Research Article Open Access
Biogas Recovery from Hyper-Thermophilic Anaerobic Co-Digestion of Thickened Waste Activated Sludge, Organic Fraction of Municipal Solid Waste and Fat, Oil and Grease
Alqaralleh R*, Kennedy K, Delatolla R, and Sartaj M
Department of Civil Engineering, University of Ottawa, Ottawa, ON, Canada
- *Corresponding Author:
- Alqaralleh R
Department of Civil Engineering
University of Ottawa, Ottawa, ON, Canada
Tel: 16135625700
E-mail: raniaqaralleh@yahoo.com
Received Date: August 03, 2017; Accepted Date: August 24, 2017; Published Date: August 28, 2017
Citation: Alqaralleh R, Kennedy K, Delatolla R, Sartaj M (2017) Biogas Recovery from Hyper-Thermophilic Anaerobic Co-Digestion of Thickened Waste Activated Sludge, Organic Fraction of Municipal Solid Waste and Fat, Oil and Grease. J Bioremediat Biodegrad 8:408. doi: 10.4172/2155-6199.1000408
Copyright: © 2017 Alqaralleh R, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The use of organic fraction of municipal solid waste and Fat Oil and Grease (FOG) as co-substrates for thickened waste activated sludge anaerobic digestion has the potential to improve the biodegradation process and significantly enhance biogas production and methane yields. This will not only help convert these potential waste streams from landfills increasing the longevity of existing landfills, but also provide a sustainable waste to energy waste management method. In this study the anaerobic co-digestion of organic fraction of municipal solid waste, with thickened waste activated sludge (50:50, w/w based on total volatile solids) was investigated using anaerobic digestion thermophilic and hyper-thermophilic biochemical methane potential (BMP) assays. The hyper-thermophilic BMP assays outperformed the thermophilic BMP assays by providing faster biogas production rates, higher cumulative biogas productions and methane yields. Additionally, 10, 20 and 30% FOG (based on total volatile solids) were added to the co-digestion mixtures in order to boost the biogas production and methane yield in three hyperthermophilic assays. 30% FOG in the co-digestion mixture enhanced the biogas methane content for sample TWAS:OFMSW:30%FOG(H) to 66.4% compared to 60.1% for the control sample TWAS(T), and accordingly improved the methane yield to be 84.4% higher than the methane yield of the control.
Keywords
Thickened waste activated sludge; Organic fraction of municipal solid waste; FOG; Hyper-thermophilic anaerobic digestion; Methane yield
Introduction
Renewable energy industries have been developed incredibly fast in the recent years [1]. Biogas harvested from organic wastes is a potential renewable source of energy alternative to fossil fuels. Anaerobic digestion (AD) is a widely-applied method for generating biogas from organic waste. However, the biogas utilization in wastewater treatment plants (WWTPs) is still limited; for example, in the US less than 10% of the WWTPs produce biogas for beneficial use such as heat and power generation [2]. The slow biogas production rate during the AD process, biogas low energy content (low methane%), and the high cost to upgrade the produced biogas are some of the critical challenges that WWTPs industry is facing to deploy biogas production and utilization [2,3]. Sewage sludge in the form of primary sludge (PS), waste activated sludge (WAS) and thickened waste activated sludge (TWAS) is the most popular waste that is treated using AD. However, sludge low (C/N) ratio that generally ranges from 6 to 9 negatively impact the efficiency of AD process especially under the traditional mesophilic conditions [4,5]. Organic fraction of municipal solid waste (OFMSW) has high C/N ratios, when co-digested with TWAS, OFMSW will balance the overall C/N ratio in the mixture for more stable AD process. Additionally, adding FOG to the co-digestion mixture proved to be an effective way to boost the biogas production and increase methane yields resulting from the AD process. This is due to FOG high theoretical methane potential (0.70-1.43 m3 CH4/kg volatile solids) [6,7].
Hyper-thermophilic digestion at 70 ± 1°C temperature is a relatively new approach in the sludge/TWAS AD. Alqaralleh et al. used the hyperthermophilic method for the co-digestion of TWAS and FOG. The results showed that hyper-thermophilic digestion improved the overall anaerobic co-digestion process which led to higher biogas production, higher methane yield, as well as increasing the ability of the digester to digest mixtures with high FOG contents (up to 60% based on TVS) without causing adverse effects on the AD process [4].
Therefore, the present study aimed to investigate the hyperthermophilic AD of binary mixture of TWAS: OFMSW (50:50, w/w) and trinary mixtures of TWAS: OFMSW and FOG with different FOG% (10, 20 and 30%). The performance of the binary and trinary mixtures hyper-thermophilic co-digestion were investigated in comparison with the thermophilic mono-digestion of TWAS. Linear and nonlinear regression models were used to fit the experimental results from different co-digestion samples and conditions to better understand and compare the co-digestion results.
Materials and Methods
Substrates and inoculum
Thickened waste activated sludge (TWAS) was obtained from Robert O. Pickard Environmental Center (ROPEC), Ottawa, ON, Canada. It contained 5% TS of which about 72% were VS. FOG sample was provided from the Organic Resources Management Inc. (ORMI), Ottawa, ON, Canada. ORMI provides grease trap cleaning services for different wastewater treatment plants in Ontario.
Organic fraction of municipal solid waste (OFMSW) to minimize the day-to-day variation in the organic waste, a simulated organic fraction of municipal solid waste was used in the current study to provide a homogeneous waste composition that is representative for the kitchen waste in Canada and North America based on the Canadian Food Guide and the USDA Food Patterns [8-10]. The simulated OFMSW used in this study consists of bread (6 wt%), cooked rice (12 wt%), cooked pasta (12 wt%), apples (11 wt%), bananas (11 wt%), cabbage (11 wt%), carrots (11 wt%), ground beef (10 wt%), fish (10 wt%), and boiled eggs with shells (6 wt%). All waste components were prepared fresh and mixed in a food processor to generate a particle size ranges from 1 to 5 mm prior to use. The prepared OFMSW had a total solids concentration of 17.3 ± 0.2% (w/w).
All three substrates TWAS, FOG and OFMSW were stored at 4°C prior to use. Thermophilic anaerobic inoculum (55°C) was obtained from the effluent of a 10 L thermophilic anaerobic digester fed with TWAS at a 20 days hydraulic retention time (HRT) in our research lab. Hyper-thermophilic anaerobic inoculum (70°C) was the effluent from a 2 L hyper-thermophilic anaerobic digester digesting TWAS at HRT of 2 days. The characteristics of substrates and inoculums used in this study are listed in Table 1.
| Parameter | TWAS | M-OFMSW | FOG | Thermophilic inoculum | Hyper-thermophilic inoculum |
|---|---|---|---|---|---|
| pH | 6.8±0.1 | 4.7±0.1 | 4.1±0.1 | 7.7±0.1 | 6.8± 0.1 |
| TS (%) | 4.9±0.1 | 17.3±0.2 | 29.2±0.2 | 3.2±0.1 | 3.3±0.3 |
| VS (%) | 3.6±0.1 | 10.7±0.1 | 28.3± 0.2 | 1.6±0.3 | 1.8±0.2 |
| VS/TS | 0.72±0.01 | 0.61±0.2 | 0.97±0.03 | 0.49±0.02 | 0.55±0.02 |
Data represents the arithmetic mean ± standard deviation (n=4)
Table 1: Characteristics of substrates and inoculum.
BMP assays
The BMP batch tests were performed in 250 ml Kimax glass bottles with 160 ml working volume. All BMP assays were run in triplicates except for inoculum assays were run in duplicates. The inoculums and substrates were added to the bottles to obtain approximately 1:1 substrate to inoculum ratio (S:I) based on the total volatile solids (TVS). Equal amounts of NaHCO3 and KHCO3 were added to each BMP bottle to maintain alkalinity of 4000-6000 mg/L as CaCO3. Before finally sealing the BMP bottles, each bottle was purged with N2 gas for 3 minutes to remove the O2 gas from the headspace and maintain anaerobic condition. The BMP bottles were kept in a temperaturecontrolled shaker at 55 ± 1°C and 100 rpm for the thermophilic BMP assays. For the hyper-thermophilic BMP assays BMP bottles were first kept in a 70 ± 1°C incubator for 2 days, and then moved to the 55 ± 1°C and 100 rpm shaker for the BMP test duration. The composition of BMP bottles is represented in Table 2 below.
| Sample | Test Condition | Inoculum 55°C (ml) | Inoculum 70°C (ml) | TWAS(%)a | OFMSW (%) | FOG (%) |
|---|---|---|---|---|---|---|
| Blank 55°C | Thermophilic, 55°C | 70 | 0 | 0 | 0 | 0 |
| Blank 70°C | Thermophilic, 55°C | 0 | 60 | 0 | 0 | 0 |
| TWAS (T) | Thermophilic, 55°C | 70 | 0 | 100 | 0 | 0 |
| TWAS:OFMSW (T) | Thermophilic, 55°C | 70 | 0 | 50 | 50 | 0 |
| TWAS:OFMSW (H) | Hyper-thermophilic, 70°C/ Thermophilic, 55°C b | 35 | 30 | 50 | 50 | 0 |
| TWAS:OFMSW: 10%FOG (H) | Hyper-thermophilic, 70°C/ Thermophilic, 55°C | 35 | 30 | 45 | 45 | 10 |
| TWAS:OFMSW: 20%FOG (H) | Hyper-thermophilic, 70°C/ Thermophilic, 55°C | 35 | 30 | 40 | 40 | 20 |
| TWAS:OFMSW: 30%FOG (H) | Hyper-thermophilic, 70°C/ Thermophilic, 55°C | 35 | 30 | 35 | 35 | 30 |
aThe % is the weight percentage based on the total volatile solids (TVS) in the co-digestion mixture. bThe BMP assay bottles were kept for 2 days in the hyper-thermophilic conditions (70°C) then moved to the thermophilic conditions (55°C) for the rest of the assay duration
Table 2: Inoculum and digestion mixtures used for the BMP assays.
The 50:50 ratio (based on TVS) for the TWAS: OFMSW mixture was used in our experiments as recommended by Liu et al. in order to get the best synergetic effect of OFMSW in the co-digestion process and avoid ammonia accumulation that may occur when high OFMSW concentrations are used in the co-digestion mixture [11].
Analytical methods
The analysis for the BMP assays was done in triplicates, using standard methods [12]. Total and volatile solids were determined according to standard method 2540G. Alkalinity analysis was measured based on standard method 2320B using a Fisher Accument® XL25 pH meter. Biogas was measured using a manometer every 24 hour the first 7 days of the experiment and it was measured occasionally after the biogas production slowed down until the end of the BMP assay (40 days). The volume of the biogas was corrected to the standard ambient temperature and pressure conditions (STP, 25°C and 1 atm). The net biogas volumes for samples were calculated by subtracting the biogas produced in the inoculum bottles from the biogas produced in each of the samples bottles. The biogas composition (methane%) was monitored weekly using a Hewlett Packard 5710A gas chromatograph.
Data analysis
The reaction curve model (RC) was used for the non-linear regression to evaluate the anaerobic digestion performance and estimate the lag phase time (λ, h), predict the ultimate cumulative biogas yield (B0, mL/g TVS), and find the maximum biogas production rate (Rm, mL/gTVS.h) for each of the BMP conditions tested in this study. The RC model represented by Equation 1 has been used successfully in literature for anaerobic digestion evaluation [4,13].
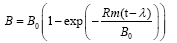 (1)
(1)
where, B0 is the ultimate biogas yield (mL/g TVS), B is the cumulative biogas yield (mL/g TVS), t is incubation time (h), Rm is the maximum biogas production rate (mL/g TVS.h), and λ is the lag phase duration time (h).
The first-order equation (Equation 2) was used to estimate the apparent hydrolysis rate coefficient (first-order biogas production rate) (k, h-1) for each of the BMP conditions [14].
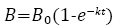 (2)
(2)
where k is the first-order apparent hydrolysis rate (h-1), B0, B and t have the same definition as defined in the RC model above (Equation 1).
Statistical analysis of the collected data included t-test (p-value=0.05), one-way analysis of variance (ANOVA), Pearson correlation index (R2) and degree of freedom.
Results and Discussion
Biogas production and methane yields
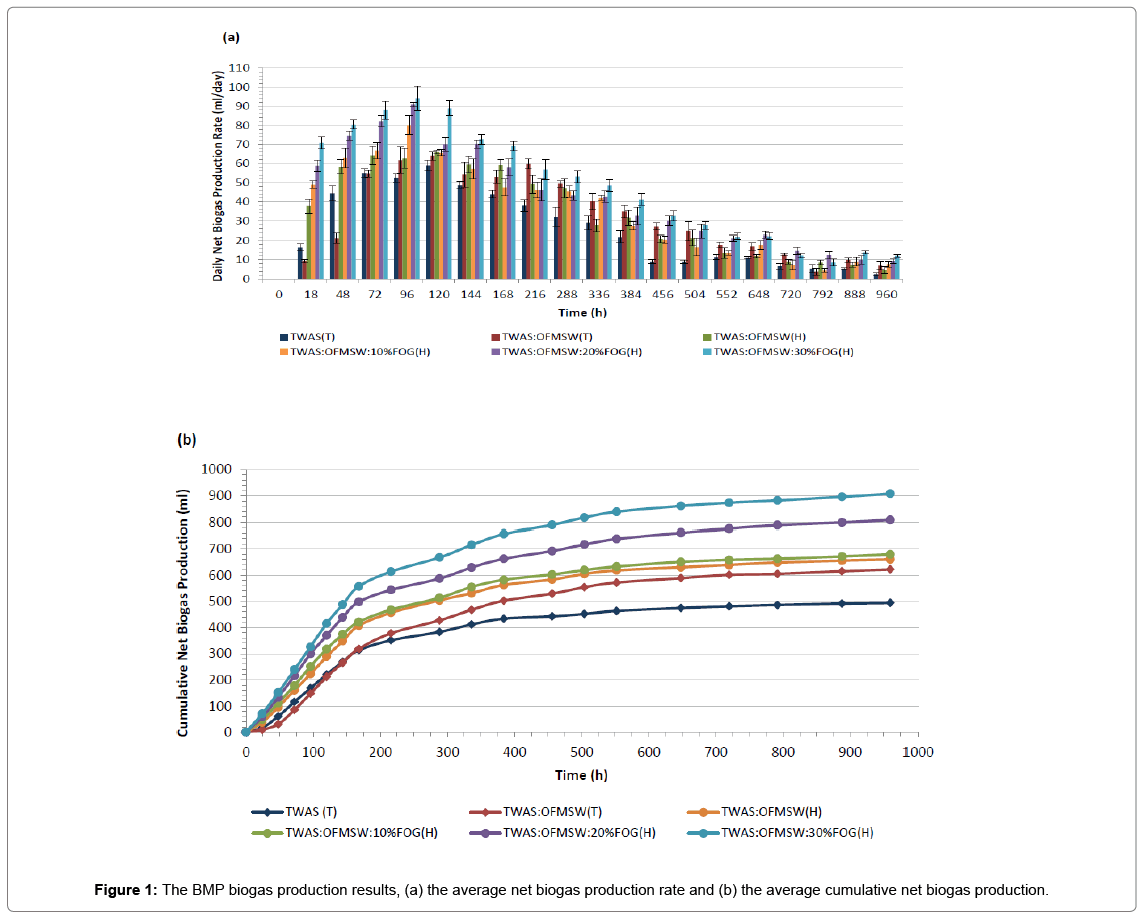
The average biogas production rates and the accumulative biogas production results for all BMP assays are illustrated in Figures 1a and 1b, respectively. In addition to Table 3 that represents the average biogas productions, methane contents, methane yields and the improvement in methane yields compared to the control sample TWAS(T).
| Parameter | Unit | TWAS(T)/Control | TWAS-OFMSW(T) | TWAS-OFMSW(H) | TWAS-OFMSW-10%FOG | TWAS-OFMSW-20%FOG | TWAS-OFMSW-30%FOG |
|---|---|---|---|---|---|---|---|
| Cumulative biogas* | (ml) | 494.0 ±15.9 | 622.6 ± 37.4 | 660.3 ± 20.2 | 679.5 ± 48.7 | 807.7 ± 30.9 | 908.1 ± 27.8 |
| CH4 | (%) | 60.1 ± 0.8 | 60.6 ± 0.4 | 61.8 ± 0.7 | 63.3 ± 0.8 | 65.9 ± 0.5 | 66.4 ± 0.6 |
| CH4 Yield | (ml/g VSadded) | 297.0 ± 9.6 | 342.7 ± 20.6 | 370.7 ± 11.3 | 430.3 ±3 0.8 | 484.1 ± 18.5 | 547.9 ± 16.8 |
| CH4 Yield Improvementa | (%) | ----- | 13.3 | 24.8 | 44.9 | 63 | 84.4 |
*Net biogas and methane production in (ml). Data represents arithmetic mean ± standard deviation of replicate samples. aMethane yield improvement calculated compared to the control sample (TWAS(T))
Table 3: The average BMP tests results (accumulative net biogas production, methane content, methane yield and methane yield improvement).
Comparing TWAS: OFMSW (T) sample with the control sample TWAS(T) in order to evaluate the effect of 50% (w/w, based on TVS) OFMSW as a co-substrate in the co-digestion mixture. First from Figure 1a we notice that TWAS: OFMSW(T) sample had lower biogas production rate at the beginning of the BMP assay compared to TWAS(T) sample. This can be related to the fact that the inoculum used in the BMP assays was acclimated to TWAS digestion, but not to OFMSW. Hence the microorganisms were not adapted to OFMSW that probably caused a delay in starting the digestion process for the sample with OFMSW. However, TWAS: OFMSW(T) sample biogas production rate recovered around the day 3 (72 h) and outperformed the TWAS(T) sample until the end of the test resulting in a cumulative biogas production of 622.6 ± 37.4 ml compared to 494.0 ± 15.9 ml the cumulative biogas production of the control sample TWAS(T). Additionally, from Table 3 below, we can observe that the addition of OFMSW to the digestion mixture did not cause any change in the biogas methane content since both samples TWAS: OFMSW(T) and TWAS(T) had similar (with no statistically significant difference) methane content in the rage of (60.1-60.6)%. By the end of the BMP assay the net methane yield for TWAS: OFMSW(T) sample was 13.3% higher than the methane yield of the control TWAS(T). This increase in biogas production and methane yield confirms the positive effect of OFMSW as a co-substrate for the TWAS AD. However due to the high solids nature of OFMSW it is often suggested in literature the use of a pre-treatment method that will help solubilizing the co-digestion mixture before AD to help getting the maximum benefits from the codigestion process [15,16].
As from sample TWAS: OFMSW(H) results, we can notice clearly that this hyper-thermophilic digested sample started the BMP with much higher biogas production rate compared to TWAS(T) and TWAS: OFMSW(T). Thanks to the 2 days hyper-thermophilic digestion of TWAS: OFMSW(H) sample at a temperature of 70 ± 1°C prior to the following thermophilic digestion. The 2 days hyper-thermophilic step is believed to help solubilizing the co-digestion mixture and increase the soluble organic matter that become available to be utilized by the microorganisms and converted to biogas. TWAS: OFMSW(H) sample ended the BMP assay producing 660.3 ± 20.2 ml biogas with 61.8 ± 0.7% methane. This methane content (%) is slightly higher than the methane content for both samples digested under the regular thermophilic conditions; TWAS(T) and TWAS: OFMSW(T). The methane yield of TWAS: OFMSW(H) sample was 370.7 ± 11.3 ml CH4/g TVS at the end of the BMP assay and this is a 24.8% increase in the methane yield compared to the control sample TWAS(T).
The next three samples are samples that included 10, 20 and 30% FOG (as a percentage of TVS of the co-digestion mixture). It is evident from Figures 1a and 1b, that these three samples had the highest biogas production rates during the first 5 days of digestion (120 h). Higher FOG% contributed to higher biogas production rate and higher accumulative biogas production. The addition of 10% FOG (w/w) to the TWAS: OFMSW co-digestion mixture increased the biogas methane content to 63.3 ± 0.8% which is a statistically significant increase in the biogas methane content compared to samples that did not contain FOG. Increasing FOG% in the co-digestion mixture to 20 and 30% resulted in a further increase in the biogas methane content to 65.9 ± 0.5% and 66.4 ± 0.6%, respectively. This noticed boost in methane content caused by FOG addition to the co-digestion mixtures led to a significant increase in methane yields to reach as high as 547.9 ± 16.8 ml CH4/g VS added for the TWAS: OFMSW:30%FOG sample, and this represents 84.4 higher methane yield compared to the control sample TWAS(T).
Linear and non-linear regression
To accurately evaluate the co-digestion performance parameters for the AD digestion, process the non-linear regression was utilized using the RC equation (Equation 1). Also, linear regression using the firstorder equation (Equation 2) was used to estimate the hydrolysis rate coefficient k (h-1) for the biogas production results. Table 4 represents the linear and non-linear regression results.
| Sample | Measured Biogas Yielda | RC model | FO eq. | ||||
|---|---|---|---|---|---|---|---|
| (ml/g VS) | B0 (ml/gVS) | λ (h) | Rm(ml/gVS.h) | R2 | Δb (%) | K (h-1) | |
| TWAS (T) | 494.0 | 492.9 | 11.0 | 2.7 | 0.992 | 0.2 | 4.97 E-3 |
| TWAS-OFMSW(T) | 622.6 | 639.8 | 17.9 | 2.6 | 0.991 | 2.8 | 3.49 E-3 |
| TWASOFMSW(H) | 660.3 | 658.8 | 7.5 | 3.4 | 0.995 | 0.2 | 4.84 E-3 |
| TWAS-OFMSW-10%FOG(H) | 679.5 | 672.9 | 4.8 | 3.6 | 0.996 | 1.0 | 5.13 E-3 |
| TWAS-OFMSW-20%FOG(H) | 807.7 | 793.1 | 2.5 | 4.1 | 0.994 | 1.8 | 4.99 E-3 |
| TWAS-OFMSW-30%FOG | 908.1 | 895.8 | 3.1 | 4.6 | 0.996 | 1.4 | 4.98 E-3 |
aAverage net biogas yield as ml biogas/g VS added calculated from the BMP assays results. bΔ% is the calculated difference between the experimental and the predicted biogas yields
Table 4: Parameters estimated from linear and non-linear regression for the BMP assays.
From Table 4 and specifically from RC model results, we can observe that RC model fitted accurately all the BMP assay samples with high R2 values that ranged from 0.991 to 0.996. Also, the difference between the experimental and predicted biogas yields (Δ%) ranged from 0.2 to 2.8. It worth to mention here that RC model was preferred over other different linear and non-linear models used to represents the biogas production results of BMP tests by Alqaralleh et al. and Donoso-Bravo et al. In both studies RC model fitted accurately the biogas production results with Δ% ≤ 10 [4,13], could be noticed from Table 4 that the control sample TWAS(T) had 11 h estimated lag phase time before starting the biogas production. However, the thermophilic co-digestion sample TWAS: OFMSW(T) had 17.9 h estimated lag phase time. This increase in the lag phase of TWAS: OFMSW(T) sample compared to the control sample TWAS(T) is probably because the inoculum used in the BMP assays was not acclimated to OFMSW as mentioned previously, so microorganisms required longer time to start the AD process. As regard the hyper-thermophilic co-digested samples, it is evident that all hyper-thermophilic digested samples had shorter lag phase time compared to the thermophilic digested samples. Comparing TWAS: OFMSW(H) sample with TWAS: OFMSW(T) sample we can notice the effect of the 2 days hyper-thermophilic digestion step in shortening the lag phase time from 17.9 h in the thermophilic digestion of TWAS: OFMSW(T) sample to 7.5 h in the hyper-thermophilic digestion of the TWAS:OFMSW(H) sample. This is believed to be due to the solubilization of the co-digestion mixture that occur during the 2 days hyper-thermophilic digestion providing more soluble organic matter that is ready to be utilized by the methanogens in the followed thermophilic part of experiment.
From the results of the maximum methane production rate (Rm, ml/g TVS.h) it can be observed that sample TWAS: OFMSW(T) had the lowest Rm value among all other samples. On the other hand the hyperthermophilic digested sample TWAS: OFMSW(H) had significantly higher Rm compared to TWAS: OFMSW(T) sample. FOG addition clearly boosted the Rm of the three samples that contained FOG. The maximum Rm was for sample TWAS: OFMSW:30% FOG and it was 4.6 (ml biogas/g VS h).
The hydrolysis rate coefficients (k, h-1) resulted from the linear regression using the first-order equation and presented in Table 4, show the same trend as Rm discussed in RC equation results above. Adding OFMSW to the TWAS thermophilic digestion caused a drop in the hydrolysis coefficient (k) from 4.97 E-3 to 3.49 E-3 h-1. This dropin k is expected and it is related to the dense nature of the OFMSW (high TS and VS) compared to TWAS. In fact, this is the main reason why pre-treatment is always suggested before the AD of OFMSW, to help solubilizing the OFMSW and make it easier for digestion [15]. Solubilizing the waste for easier digestion is exactly what can be offered by the hyper-thermophilic step in the hyper-thermophilic digestion. This is evident by the increase in k value for TWAS: OFMSW(H) sample to 4.84 E-3 h-1 compared to 3.49 E-3 h-1 for TWAS: OFMSW(T) sample. The rest three hyper-thermophilic samples that contained FOG (TWAS: OFMSW:10%FOG, TWAS: OFMSW:20%FOG and TWAS: OFMSW:30%FOG) showed higher k values compared to both thermophilic samples and the hyper-thermophilic sample without FOG. The discussed results show that FOG addition up to 30% (w/w, based on TVS) to the co-digestion mixture helped increasing the hydrolysis rate for the co-digestion mixture and boosted the anaerobic co-digestion process resulting in obtaining higher methane productions and higher methane yields.
Conclusion
Based on the results of this study the thermophilic co-digestion of 50:50 (w/w based on TVS) mixture of TWAS and OFMSW resulted in a higher biogas production and 13.3% higher methane yield compared to the thermophilic digestion of TWAS alone. However, the presence of OFMSW in the co-digestion mixture increased the lag-phase time before the AD system started the biogas production. Hyperthermophilic anaerobic digestion proved to be a reliable alternative for the pre-treatment methods usually needed prior to AD of high solid wastes like OFMSW. The 2-days hyper-thermophilic anaerobic digestion step helped solubilizing the co-digestion mixture, providing more easy to digest matter. This solubilisation led to faster biogas production rate, higher accumulative biogas production and methane yield. Adding 10-30% FOG to the hyper-thermophilic TWAS: OFMSW mixtures significantly boosted the biogas methane content, increased the cumulative biogas production and the methane yields up to 84.4% higher than the methane yield resulted from the thermophilic digestion of TWAS alone. Linear and non-linear regression models were used to represent the entire anaerobic digestion process. The estimated parameters resulted from the regression provided valuable information for better understand the effect of OFMSW, hyper-thermophilic and FOG addition on the AD process.
Acknowledgements
The Natural Science and Engineering Research Council of Canada (NSERC) is kindly acknowledged for their financial support.
References
- Divya D, Gopinath LR, Merlin Christy P (2015) A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renewable and Sustainable Energy Reviews 42: 690-699.
- Shen Y, Linville JL, Urgun-Demirtas M, Mintz MM, Snyder SW (2015) An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: Challenges and opportunities towards energy-neutral WWTPs. Renewable and Sustainable Energy Reviews 50: 346-362.
- Gianico A, Bertanza G, Maria C, Canato M, Laera G, et al. (2015) Upgrading a wastewater treatment plant with thermophilic digestion of thermally pre-treated secondary sludge: techno-economic and environmental assessment. Journal of Cleaner Production 102: 353-361.
- Alqaralleh RM, Kennedy K, Delatolla R, Sartaj M (2016) Thermophilic and hyper-thermophilic co-digestion of waste activated sludge and fat, oil and grease: Evaluating and modelling methane production. Journal of Environmental Management 183: 551-561.
- Coelho NMG, Droste RL, Kennedy KJ (2014) Microwave Effects on Soluble Substrate and Thermophilic Digestibility of Activated Sludge. Water Environment Research 86: 210-222.
- Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, et al. (2014) A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renewable and Sustainable Energy Reviews 36: 412-427.
- Xu R, Yang Z, Chen T, Zhao L, Huang J, et al. (2015) Anaerobic co-digestion of municipal wastewater sludge with food waste with different fat, oil, and grease contents: study of reactor performance and extracellular polymeric substances. RSC Adv 5: 103547-103556.
- USDA Food Patterns (2016).
- Health Canada (2016) Canada’s Food Guide.
- Alqaralleh R, Delatolla R, Kennedy K (2015) Anaerobic digestion of simulated-organic fraction of municipal solid waste: effect of alkaline pre-treatment. International Journal of Environment and Waste Management 16: 166-185.
- Liu C, Li H, Zhang Y, Liu C (2016) Bioresource Technology Improve biogas production from low-organic-content sludge through high-solids anaerobic co-digestion with food waste. Bioresource Technology 219: 252-260.
- American Public Health Association (1998) American Water Works Association. Water Environment Federation, Standard Methods for the Examination of Water and Wastewater.
- Donoso-Bravo A, Pérez-Elvira SI, Fdz-Polanco F (2010) Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chemical Engineering Journal 160: 607-614.
- Ghatak MD, Mahanta P, Straw R (2014) Kinetic Assessment of Biogas Production from Lignocellulosic Biomasses. International Journal of Engineering and Advanced Technology 3: 244-249.
- Ara E, Sartaj M, Kennedy K (2014) Effect of microwave pre-treatment of thickened waste activated sludge on biogas production from co-digestion of organic fraction of municipal solid waste, thickened waste activated sludge and municipal sludge. Waste Management & Research: The Journal of the International Solid Wastes and Public Cleansing Association 32: 1200- 1209.
- Koch K, Helmreich B, Drewes JE (2015) Co-digestion of food waste in municipal wastewater treatment plants: Effect of different mixtures on methane yield and hydrolysis rate constant. Applied Energy 137: 250-255.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 3729
- [From(publication date):
September-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 2798
- PDF downloads : 931