Research Article Open Access
Biodegradation of Hexachlorocyclohexane (HCH) Isomers by White Rot Fungus, Pleurotus florida
| Soudamini Mohapatra1* and Meera Pandey2 | |
| 1Pesticide Residue Laboratory, Indian Institute of Horticultural Research, Hessaraghatta Lake, India | |
| 2Mushroom Culture Laboratory, Indian Institute of Horticultural Research, Hessaraghatta Lake, India | |
| Corresponding Author : | Soudamini Mohapatra Pesticide Residue Laboratory Indian Institute of Horticultural Research Hessaraghatta Lake, Bangalore 560089,India Tel/Fax: 91 80 28446649 E-mail: Soudamini_mohapatra@rediffmail.com |
| Received December 23, 2014; Accepted February 24, 2015; Published February 27, 2015 | |
| Citation: Mohapatra S, Pandey M (2015) Biodegradation of Hexachlorocyclohexane (HCH) Isomers by White Rot Fungus, Pleurotus florida. J Bioremed Biodeg 6:280. doi:10.4172/2155-6199.1000280 | |
| Copyright: © 2015 Mohapatra S, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Hexachlorocyclohexane (HCH) isomers are reported to persist in the environment long after their usage is discontinued. Pleurotus florida, a white rot fungus, was found to degrade not only γ-HCH but also its more persistent isomers individually as well as a mixture in a Raper’s complete medium. Within 30 days γ-HCH degraded completely, whereas other 3 isomers degraded 92-99%. The degradation rate was γ-HCH>β-HCH>α- and δ-HCH. The mycelium biomass was free from γ-HCH residues but accumulated about 3% residues of other 3 isomers. Presence of intermediate metabolites was not detected indicating complete mineralization of HCH isomers. Ability of P. florida to degrade HCH isomers was further studied in soil by amendment with spent mushroom substrate (SMS). SMS addition could marginally increase degradation of α-, β- and δ-HCH, but significantly increased degradation of γ-HCH. When the study was repeated similar trend was observed. The half-life of degradation of γ-HCH was 439-570 days in un-amended soil while 37-42 days in SMS amended soil. For other 3 stable isomers the half-life was reduced from 686-828 to 88-125 days by SMS amendments. These results indicate that SMS from P. florida cultivation can be utilized for bioremediation of HCH contaminated site.
| Keywords |
| Degradation; Hexachlorocyclohexane; Metabolites; Pleurotus florida; Spent mushroom substrate |
| Abbreviations |
| HCH: Hexachlorocyclohexane; LOD: Limit of Detection; P: Pleurotus |
| Introduction |
| Hexachlorocyclohexane is an organochlorine insecticide which has been extensively used in agriculture in the past. It is still used for public health and medical purposes. Hexachlorocyclohexane is the term used for all 8-isomers of HCH [1]. Among the isomers, only gammahexachlorocyclohexane (γ-HCH), commonly known as lindane, has insecticidal properties. During the production of γ-HCH (lindane) upto 85% of the product consisted of other isomers, mainly α-, β- and δ-HCH [2]. These isomers don’t have insecticidal properties, therefore dumped as waste causing serious environmental pollution. Use of γ-HCH has been banned all over the world. But its continuous use has led to its accumulation in the environment [3]. All isomers of HCH have been detected in environmental samples world over. The β-isomer is consistently found in higher concentration in human fat and blood whereas α- and γ-HCH are most prevalent isomers in soil [4]. All four isomer of HCH are toxic and considered worldwide pollutants. Biodegradation of HCH isomers has been studied in soil [5], soil slurry [6] etc. A lot of information is available on the biodegradation of γ-HCH and other isomers of HCH using pure bacterial cultures, Pseudomonas aeruginosa, ITRC-5, Xanthomonas species, Clostridium rectum, Pandoraea spp etc [7-10]. Studies have also been carried out on the degradation of HCH isomers using sewage sludge under aerobic and anaerobic conditions [11-13]. It was observed that the isomers β- and δ-HCH were resistant to biodegradation under aerobic and anaerobic conditions, but α- and γ-HCH degraded upto 90%. Besides bacterial cultures, some fungi have also been found to degrade HCH isomers. White rot fungus, Phanerochaete chrysosporium and a non-white rot fungus Conidiobolus were capable of degrading HCH isomers [14-16]. Pleurotus ostreatus has been reported to be used in bioremediation of lindane contaminated site [17]. Information on the complete biodegradation of all isomers of HCH using a single microbial culture is rare. Also in majority of the studies intermediate metabolite formation has been reported [8]. Pleurotus florida, belonging to the genus Pleurotus, is a white rot fungus which has been commercially exploited for mushroom cultivation. The species can be easily cultivated on paddy straw. Members of this genus are commonly referred to as white oyster mushrooms, which are prime edible mushrooms. P. florida is able to grow on various media and at a wide range of temperature (15-30°C). Preliminary studies conducted in the laboratory showed that P. florida could accelerate degradation of γ-HCH both in medium and soil. But the other 3 isomers are known to be more persistent than γ-HCH. In addition to that if γ-HCH application is given for any purpose the other isomers are likely to enter as impurities. The purpose of our study was to find a suitable microorganism that can be used for bioremediation of all HCH isomers together and individually. Therefore in the present study the ability of P. florida was evaluated to degrade α-, β-, γ- and δ-isomers of HCH individually and as a mixture in medium as well as in soil. |
| Materials and Methods |
| Chemicals |
| Analytical grade HCH isomers i.e. α-HCH (purity 98.3%), β-HCH (purity 99.2%), γ-HCH (purity 99%) and δ-HCH (purity 98.5%) were procured from Accu Standard, Germany. The degradation products 1,3 dichlorobenzene (purity 99.4%), 1,2-dichlorobenzene (purity 99.9%), 1,2,4-trichlorobenzene (purity 99.5%), 1,2,3-trichlorobenzene (purity 99.9%), 1,2,4,5-tetrachlorobenzene (purity 99.4%), 1,2,3,5-tetrachlorobenzene (purity 97.5%), 1,2,3,4-tetrachlorobenzene (purity 99.0%), and pentachlorobenzene (purity 99.8%) were procured from Sigma-Aldrich, Germany. All chemicals and reagents used were of analytical grade. |
| Standard solution |
| Stock solutions (1000 μg mL-1) of α-, β-, γ-, δ-HCH and all the metabolites were prepared in n-hexane. Working standards were prepared by further dilutions. Mixture of the above 4 isomers were also prepared. All the HCH isomers were analyzed by gas chromatograph at a concentration range of 0.01-1.0 μg mL-1 to study the linearity and limit of detection (LOD). |
| Pleurotus florida |
| Pleurotus florida used in the present study was obtained from the mushroom germplasm repository of Indian Institute of Horticultural Research, Hessaraghatta, Bangalore, India. The cultures of P. florida was maintained on malt extract agar medium (malt extract 30 g; mycological peptone 5 g; agar 15 g; distilled water 1 L) through regular sub culture and tissue culture. |
| In vitro degradation of HCH isomers |
| The in vitro degradation study of HCH isomers by P. florida was carried out in Raper’s complete medium (glucose, 20 g; peptone, 2 g; yeast extract 2 g; MgSO4.7H2O 0.5 g; KH2PO4 0.4 g; K2HPO4 1 g; distilled water 1 litre). Hundred mL portions of the sterilized medium were taken in 250 mL conical flasks. Each flask was inoculated with one fungal disc (8 mm diameter) obtained from the periphery of fully grown 7 day old cultures. The flasks containing P. florida was amended with 100 μg (1 μg/mL) of α-, β-, γ- and δ-HCH individually. In one set of flasks all four HCH isomers were added together, at a concentration of 100 μg each (total 400 μg). Flasks without P. florida but with HCH isomers individually and as a mixture were kept as controls. Flasks inoculated with only fungal cultures also served as control. The culture was incubated as static culture at room temperatures (25 ± 2°C). Degradation of HCH isomers in the medium was studied on 0, 10, 20 and 30 days after incubation. |
| Degradation of HCH isomers in soil |
| Degradation of HCH isomers by P. florida in soil was carried out in the following manner. In a 500 mL conical flask 100 g soil was taken and mixed with 20 g spent mushroom substrate (SMS) on which P. florida was grown. Stock solutions of mixture of α-, β-, γ- and δ-HCH were diluted with tap water. Each flask received 50 mL portions of water containing HCH mixture. The concentration of each isomer was maintained @ 1 μg g-1 soil. Soil without SMS served as control. Soil with SMS but without mixture of HCH isomers also served as control. The soils were kept at ambient (room) temperature (26 ± 2°C) and analyzed for HCH isomers on 0, 10, 20, 30, 40, 50 and 60 days. |
| Extraction of HCH isomers in medium |
| On every sampling day 5 replicates from each medium treatment were taken for analysis. The mycelial mats were separated from the culture medium and both were extracted and analyzed separately. The medium was transferred into a 500 mL conical flask the mycelial mats were washed repeatedly with distilled water (100 mL) and the washings were added to the culture medium. The mycelial mats were further washed repeatedly with ethyl acetate (50 mL) to remove any traces of HCH isomers sticking to it. The ethyl acetate washings were added back to the medium. The washed mycelial mats were homogenized thoroughly with a mortar and pestle after adding 25 g of anhydrous sodium sulphate. It was further extracted with 25 mL hexane : ethyl acetate; 75 : 25 (v v-1) by shaking on a mechanical shaker for 2 h. The process was repeated twice. The extracts were combined and evaporated using a rotary vacuum evaporator (Heidolph Laborta 4002, Germany) under vacuum and dissolved in 2 mL of n-hexane for analysis by gas chromatograph (GC) and GC-MS. The culture medium was extracted with 50 mL hexane: ethyl acetate; 75: 25 (v v-1) by shaking on a mechanical shaker for 2 h. The process was repeated twice. The combined extracts were evaporated using a rotary vacuum evaporator under vacuum and redissolved in 5 mL of n-hexane for analysis by gas chromatograph (GC) and gas chromatograph mass spectrometry (GC-MS). The extracts were further diluted with n-hexane as and when it was required to make it suitable for GC analysis. On the 10th day of treatment the growth of mycelium was not sufficient to separate it from the medium. Therefore the entire content was extracted as culture medium. The control flasks without P. florida were also extracted as culture medium. Throughout the analysis period on every sampling day medium and mycelial mats from flasks inoculated with only fungal cultures were extracted as described above and analyzed by GC and GC-MS for comparison. |
| Extraction of HCH isomers in soil |
| On every sampling day 5 replicates of the treated soil was taken for analysis. HCH isomers from soil were extracted with 100 mL hexane: ethyl acetate; 75: 25 (v v-1) after adding 40 mL distilled water. The flasks were kept for shaking for 1 hour and filtered in Buchner funnel under vacuum. The soil was further rinsed with 2 × 20 mL of the solvent mixture. The combined extracts were evaporated under vacuum; the aqueous fraction was partitioned with 3 × 30 mL solvent mixture. The fraction thus obtained was concentrated and redissolved in 10 ml of n-hexane for analysis by GC and GC-MS. The final extracts were diluted whenever required to make it suitable for GC analysis. The control soil samples were extracted in a similar manner. |
| Analysis by GC |
| A Gas Chromatograph Bruker GC-450 equipped with electron capture detector (ECD) was used for analysis of all HCH isomers and 8 metabolites. One μl of sample was injected with an auto sampler. A capillary column, Varian VF-1701MS (30 m × 0.25 mm i.d. × 0.25 μm; stationary phase, 14% cyanopropyl-phenyl 86% dimethyl polysiloxane) was used. The injector was kept in the split mode at a split ratio of 10. Ultra high pure nitrogen was used as carrier gas at a flow rate of 1.0 mL min-1. The column oven temperature was initially maintained at 90°C with a hold time of 5 min and programmed @ 2°C min-1 to 120°C and @ 4°C min-1 to 180°C with a hold time of 10 min. Injector and detector temperatures were maintained at 280°C and 300°C. Under the above conditions retention times of α-HCH, γ-HCH, β-HCH, δ-HCH were 32.9, 33.8, 34.7 and 35.1 min, respectively. The retention time of the degradation products, 1,3-dichlorobenzene; 1,2-dichlorobenzene; 1,2,4-trichlorobenzene; 1,2,3-trichlorobenzene; 1,2,3,5-tetrachlorobenzene; 1,2,4,5-tetrachlorobenzene; 1,2,3,4-tetrachlorobenzene, pentachlorobenzene were 9.0, 9.6, 13.8, 15.1, 20.3, 20.4, 22.3 and 27.9 min, respectively. |
| Analysis by GC-MS |
| For analysis by GC-MS Shimadzu QP2010 Plus equipment, with a QP2010 Plus detector was used. The GC-MS was operated in full scan mode (mass acquisition range m/z 40-450) using ionization energy of 70 eV. The gas chromatograph was operated in split less mode with injector temperature of 250°C and ion source temperature of 200°C. A capillary column, Agilent DB-5ms (30 m × 0.25 mm i.d. 0.25 μm; stationary phase, 5% phenyl 95% methyl polysiloxane) was used for analysis. The column oven temperature was initially maintained at 80°C with a hold time of 1 min and programmed at 2°C/min to 250°C withhold time of 5 min. Helium was used as carrier gas with a column flow rate of 1 mL min-1. The initial solvent cut time was 4 min. Under the above conditions retention times of α-HCH, γ-HCH, β-HCH, δ-HCH, were 26.57, 29.06, 31.20 and 34.15 min, respectively. The retention time of the degradation products 1,3-dichlorobenzene; 1,2-dichlorobenzene; 1,2,4-trichlorobenzene; 1,2,3-trichlorobenzene; 1,2,3,5-tetrachlorobenzene; 1,2,4,5-tetrachlorobenzene; 1,2,3,4-tetrachlorobenzene, pentachlorobenzene were 4.06, 4.15, 7.81, 9.61, 13.36 13.61, 16.81 and 21.47 min, respectively. The MS spectrum was compared with NIST mass spectra data base and found to match more than 95% for each compound. |
| Recovery study |
| Before carrying out extraction and analysis of inoculated samples a recovery study was carried by spiking Raper’s complete medium/soil with α-, γ-, β- and δ-HCH at a concentration of 0.01, 0.1 and 1.0 μg mL-1 (μg g-1). The method followed was the same as described above. |
| Results |
| Recovery of HCH isomers |
| Recovery of α-, γ-, β- and δ-HCH from Raper’s complete medium and soil is given in Table 1. Recovery of all isomers was above 95% at all concentration levels with the RSD values within 2.3-7.3%. Analysis of HCH isomers at different levels showed that the calibration curve was linear in the concentration range of 0.01-1.0 μg mL-1 and the correlation coefficient, R2>0.99 for all the 4 analytes. |
| Degradation of HCH isomers in medium |
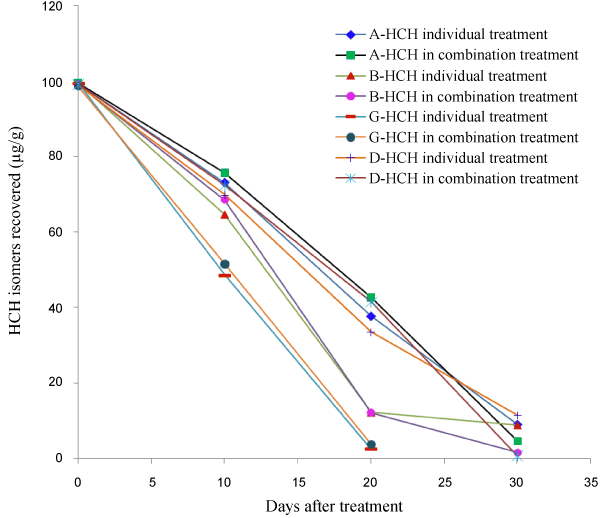
| The study conducted on the degradation of HCH isomers as individual treatment and as a mixture of isomers in the Raper’s complete medium showed that P. florida was capable of degrading all isomers of HCH, though γ-HCH degradation was faster than others. Within 10 days of inoculation about 50% of the added γ-HCH had disappeared from the medium inoculated with P. florida. During this period about 25-30% of both α- and δ-HCH had degraded from medium treated with the fungal culture as individual treatment. Degradation of β-HCH was comparatively faster with about 35% degradation during the initial 10 days. When P. florida was inoculated to the medium containing mixture of all four isomers the degradation pattern was about the same as individual treatments during the initial 10 days (Table 2). After a period of 20 days major portion of the applied γ-HCH (about 97%) had degraded whether treated individually or as a mixture with other isomers. The mycelium biomass from γ-HCH treated medium was also free from its residues. β-HCH, which is known to be highly stable in the environment, also degraded very fast, with about 91% being lost from the treated medium after 20 days. A small amount (3.23%) of β-HCH was found adsorbed by the mycelium. However degradation of other isomers, i.e. α- and δ-HCH was not as fast as the other 2 isomers. About 65% applied α-HCH was lost from medium when applied individually, with 2.91% accumulation in the mycelium. When treated in combination with other isomers 67.3% was lost from medium, but substantially high, about 10% was recovered from the mycelium. In case of δ-HCH about 35% was recovered from medium plus mycelium when treated individually and about 42% was recovered when treated in combination (Table 2). |
| After a period of 30 days complete degradation of γ-HCH had occurred from the treated medium both from individual and combination treatment with no accumulation in the mycelium. Residues of the other 3 isomers in the medium and mycelium together for α-, β- and δ-HCH were 8.99, 8.77 and 11.50%, respectively after 30 days when treated individually. These 3 isomers seemed to degrade faster when treated as a mixture compared to individual treatment. From combination treatment 4.6 and 1.58%, α- and β-HCH, respectively was recovered from medium and mycelium together. Only 0.46% added δ-HCH was recovered from medium and the mycelium was residue free from combination treatment after 30 days. The results show that γ-HCH degradation was fastest among the 4 isomers and the degradation rate was not affected whether the treatment was carried out individually or in combination with other isomers. For the other isomers slightly faster degradation was observed in the initial stages from individual treatment. But after 30 days it had reversed, i.e. degradation of α-, β- and δ-HCH isomers was faster when they were treated in combination. But overall it was observed that P. florida not only degraded α- and γ-HCH, it degraded the more persistent β- and δ-isomers equally fast. The analysis of extracts from medium/mycelium did not show any additional peak corresponding to any of the metabolites mentioned above. This was also confirmed by analyzing the extracts by GC-MS. Degradation of HCH isomers in Raper’s complete medium is given in Figure 1. |
| Degradation of HCH isomers in soil |
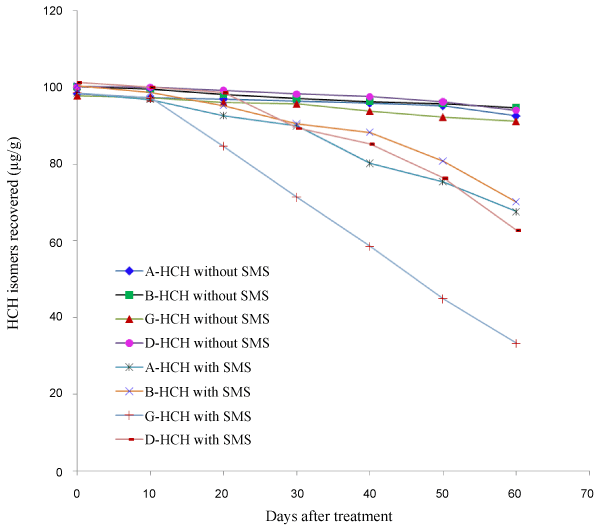
| Degradation of HCH isomers was slow in soil without SMS compared to soil with SMS. It was also observed that there was difference in the rate of degradation of all isomers in the presence of SMS (Table 3). In the initial 30 days period there was no significance difference in the rate of degradation of all isomers. But after 40 days period about 12-17% of α-, β- and δ-HCH was lost from soil while about 38% γ-HCH was lost. After a period of 60 days about 61-67% γ-HCH was lost from soil with SMS compared to about 20-30% loss of the other isomers. In soil without SMS the loss of HCH residues were about 4-9% in 60 days. The experiment was repeated and a similar trend was observed (Table 4). Degradation of HCH isomers from soil is given in Figure 2. |
| Dissipation kinetics of HCH isomers in soil |
| Residue dissipation of HCH isomers was studied by subjecting the data to first order kinetics; Ct=C0 e-kt, where Ct is the concentration at time t; C0 is the initial concentration, k is the rate constant for dissipation and t is the time. The half-life was calculated from the k value using the formula; t1/2=ln(2)/k. It was observed that the dissipation of all HCH isomers followed first order rate kinetics. In soil without SMS the halflife of degradation of α-, β- and δ-HCH was in the range of 748-828 days. But when SMS was added to the soil it was substantially reduced to the range of 88-125 days. For γ-HCH SMS addition reduced the halflives from the range of 439-570 days to 37-42 days only. The half-life of degradation, regression equation and regression coefficients are given in Tables 5 and 6. |
| Discussion |
| In a study conducted using anaerobic bioreactor to degrade HCH isomers in soil slurry cultures it was observed that α- and γ-HCH completely degraded within 10 days; β- and δ-HCH were removed only after 50 days when applied at the concentration of 25-100 mg HCH kg-1 [18]. A Pseudomonas sp could readily degrade α-, β- and γ-HCH in a medium but degraded α- and γ-HCH in soil, but not β-HCH [19]. Biodegradation of HCH isomers in contaminated soils has been carried out using an isolate Pseudomonas aeruginosa ITRC-5. It was reported that the isolate could degrade > than 98% α- and γ-HCH, 76% δ-HCH, but only 17% β-HCH after 15 days [20]. From all the studies mentioned above it was evident that among the 4 HCH isomers α- and γ- are easily degraded, but not β- and δ-HCH. Whereas in the present study. P. florida degraded all isomers equally efficiently in medium, though γ-HCH degradation was faster compared to the rest. Ability of whiterot fungus, Bjerkandera adusta to degrade HCH isomers has been studied in a soil slurry batch bioreactor [21]. The fungus significantly degraded the HCH isomers from the soil slurry in the following order: alpha ~ gamma>delta>beta -HCH. After a 30 day period 94.5%, 78.5% and 66.1% for γ-, α- and δ-HCH isomers, respectively degraded at an optimal condition of 100 g soil L-1 and 100 mg total HCH L-1. In a recent study nano scale Fe-Pd bimetallic particles have been used to dechlorinate lindane to form cyclohexane at a concentration of 5 mg L-1 [22]. But complete degradation of lindane is not achieved. The present study in a Raper’s complete medium P. florida had the ability to degrade all isomers efficiently and the degradation is accelerated to some extent when all the isomers are added together. |
| When the study was extended to soil with addition of spent mushroom substrate from Pleurotus florida cultivation the degradation of HCH isomers was not as fast as it was in the medium. But SMS addition could increase degradation of HCH isomers to some extent, with γ-HCH being degraded to the highest extent. When the fungus was directly added to a homogenous medium the inoculums were in a concentrated form and availability of the insecticide was also more which could have been used for its growth. Soil is a heterogeneous medium and only SMS was used where inoculums were less. This could have affected the degradation of HCH isomers. The build-up of the fungus on soil took time which was probably the reason for slow degradation of the HCH isomers in the initial stage. Nevertheless the half-life decreased from about 740 days to 106-125 days even for the most persistent β-HCH in the presence of SMS. |
| Conclusion |
| Though several researchers have reported degradation of HCH isomers using various substrates the ultimate aim has been to remove these persistent pesticides from the environment. Pseudomonas sp has been reported to degrade other isomers of HCH in soil but not β-HCH [19]. But in the present study when SMS from P. florida cultivation was amended with soil degradation of persistent isomers like α-, β- and δ-HCH was accelerated in addition to the less persistent γ-HCH. Large amount of SMS is left after harvest of mushroom. The SMS is composed of the mycelium of P. florida along with degraded straw. This fungus is able to grow on soil when the spent mushroom substrate (SMS) is mixed. Therefore it can be used for bioremediation of HCH contaminated sites. |
| Acknowledgements |
| The author thanks Director, Indian Institute of Horticultural Research, Bangalore, India for providing the facilities to carry out the work. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16231
- [From(publication date):
March-2015 - Jul 19, 2025] - Breakdown by view type
- HTML page views : 11556
- PDF downloads : 4675
