Research Article Open Access
Biodegradation of Feather by Microsporum fulvum Singly or in Combination with Other Fungi
| Ajay Kumar1*, Charu Singh1 and Pragati Saini2 | |
| 1Department of Microbiology, School of Life Sciences, ITM University, India | |
| 2Department of Microbiology, Kamla Raje Government PG College, India | |
| Corresponding Author : | Ajay Kumar Department of Microbiology School of Life Sciences ITM University, India Tel: 0751 243 2988 E-mail: kumarajayitm@gmail.com |
| Received August 13, 2014; Accepted November 25, 2014; Published November 28, 2014 | |
| Citation: Kumar A, Singh C, Saini P (2014) Biodegradation of Feather by Microsporum fulvum Singly or in Combination with Other Fungi. J Bioremed Biodeg 5:265. doi:10.4172/2155-6199.1000265 | |
| Copyright: © 2014 Kumar A, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The course of feather degradation by Microsporum fulvum singly and in combination with Chrysosporium tropicum, C. vallenarense, C. quenslandicum and Microsporum boullardii was studied by measuring protein, amino acids, keratinase enzyme released in the culture medium and weight loss of feather by M. fulvum and the effect of addition of M. fulvum on continued degradation of feather by C. tropicum, C. vallenarense, C. quenslandicum and M. boullardii were also studied. The synergistic action of M. fulvum and C. tropicum on feather degradation was found to be more effective. The biodegradation ability of M. fulvum can be effective by C. tropicum and C. vallenarense if the latter fungi join midway of keratinolysis. M. fulvum was also effective on the biodegradation ability of C. tropicum. M. fulvum did not act as follower fungus in wool degradation.
| Keywords |
| Combined effect; Chrysosporium tropicum; Feather degradation; Keratinolytic activity; Microsporum fulvum |
| Introduction |
| Keratin is the cornified part of the epidermis of vertebrates and is characterized by having higher resistance to attack by proteolytic enzymes. Keratin is a major component of feathers [1] It is an insoluble structural protein in epithelial cells of vertebrates [2]. Keratin is also very rich in amino acids like Leucine and Serine [3]. These cornified appendages include feathers, hairs, horns, hooves nails, claws and scales [4,5]. Keratin is divided into two types. α- Keratin (It present in wool, hair, and horn. It is in the form of folder chain). β-Keratin (It present in feather in the form of polypeptide chain) [6]. Bird’s feather and animal hairs are the most troublesome waste products and studies concerning their utilization are of great economic and ecological value [7]. |
| The soil inhabiting keratinophilic fungi have special affinity for keratin. To hydrolyze the keratin by synthesizing specific class of extracellular enzymes called keratinases, which degrade keratin into small peptide that can be utilized by the cells [8]. The ability of these fungi to invade and parasitize the cornefied tissue is closely associated and depends on the utilization of keratin by enzymatic digestion. Under natural conditions the degradation of keratin protein occurs microbiologically as a result of the enzymatic and mechanical interaction of the medium and by the high activity of proteolytic enzymes in the culture fluid, the cleavage products with the characters of amino acids, peptides and proteins were also established. Studies on biodegradation of keratin are limited to very little numbers of fungi [9-14]. |
| Fungi are an important component of the soil microbiota more in abundance than bacteria; their population depends upon soil depth and nutrient conditions [15]. The study presented here, therefore, provides the evidence that in the course of hair degradation. Microsporum fulvum can rapidly attack and utilize keratinous substrates acting singly or in combination with other fungi and in sequence. A series of experiments was performed to see if less keratinolytic Chrysosporium tropicum, C. vallenarense, C. quenslandicum, M. boullardii and a common geophilic dermatophyte could be effective on the amount of feather decomposed by M. fulvum. It would be expected to occur because some of these might be utilizing protein released as its breakdown. |
| In doing so, should relieve competitive inhibition keratinase action and also relieve repression of keratinase synthesis. Further experiments were performed to see if the feather breakdown by M. fulvum become limited and addition of other fungi can result in further weight loss, change ion pH, release of protein, amino acids and keratinase enzyme. |
| Materials and Methods |
| Screening of fungi for their keratinolytic ability |
| Preliminary screening of isolated fungi was carried on the basis of their keratinolytic ability [14]. On the basis of the preliminary screening, the keratinolytic fungi were given below in increasing order of their keratinolytic activity: Chrysosporium quenslandicum, Microsporum boullardii, M. gypseum, Trichophyton mentagrophytes, C. vallenarense, C. tropicum, and M. fulvum. These results revealed that M. fulvum was highly and C. quenslandicum was least keratinolytic. Thus, C. quenslandicum, C. tropicum, C. vallenarense, M. boullardii and M. fulvum were selected as treated fungi for keratinolytic ability in this study. |
| Preparation of feather and inoculums |
| In this method, the feather was washed with sterilized water, cut in 2 cm long pieces, sterilized in an autoclave for 15 minutes at 15 lbs pressure and used as substrate. Mineral medium containing K2HPO4-0.005 grm, MgSO4-0.25 grm, ZnSO4-0.005 grm, CaCl2-0.025 grm, FeSO4.7H2O-0.005 grm, dextrose-30.0 grm and pH - 6.5 per liter of distilled water was used in all the experiments. |
| Inoculum was the conidial suspension from the surface of 6 days old culture on mineral medium. The conidial suspension was obtained from tubes by brushing conidia in 5 ml of sterilized distilled water and 2 ml of conidial suspension (200 conidia/ml) was added to each flask. Each 250 ml Erlenmeyer flask received 250 mg of feather substrate. The cultures were incubated in stationary condition at 270C. The following controls were also run. |
| 1. Keratin control-50 ml of mineral medium and 250 mg of feather |
| 2. Fungus conrol-50 ml of mineral medium and fungus inoculum |
| 3. Test sample-50 ml of mineral medium, 250 mg of feather substrate and fungal inoculum. |
| Substrate decomposition and release of different metabolic bi-products into the medium |
| In preliminary study, the conidial suspension of M. fulvum was inoculated singly & filtered after 2, 4, 6, 8 & 10 days of incubation. In the first set of experiment, the conidial suspension of each fungus i.e. C. tropicum, C. vallenarense, C. quenslandicum and M. boullardii were inoculated with M. fulvum separated and filtered after 1, 2, 3 & 4 week. In the second experiment, M. fulvum was inoculated first and flasks received a second inoculum prepared as above, of four different fungi after 2 weeks and filtered after 4 weeks. |
| Determination of weight loss of feather substrate |
| The weight loss of the substrate was calculated by filtering the aliquots present in the medium after through Whatman’s filter paper no. 42 [9]. |
| Determination of soluble protein |
| Protein determination from filtrates was carried out from the flasks of all the three experimental sets after different incubation period. The filtrate from each flask was centrifuged at 4000 rpm for 5 minutes and the supernatants were assayed for protein using Folin-Ciocalteu reagent (Lowry 1951). The developing colour was read at 660 nm on UV-Vis spectrophotometer (Elico-169, India). Freshly prepared bovine serum albumin (BSA) was used as standard. |
| Determination of amino acids |
| Cysteine: Cysteine was quantitatively estimated by Ramakrishnan’s method [16]. The absorbance was measured at 510 nm on UV-Vis spectrophotometer. Freshly prepared cysteine was used as standard. |
| Cystine: Cystine was quantitatively estimated by Ramakrishnan’s method [16]. The absorbance was measured at 510 nm on UV-Vis spectrophotometer. Freshly prepared cystine was used as standard. |
| Methionine: Methionine was analyzed by Singh [17]. The absorbance was measured at 356 nm on UV-Vis spectrophotometer. Freshly prepared methionine was used as standard. |
| Keratinase enzyme: It was determined by the method of Noval & Nickerson [18]. The absorbance was measured at 280 nm on UV-Vis spectrophotometer. Freshly prepared keratinase enzyme was used as standard. |
| The results were expressed as net value i.e. the measured value in the test sample minus the sum of values keratin control (KC)+fungal control (FC). All the experiments were carried out in triplicate at 27 ± 2Ã?Â?C with appropriate controls. |
| Results and Discussion |
| Keratinolytic activity of M. fulvum |
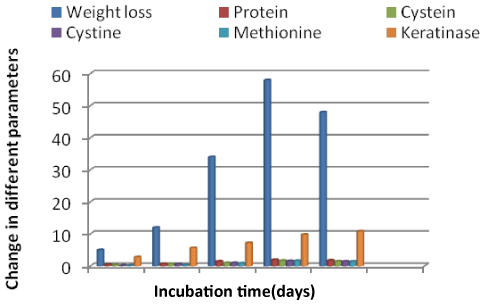
| M. fulvum was found strongly keratinolytic on preliminary study when used singly & released maximum protein (1.92 μg/ml) on 8th day and 1.75 μg/ml on 10th day in the culture filtrate. The maximum amount of amino acids i.e. cysteine, cystine & methionine were also released on 8th day 1.68, 1.58 & 1.62 μg/ml respectively. The enzyme keratinase was found maximum (9.8 μg/ml) on 8th day which is shown in Figure 1. |
| In the first set of experiment, the keratinolytic activity of M. fulvum was very much reduced with M. boullardii, C. vallenarense and C. quenslandicum. Similar results were observed during the release of protein, amino acids and keratinase enzyme. This fungus in collaboration with C. tropicum showed good keratinolytic activity (78.4% on 3rd week) and production of different metabolic bi-products. They showed maximum weight loss and production of bi-products (protein &amino acids) in 3 weeks and maximum keratinase enzyme production in 4 week (Figure 2A-2E). |
| The synergistic action of M. fulvum & C. tropicum in feather degradation was found to be more effective as it caused greater weight loss and production of different bi-products in 3 week. M. fulvum showed differential results with M. boullardii & C. quenslandicum. The weight loss of feather fluctuated in a very close range showing a slight decrease in two week time with a further increase at the end of incubation. The exact explanation for this fluctuation has not yet been found out and more information is needed to clarify it. The pattern of protein, amino acids and keratinase enzyme values were similar to weight loss trend which increase upto 3 weeks and then rapid decrease after 4 weeks. |
| The keratin substrates revealed different rates of keratin degradation with the fungi. It is generally believed that keratin degradation is due to the enzymatic action of the fungi, which is indicated by the substrate weight loss, as well as by the release of soluble products into the culture medium [12]. However, the distinctive feature of keratin is its high cysteine content, that makes it more resistant to enzymatic digestion [19].The enzyme was found to be a halophilic serine proteinase with unique substrate specificity [20]. The keratinolytic nature of the examined fungi towards different substrates may be related to their ability to produce cysteine as reported in other studies [19,21]. Some groups have emphasized the specific properties of fungal enzymes able to digest keratin, a substrate extremely resistant to the action of physical and chemical agents [22,23] and others have isolated and characterized keratinolytic enzymes of various species of dermatophytes [24] and their role in virulence. The present study has revealed that M. fulvum was found strongly keratinolytic when used singly & released maximum protein on 8th &10th day. The maximum amount of amino acids (cysteine, cystine & methionine) & enzyme keratinase were also released on 8th day. This is in agreement with the study of Muhsin TM, Hadi [25] who also reported that Microsporum species released high protein in culture medium. The keratinolytic activity of M. fulvum was very much reduced in combination with M. boullardii, C. vallenarense and C. quenslandicum. Similar results were observed during the release of protein, amino acids and keratinase enzyme. While M. fulvum in collaboration with C. tropicum showed good keratinolytic activity and production of different metabolic biproducts in 3 weeks and maximum keratinase enzyme production in 4 week. The amount of keratinolytic enzyme in the culture fluid was dependent on the initial pH of the culture medium [26]. In the study of extracellular keratinase from Trichophyton sp. detected the highest enzyme production was registered at 35°C (5.0 KU/ml) [27]. In other study Anbu et al. [28] detected the highest keratinase activity by Scopulariopsis brevicaulis (3.2 KU/mL) and Trichophyton mentagrophytes (2.7 KU/mL) in the culture medium with chicken feathers during 5 week of cultivation. According to Gupta and Ramnani [29] microbial keratinases have become biotechnologically important since their target the hydrolysis of highly rigid, strongly cross/linked structural polypeptide keratin recalcitrant to the commonly known proteolytic enzymes trypsin, pepsin and papain. |
| Conclusion |
| Keratinolytic activity has an important role in degradation of feather in natural environment. According the results, fungi that colonize & become most active later in a succession must be able both to tolerate growth inhibitory substances produced by prior colonist and to obtain nitrogen and other minerals that may already have been utilized by prior colonists. In the conditions used here M. fulvum did not act as follower even though it could degrade keratin. M. fulvum cannot obtain nitrogen that if needs for growth if C. tropicum and C. vallenarense already exploited that nitrogen. This may also explain why M. fulvum is not as dominant as C. tropicum in a distributional study specific for Chrysosporium. |
| Acknowledgements |
| The authors are grateful to Dean Prof. G.T. Kulkarni, and Dr. Kuldip Dwivedi, Head Department of Life Sciences, ITM University, Gwalior, for their encouragement and laboratory facilities. |
References
|
Figures at a glance
 |
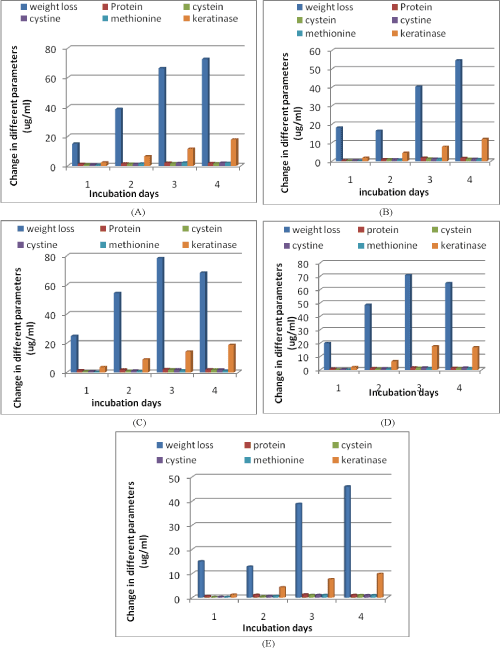 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14230
- [From(publication date):
December-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9693
- PDF downloads : 4537
