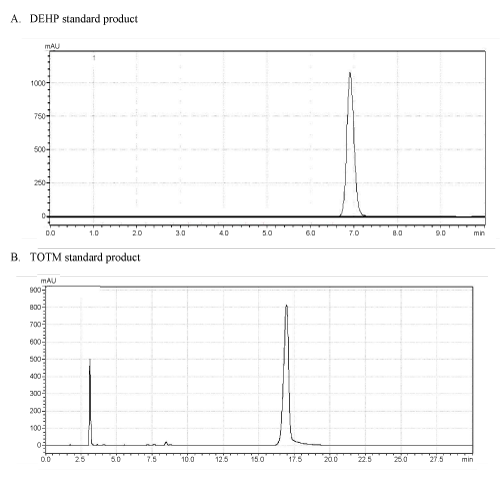
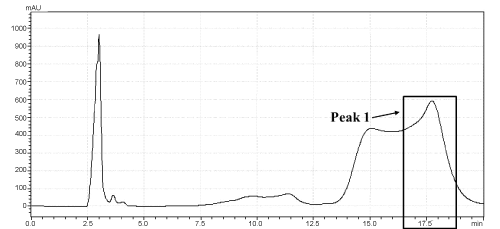
| As shown in Figure 1, the retention times of DEHP standard product (A) and TOTM standard product (B) in similar chromatographic conditions were approximately 6.9 min and 17.0 min, respectively. The test nutriment which immersed a TOTM-plasticized PVC feeding tube was concentrated, and this concentrate was analyzed by HPLC. This concentrate contained a peak showing the same retention time as TOTM standard product, and area of this peak was named Peak-1 (Figure 2). The concentration of TOTM in the Peak-1 increased in a time-dependent manner. The cumulative amount of TOTM released from the PVC feeding tube up to 4h in the Peak-1 was less than 100 μg. On the other hand, the concentrate of the control nutrient did not show a TOTM peak. | |
| Analysis of TOTM and Peak-1 by LC/MS | |
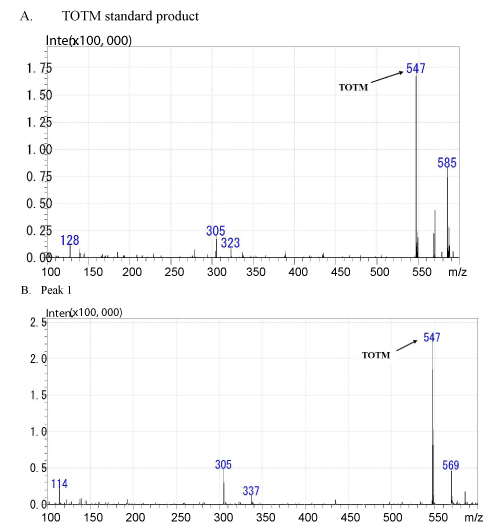
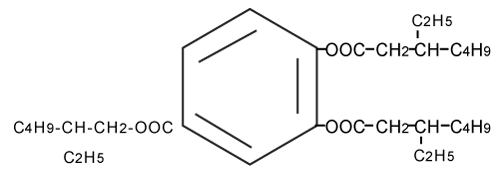
| As shown in Figure 3A, the molecular ion peak of TOTM standard product was obtained at m/z 547 (M+) by LC/MS. The elementary analysis of TOTM generated C33H54O6 as the molecular formula (Figure 4). As shown in Figure 3B, a molecular ion peak similar to TOTM was observed in the Peak-1. | |
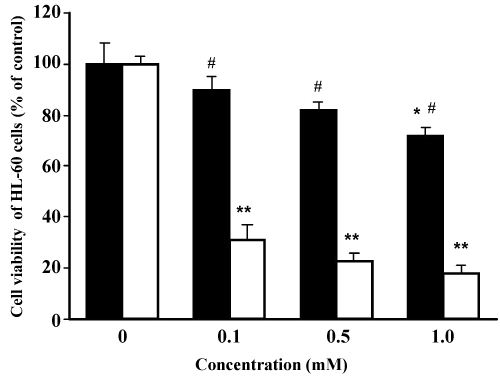
| Effects of TOTM and DEHP on HL-60 cell viability | |
| As shown in Figure 5, HL-60 cell viability at 1.0 mM TOTM was 78 ± 7%, and the value was significantly less than that of the control. In contrast, HL-60 cell viability at 0.1 mM DEHP decreased dramatically to 32 ± 8%. Cell viability at 1.0 mM DEHP was 21 ± 6%, and this value was significantly less than that at 1.0 mM TOTM. | |
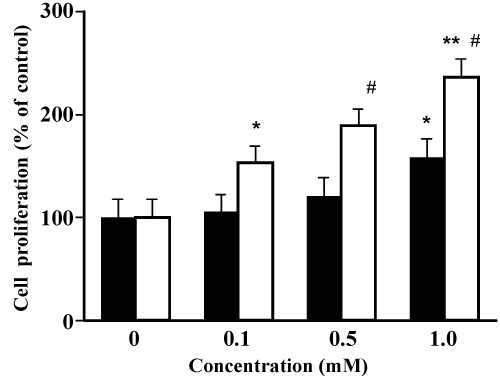
| Effects of TOTM and DEHP on MCF-7 cell proliferation | |
| As shown in Figure 6, TOTM-treated MCF-7 cell proliferation increased in a concentration-dependent manner. MCF-7 cell proliferation at 1.0 mM TOTM was 155 ± 8%, and this value was significantly greater than that of the control. On the other hand, proliferative effects of DEHP on MCF-7 cells were observed at 0.1 mM concentration, and this value (151 ± 7%) was significantly greater than that of the control. Proliferative effects of DEHP on MCF-7 cells were approximately 10-fold greater compared with those of TOTM. | |
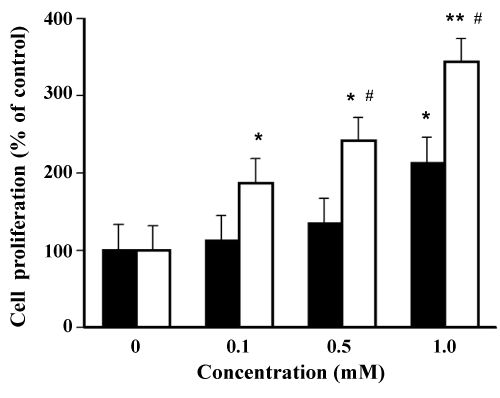
| Stimulating action of plasticizers to ERα | |
| As shown in Figure 7, the formation of ERα-TOTM complex to SRC1 peptide (% ligand intensity) at 1.0 mM TOTM was 212 ± 17%, and this value was significantly greater than that of the control. In contrast, the ligand intensity (%) induced by 0.1 mM DEHP treatment was significantly greater than that of the control, and this value (358 ± 28%) was significantly greater than that of the control. The ligand intensity (%) of DEHP increased in a concentration-dependent manner. Stimulating action of DEHP to ERα was approximately 10- fold greater compared with those of TOTM. | |
| Discussion | |
| First, we clarified the leachability of TOTM from TOTM-plasticized PVC feeding tubes when this feeding tube was incubated with liquid nutriment (Ensure Liquid) that included salad oil. In contrast, we did not observe TOTM in the extract of the control nutrient, namely the liquid nutriment without the salad oil. These phenomena suggest that salad oil leaches TOTM from the feeding tube. The concentration of TOTM in the extracts was confirmed by HPLC and LC/MS. There have been some reports concerning the leachability of TOTM from TOTMplasticized PVC medical devices. According to Ito et al. [46], in the case of Prograf and Florid-F, there is a significant difference between the concentration of TOTM released from gamma-ray-sterilized PVC/ TOTM tubes and that from unsterilized control tubes. Flaminio et al. [50] measured plasma concentrations of TOTM and/or its metabolites and demonstrated that TOTM might leach from TOTM-containing dialysis tubes, although the amount of TOTM released from the TOTM-containing dialysis tubes was less than the amount of DEHP released from the DEHP-containing dialysis tubes. | |
| Next, we investigated the anti-proliferative effects of TOTM and DEHP in HL-60 cells using the MTT assay and determined that 1.0 mM TOTM inhibited HL-60 cell viability. In contrast, the inhibitory effects of DEHP were 5-fold stronger than those of TOTM. Our results have been supported by the reports of Iwata et al. [26] and Lee et al. [28]. Other reports have indicated that DEHP causes a spectrum of toxic effects in developing and adult animals and in multiple organ systems including the liver, reproductive tract (i.e., testes, ovaries, and secondary sex organs) kidneys, lungs, and heart [2,15,51,52]. Furthermore, Latini et al. [53] indicated that DEHP has been shown to cause reproductive and developmental toxicity and is suspected to be an endocrine disruptor. More research may be necessary to clarify the toxic effects associated with TOTM exposure in humans. | |
| We investigated the proliferative effect of plasticizers using MCF- 7 cells. Many in vitro studies have reported the estrogenic effect of some phthalates by ERα-binding assays, proliferation assays using MCF-7 cells, and ERα-dependent transcription assays [38-42,54]. Moreover, these estrogenic responses at 10-5 M were suppressed by the ER antagonist, tamoxifen, suggesting that the estrogenic activities of phthalates might be induced by binding to ERα. In the present study, 1.0 mM TOTM-treated MCF-7 cells exhibited a significant increase in cell proliferation. Therefore, 1.0 mM TOTM may possess a proliferative effect in MCF-7 cells. In contrast, the proliferative effect of DEHP was approximately 10-fold stronger than that of TOTM because this effect was observed at 0.1 mM DEHP. Our results may be supported by Choi and Jeung [55]. We have considered that TOTM as well as DEHP may affect cell proliferation, apoptosis, and cell survival via estrogendependent signaling through ERα [37]. Furthermore, we investigated the endocrine-disrupting risks associated with these two plasticizers by measuring the affinity of plasticizers to ER proteins [56]. In our results, the estrogenic activity of TOTM was observed at 1.0 mM, and this effect is significantly weaker than that of DEHP. This is the first report concerning the estrogenic activity of TOTM via ERα activation. However, more research concerning the in vivo kinetics of TOTM and /or its metabolites are required to clarify the effects of TOTM in humans. | |
| In our results, TOTM as a plasticizer demonstrated acute toxicity to HL-60 cells. However, acute toxicity of TOTM was approximately 10-fold weaker than that of DEHP. On the other hand, proliferative effects of TOTM on MCF-7 cells may be correlated to the activation of ERα. Furthermore, these effects of TOTM was approximately 10-fold weaker than that of DEHP. Several reports supported these effects of DEHP [39,41,45,57]. | |
| Conclusion | |
| We have discovered that TOTM from PVC feeding tubes was leached by exposure to salad oil. Furthermore, it has been demonstrated that TOTM may possess cell toxicity and estrogenic activity through a receptor-mediated signaling pathway. However, these reactions of TOTM are significantly weaker compared with that of DEHP. Therefore, additional research may be necessary to confirm and extend these observations. | |
| Acknowledgements | |
| We would like to thank PhD. H. Saitou for the excellent technical assistance provided during the course of these studies. | |
| References | |
References
- FDA (2001) Center for Devices and Radiological Health, U.S. Food and Drug Administration.
- Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M (2001) Health risks posed by use of Di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am J Ind Med 39: 100-111.
- Kambia K, Dine T, Azar R, Gressier B, Luyckx M, et al. (2001) Comparative study of the leachability of di(2-ethylhexyl) phthalate and tri(2-ethylhexyl) trimellitate from haemodialysis tubing. Int J Pharm 229: 139-146.
- Takehisa H, Naoko E, Masahiko S, Katsuhide T, Moriyuki O, et al. (2005) Release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing used for intravenous administration and the plasticized PVC membrane. Int J Pharm 297: 30-37.
- Ito R, Seshimo F, Haishima Y, Hasegawa C, Isama K, et al. (2005) Reducing the migration of di-2-ethylhexyl phthalate from polyvinyl chloride medical devices. Int J Pharm 303: 104-112.
- Venkataramanan R, Burckart GJ, Ptachcinski RJ, Blaha R, Logue LW, et al. (1986) Leaching of diethylhexyl phthalate from polyvinyl chloride bags into intravenous cyclosporine solution. Am J Hosp Pharm 43: 2800-2802.
- Pearson SD, Trissel LA (1993) Leaching of diethylhexyl phthalate from polyvinyl chloride containers by selected drugs and formulation components. Am J Hosp Pharm 50: 1405-1409.
- Faouzi M el-A, Dine T, Luyckx M, Brunet C, Mallevais ML, et al. (1995) Stability, compatibility and plasticizer extraction of miconazole injection added to infusion solutions and stored in PVC containers. J Pharm Biomed Anal 13: 1363-1372.
- Demoré B, Vigneron J, Perrin A, Hoffman MA, Hoffman M (2002) Leaching of diethylhexyl phthalate from polyvinyl chloride bags into intravenous etoposide solution. J Clin Pharm Ther 27: 139-142.
- Takehisa H, Naoko E, Masahiko S, Katsuhide T, Moriyuki O, et al. (2005) Release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing used for intravenous administration and the plasticized PVC membrane. Int J Pharm 297: 30-37.
- Muramatsu E, Hanawa T, Suzuki M, Tanaka M, Kawano K, et al. (2000) Investigation of the effect of the coexistence of surfactant on the release behavior of diethylhexyl phthalate from the polyvinyl chloride tubing. Jpn J Hosp Pharm 25: 471-477.
- Hanawa T, Muramatsu E, Asakawa K, Suzuki M, Tanaka M, et al. (2000) Investigation of the release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing for intravenous administration. Int J Pharm 210: 109-115.
- Subotic U, Hannmann T, Kiss M, Brade J, Breitkopf K, et al. (2007) Extraction of the plasticizers diethylhexylphthalate and polyadipate from polyvinylchloride nasogastric tubes through gastric juice and feeding solution. J Pediatr Gastroenterol Nutr 44: 71-76.
- Tanaka M, Kawano K, Hanawa T, Suzuki M, Nakajima S (2002) Dissolusion of di-2-ethylhexyl phthalate from polyvinyl chloride tube during enteral tube feeding. Jpn J Pharm Health Care Sci 28: 152-156.
- Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, et al. (1997) Subchronic oral toxicity of di-n-octyl phthalate and di(2-Ethylhexyl) phthalate in the rat. Food Chem Toxicol 35: 225-239.
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, et al. (2000) The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58: 339-349.
- Li LH, Jester WF Jr, Laslett AL, Orth JM (2000) A single dose of Di-(2-ethylhexyl) phthalate in neonatal rats alters gonocytes, reduces sertoli cell proliferation, and decreases cyclin D2 expression. Toxicol Appl Pharmacol 166: 222-229.
- Kang JS, Morimura K, Toda C, Wanibuchi H, Wei M, et al. (2006) Testicular toxicity of DEHP, but not DEHA, is elevated under conditions of thioacetamide-induced liver damage. Reprod Toxicol 21: 253-259.
- Han SW, Lee H, Han SY, Lim DS, Jung KK, et al. (2009) An exposure assessment of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) in human semen. J Toxicol Environ Health A 72: 1463-1469.
- Martinasso G, Maggiora M, Trombetta A, Canuto RA, Muzio G (2006) Effects of di(2-ethylhexyl) phthalate, a widely used peroxisome proliferator and plasticizer, on cell growth in the human keratinocyte cell line NCTC 2544. J Toxicol Environ Health A 69: 353-365.
- McKee RH, Butala JH, David RM, Gans G (2004) NTP center for the evaluation of risks to human reproduction reports on phthalates: addressing the data gaps. Reprod Toxicol 18: 1-22.
- National Institute of Technology and Evaluation (2003) —Summary of the interim report— Bis (2-ethylhexyl) phthalate.
- Japan Medical Devices Manufacturers Association.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
- Plumb JA, Milroy R, Kaye SB (1989) Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res 49: 4435-4440.
- Iwata K, Ogata S, Okumura K, Taguchi H (2003) Induction of differentiation in human promyelocytic leukemia HL-60 cell line by niacin-related compounds. Biosci Biotechnol Biochem 67: 1132-1135.
- Ninomiya M, Nishida K, Tanaka K, Watanabe K, Koketsu M (2013) Structure-activity relationship studies of 5,7-dihydroxyflavones as naturally occurring inhibitors of cell proliferation in human leukemia HL-60 cells. J Nat Med 67: 460-467.
- Lee KH, Hong HS, Lee CH, Kim CH (2000) Induction of apoptosis in human leukaemic cell lines K562, HL60 and U937 by diethylhexylphthalate isolated from Aloe vera Linne. J Pharm Pharmacol 52: 1037-1041.
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, et al. (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863-870.
- Hanstein B, Liu H, Yancisin MC, Brown M (1999) Functional analysis of a novel estrogen receptor-beta isoform. Mol Endocrinol 13: 129-137.
- Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F (2001) ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology 142: 4120-4130.
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, et al. (2004) Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 64: 423-428.
- An BS, Choi KC, Kang SK, Hwang WS, Jeung EB (2003) Novel Calbindin-D(9k) protein as a useful biomarker for environmental estrogenic compounds in the uterus of immature rats. Reprod Toxicol 17: 311-319.
- Zhang Z, Feng Y, Gao P, Wang C, Ren N (2011) Occurrence and removal efficiencies of eight EDCs and estrogenicity in a STP. J Environ Monit 13: 1366-1373.
- Colborn T (1995) Environmental estrogens: health implications for humans and wildlife. Environ Health Perspect 103 Suppl 7: 135-136.
- Lee HR, Hwang KA, Park MA, Yi BR, Jeung EB, et al. (2012) Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. Int J Mol Med 29: 883-890.
- Lee HR, Kim TH, Choi KC (2012) Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res 28: 71-76.
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP (1995) A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect 103: 582-587.
- Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, et al. (1995) The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect 103 Suppl 7: 113-122.
- Harris CA, Henttu P, Parker MG, Sumpter JP (1997) The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105: 802-811.
- Zacharewski TR, Meek MD, Clemons JH, Wu ZF, Fielden MR, et al. (1998) Examination of the in vitro and in vivo estrogenic activities of eight commercial phthalate esters. Toxicol Sci 46: 282-293.
- Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, et al. (1999) Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect 107 Suppl 1: 89-108.
- Levenson AS, Jordan VC (1997) MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res 57: 3071-3078.
- Seeger H, Diesing D, Gückel B, Wallwiener D, Mueck AO, et al. (2003) Effect of tamoxifen and 2-methoxyestradiol alone and in combination on human breast cancer cell proliferation. J Steroid Biochem Mol Biol 84: 255-257.
- Okubo T, Suzuki T, Yokoyama Y, Kano K, Kano I (2003) Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol Pharm Bull 26: 1219-1224.
- Ito R, Miura N, Iguchi H, Nakamura H, Ushiro M, et al. (2008) Determination of tris(2-ethylhexyl)trimellitate released from PVC tube by LC-MS/MS. Int J Pharm 360: 91-95.
- Weber Lozada K, Keri RA (2011) Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol Reprod 85: 490-497.
- Tremblay A, Tremblay GB, Labrie F, Giguère V (1999) Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell 3: 513-519.
- Tremblay A, Giguère V (2001) Contribution of steroid receptor coactivator-1 and CREB binding protein in ligand-independent activity of estrogen receptor beta. J Steroid Biochem Mol Biol 77: 19-27.
- Flaminio LM, De Angelis L, Ferazza M, Marinovich M, Galli G, et al. (1988) Leachability of a new plasticizer tri-(2-ethylhexyl)-trimellitate from haemodialysis tubing. Int J Artif Organs 11: 435-439.
- Lamb JC 4th, Chapin RE, Teague J, Lawton AD, Reel JR (1987) Reproductive effects of four phthalic acid esters in the mouse. Toxicol Appl Pharmacol 88: 255-269.
- Lovekamp-Swan T, Davis BJ (2003) Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 111: 139-145.
- Latini G, Verrotti A, De Felice C (2004) DI-2-ethylhexyl phthalate and endocrine disruption: a review. Curr Drug Targets Immune Endocr Metabol Disord 4: 37-40.
- Kim IY, Han SY, Moon A (2004) Phthalates inhibit tamoxifen-induced apoptosis in MCF-7 human breast cancer cells. J Toxicol Environ Health A 67: 2025-2035.
- Choi KC, Jeung EB (2003) The biomarker and endocrine disruptors in mammals. J Reprod Dev 49: 337-345.
- Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, et al. (2008) Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: comparative study with 65 selected chemicals. Toxicol In Vitro 22: 225-231.
- Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, et al. (2005) Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology 210: 223-233.
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 16001
- [From(publication date):
specialissue-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11307
- PDF downloads : 4694
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
