Biodegradation of Chlorpyrifos by Bacterial Strains Isolated from Agricultural Soils of Visakhapatnam District
Received: 30-Jan-2019 / Accepted Date: 19-Mar-2019 / Published Date: 26-Mar-2019
Abstract
Chlorpyrifos a broad-spectrum insecticide is effective in controlling cutworms, corn root worms, cockroaches, flies, termites, fire ants, beetles and lice. Though the use of Chlorpyrifos is banned in many developed countries, it is still widely used in developing countries like India. The widespread use of Chlorpyrifos is found to accumulate in soil and also gets leached into water bodies, making the environment polluted and is found to be toxic to non target organisms. The present study reports the bioremediation studies of Chlorpyrifos in agricultural soils of Visakhapatnam district. Bacterial strains were isolated from agricultural soils from different land use patterns of Visakhapatnam dist. The isolates were identified as Bacillus aryabhattai, Bacillus drentensis, Bacillus firmus, and Staphylococcus vitulinus. The degradation potential of the organisms were studied using Chlorpyrifos as the only carbon source and also the influence of Cow dung manure and NPK fertilizer on the growth and degradation capacity of organisms were studied. Degradation potential in media for individual organism as well as consortia was studied using spectrophotometric analysis. The degradation of microbial consortia in soil was detected using LC-MS to know the intermediate metabolites formed during degradation. It was found that the bacterial consortium has the highest degradation potential compared to individual organisms and also Bacillus drentensis is found to degrade Chlorpyrifos effectively compared to other organisms.
Keywords: Bioremediation; Chlorpyrifos; Cow dung manure; NPK fertilizer; Spectrophotometric analysis; LC-MS
Abbreviations
OP-Organo-Phosphorous; CDM-Cow Dung Manure; NPK fertilizer–Nitrogen, Phosphrous, Potassium Fertilizer; AChE-Acetylcholine Esterase; TCP-3, 5, 6-trichloro-2-pyridinol
Introduction
Organo-phosphorous pesticides are generally used as insecticides and account for about 36% of the total insecticides used globally. These compounds are unstable, non-persistent and their bioaccumulation potential is relatively less, hence are considered safe to use both in household and agricultural operations to treat pests, making them the most widely used pesticides worldwide [1]. More than 36 OP pesticides have been registered for use and all run the risk of acute and sub-acute toxicity. These compounds are toxic to human and other animals since they inhibit acetylcholine esterase (AChE) and are known to disrupt normal functions of central nervous system followed by severe muscle paralysis and death [2]. Although banned in many countries, several of them, especially parathion and methyl parathion are still being used indiscriminately in India for controlling insect pests of major crops like paddy, potato, mustard, cotton and vegetables [3,4]. The adverse effect of these OP pesticides on non target beings is a growing concern that has not studied in detail [5]. Majority of the people are repeatedly exposed to low OP concentrations, and long-term epidemiologic studies reveal linkage to higher risk of cancer development [6,7]. However, continuous and excessive use of OPs has also caused not only nerve and muscular diseases in humans and animals but contamination of ecosystems in different parts of the world [8].
Chlorpyrifos (CP) is used both as a pesticide for agricultural pests control and as termiticide to treat termites in households. The use of chlorpyrifos has been vastly restricted in US and some European countries, even for agricultural purposes. However, it is still widely used in developing countries like India, where in the year 2000, it was the fourth highest consumed pesticide after monocrotophos, acephate and endosulfan [9]. Degradation of Chlorpyrifos was studied using various conventional methods which led to the release of several toxic products and accumulation of recalcitrant residuals [10]. Hence, biodegradation using native microorganisms for its removal from the environment is considered attractive [11]. Chlorpyrifos was found to be resistant to biodegradation and it remained effectively for 5-17 years in the soil. Chlorpyrifos is degraded to 3, 5, 6-trichloro-2-pyridinol (TCP) and an alkyl phosphorotioate moiety in environment [12]. The hydrolytic degradation product of Chlorpyrifos 3, 5, 6-trichloro-2-pyridinol (TCP) has anti-microbial properties and this prevents the proliferation of Chlorpyrifos degrading microorganisms [13] and also it has been classified as a toxin with estrogenic activity, persistent, mobile and is listed as potent endocrine disrupting chemical by the US EPA [14] with a half-life ranging from 65 to 360 days in soil [15]. TCP has higher water solubility than Chlorpyrifos, therefore, carried easily into the environment and contaminate soils and aquatic environments [11].
The present study is focused on the degradation potential of bacterial isolates isolated from the agricultural soils of Visakhapatnam dist. The degradation potential of individual isolates and also consortium has been studied; also the impact of cow dung manure and NPK on the growth of organisms was studied.
Materials And Methods
Collection of soil samples
Paddy field soil samples were collected from four different agriculture practicing villages of Visakhapatnam District, Andhra Pradesh, India. Control sample was taken from an area where no agriculture is practiced. The samples were collected from a top layer of 0-15 cm which is been highly exposed to pesticides. In laboratory the samples were air dried, the clods were crushed and is sieved through 2 mm stainless steel sieve and stored at 4°C until analysis. The Physicochemical properties of the soil such as pH, EC, soil texture and soil moisture were determined using UF/IFAS methods [16]. Nitrogen was estimated using Kjeldahl [17]. Phosphorus was determined by Bray and Kurtz 1943 [18] method and Potassium was estimated using methodology given by Black 1965 [19] and is prescribed in Table 1.
| Samples | pH | EC µs/cm |
Soil texture | Soil Moisture | Available (Kg/ha) | |||
|---|---|---|---|---|---|---|---|---|
| N | P | K | ||||||
| Sample 1 | 7.8 | 197 | Sandy soil | 3.7 | 165.2 | 21.2 | 270.0 | |
| Sample 2 | 7.3 | 220 | Sandy soil | 5.8 | 186.3 | 29.3 | 340.0 | |
| Sample 3 | 7.1 | 207 | Sandy soil | 5.7 | 196.2 | 32.4 | 320.2 | |
| Sample 4 | 7.5 | 217 | Sandy soil | 6.2 | 207.0 | 39.2 | 370.7 | |
| Sample 5 | 7.9 | 244 | Sandy soil | 6.8 | 170.3 | 31.5 | 283.2 | |
Table 1: Physico-chemical properties of soil samples.
Isolation of bacteria
Initially the native bacterial isolates that is present in the soil samples were identified for this serial dilution plate technique was employed and subsequent dilutions were made up to 107 times. Each diluted sample was spread over the surface of nutrient agar plates in duplicate and incubated at 37°C for 72 hours. The strains of bacteria were selectively isolated from the mixed population of microbes growing on the nutrient agar medium by single colony isolation. Isolates were selected based on their morphological characteristics such as colour, margin, elevation, and optical feature of the colonies, shape and arrangements of the vegetative cells [20]. Pure culture of the selected isolates were done using streak plate method and maintained for further studies. Biochemical analyses of all isolates were carried out according to Bergey’s Manual of Determinative Bacteriology [21]. Confirmation of the genus and species level was done using 16s rRNA sequencing, Molecular analysis was done using 16s rDNA technique using universal primer 27 F AGAGTTTGATCMTGG CTCAG 20, 1492 R TACGGYTACCTTGTTA CGACTT 22.
Chemical
Commercial grade insecticide Chlorpyrifos (50% E.C) and NPK fertilizer having ratio 19:19:19 was obtained from Sri Sai Agrochemicals Visakhapatnam. Dried cow dung manure was collected from Simhachalam Goshala, a dedicated unit for the protection of cows. All other chemicals and reagents used in this experiment were of analytical grade and procured from Hi-Media.
Enrichment of isolates
Isolates were first grown in nutrient medium supplemented with Chlorpyrifos and incubated for 24 hrs. 1% of the cultures are transferred to minimal salt media (MSM) containing 0.5 g of K2HPO4, 0.04 g of KH2PO4, 0.1 g of (NH4)2SO4, 0. 05 g of MgSO4, 0.01 g of FeSO4 and 0.5 g of NaCl/ litre at pH 7.0 containing CP as the sole source of carbon. The cultures grown on MSM were used for further studies.
Pesticide concentration
Isolates were streaked in MSM agar containing 10-500 ppm concentration of Chlorpyrifos. The plates were incubated at room temperature for 24 hours and growth was observed MSM without Chlorpyrifos, this served as control.
Growth kinetics
The growth kinetics was determined by turbidometric method. The organisms selected were grown in sterile nutrient broth containing Chlorpyrifos as sole carbon source. The growth pattern of the organisms was also determined with the addition of NPK and Cow dung manure. This is done to identify the impact of NPK and cow dung on the growth pattern. Sterile broth containing inoculates without the addition of carbon source, NPK and Cow dung manure was set as control. Absorbance was taken at 660 nm for every 10 hrs.
Bioremediation Studies
In media
The bioremediation efficacy of the selected organisms was done by growing the four organisms in nutrient broth containing Chlorpyrifos (500 mgL-1) in an erlynmayer flask (500 ml). After 24 h of incubation, about 1% of culture was transferred to minimal salt media (MSM). Chlorpyrifos was used as sole carbon source, and incubated on a rotary shaker at 150 rpm for 7 days at room temperature. Samples from culture flask were centrifuged at 3,500 rpm for 15 min to obtain cell free extract. Spectrophotometric readings of the cell free extract were taken on alternate days for about 10 days.
Bioremediation studies of the selected bacterial consortium along with addition of NPK fertilizer and cow dung manure (CDM)
The selected Bacterial consortium was grown in an erlynmayer flask (500 ml) containing nutrient broth with Chlorpyrifos (500 mgL-1) as sole carbon source. After 24 h of incubation about 1% of culture is transferred to another erlynmayer flask (500 ml) containing minimal salt media (MSM) with Chlorpyrifos. About 2.5 gms of NPK 19-19-19 was taken and diluted thoroughly in distilled water and added to MSM. To another flask about 50 gms of sterilized and dried cow dung manure [22] was taken and diluted thoroughly in distilled water and added to MSM. The flask is incubated on a rotary shaker at 150 g for 7 days at room temperature. Media containing Chlorpyrifos and NPK and Chlorpyrifos and Cow dung manure without addition of inoculates were set as control. Samples from culture flask were centrifuged at 4,200 rpm for 15 min to obtain cell free extract. Spectrophotometric readings of the cell free extract were taken on daily bases or 24 h for 7 days. All the experiments were carried out in triplicates.
In soil
The soils used for the study was taken from agricultural fields, before analysing the soils were subjected to threefold autoclaving at 15 lbs pressure and 121°C for 30 min for sterilization. 500 g of this sterilized soil was taken into plastic bags. Chlorpyrifos (500 mg/kg) was added to each soil bag and the soil was thoroughly mixed to ensure uniform concentration of pesticides. Bacterial isolates were grown in nutrient broth medium. After 24 h incubation, the organisms were transferred to individual MSM with pesticides. The organisms were inoculated with the contaminated soils at 1 × 108 cfu/g. Soil samples (15 g) were recovered and extracted with acetonitrile solvent. The extracts were analyzed by LC–MS for every 4 days. Pesticide contaminated soil without organisms were considered as control.
LC-MS
Soil samples were collected from each bag for pesticide analysis. 15 grams of soil samples were weighed into a 250 ml Erlenmeyer flask and 20 ml of HPLC grade acetonitrile was successively added and then shaken for 30 min on a rotary shaker at 120 rpm. Then, the samples were allowed to stand until the soil had settled and the clear supernatant was used to determine the pesticide concentration by LC-MS as per the following conditions:
• Column: Agilent C20 (230 × 5.4 mm),
• Detector: programmable variable wavelength UV detector,
• Flow rate: 1 mL/min,
• Mobile phase: acetonitrile+ammonium acetate (90:10),
• pH 5.5 and injection volume: 50 μL.
LC-MS determination was done to identify the intermediates and final degradation compounds.
Results
In the present study a total of 24 Bacterial isolates were identified using morphological and biochemical characterization out of which four bacterial isolates showed distinct morphological appearance. Further, biochemical results of these strains using 16s rDNA sequence analysis against BLAST search showed that the bacterial strains were Bacillus aryabhattai, Bacillus drentensis, Bacillus firmus, and Staphylococcus vitulinus (Figure 1). The sequence of the four isolates was submitted to gene bank and the following accession numbers KY399763, KY399764, KY399766, KY399767 were obtained.
Bioremediation studies
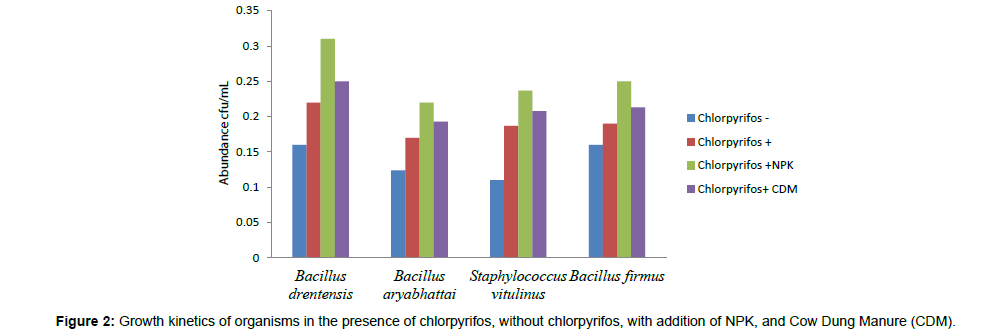
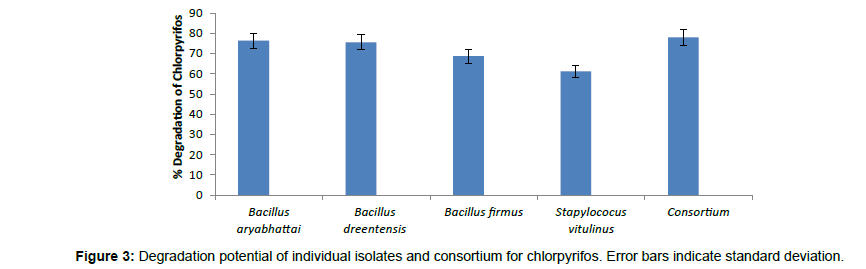
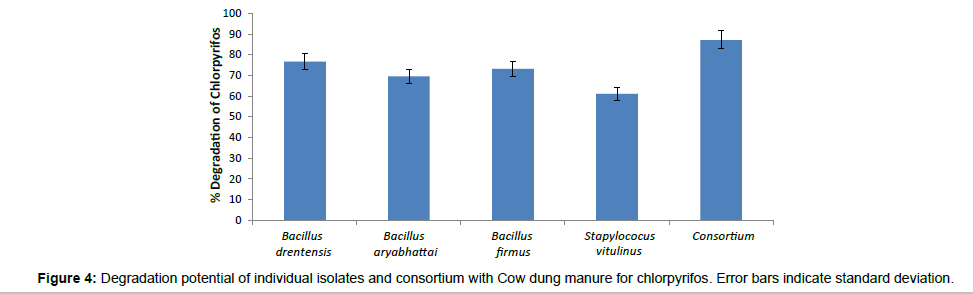
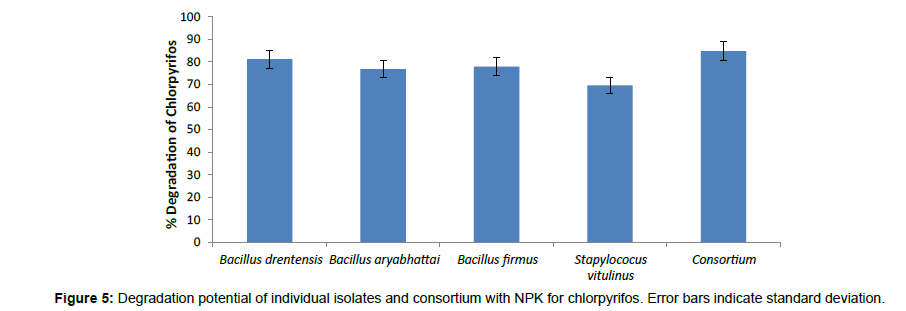
The biodegradation studies of Chlorpyrifos using four organisms Bacillus aryabhattai, Bacillus drentensis, Bacillus firmus, and Staphylococcus vitulinus shows that the organisms can effectively use Chlorpyrifos as carbon source. Table 2 depicts the tolerance levels and the abundance of growth of these four organisms for Chlorpyrifos from 10 ppm to 500 ppm, showing that Bacillus aryabhattai and Bacillus drentensis is able to grow actively till 300 ppm of pesticide concentration followed by Bacillus firmus 100 ppm and Staphylococcus vitulinus 60 ppm. Growth kinetics of the selected organisms (Figure 2) in the presence and absence of Chlorpyrifos shows that the organisms are able to utilize Chlorpyrifos as carbon source and also organisms like Bacilus aryabhattai and Bacillus firmus entered into stationary phase this might be due to the accumulation of intermediate metabolites of Chlorpyrifos and the organisms inability to utilize that for growth. Also the ability of Cow dung manure and NPK on the growth of organisms indicates that the organisms can grow well utilizing both NPK and Cow dung manure but the highest growth is observed on the addition of NPK this might be because of direct availability of large amount of extra nutrient source. The degradation potential studies of individual isolates and also consortium for Chlorpyrifos (Figure 3) with the degradation efficacy on the addition of Cow Dung Manure (Figure 4) and NPK (Figure 5) shows that all organisms are able to degrade at a higher rate when NPK is added to the medium also the control sample containing Cow dung manure and Chlorpyrifos without the addition of consortia has shown 10% degradation capacity indicating that the indigenous organisms present in cow dung manure is also able to degrade Chlorpyrifos to some extent. Among the individual isolates studied for the degradation of Chlorpyrifos B. drentensis is able to degrade Chlorpyrifos faster compared to S. vitulinus, B. firmus and B. aryabhattai. Among the four, the degradation potential of B. aryabhattai was found to be least and the degradation potential in consortium of these isolates is found to be faster compared to individual organisms.
| Conc. of chlorpyrifos (ppm) |
B. drentensis | B. aryabhattai | B. firmus | S. vitulinus |
|---|---|---|---|---|
| 10 ppm | + + | + + | + + | + + |
| 20 ppm | + + | + + | + + | + + |
| 40 ppm | + + | + + | + + | + + |
| 60 ppm | + + | + + | + + | + + |
| 80 ppm | + + | + + | + + | + |
| 100 ppm | + + | + + | + + | + |
| 200 ppm | + + | + + | + | + |
| 300 ppm | + + | + + | + | + |
| 400 ppm | + | + | + | + |
| 500 ppm | + | + | + | + |
| + + Abundant growth, + light growth. | ||||
Table 2: Tolerance of chlorpyrifos concentration by the four isolates.
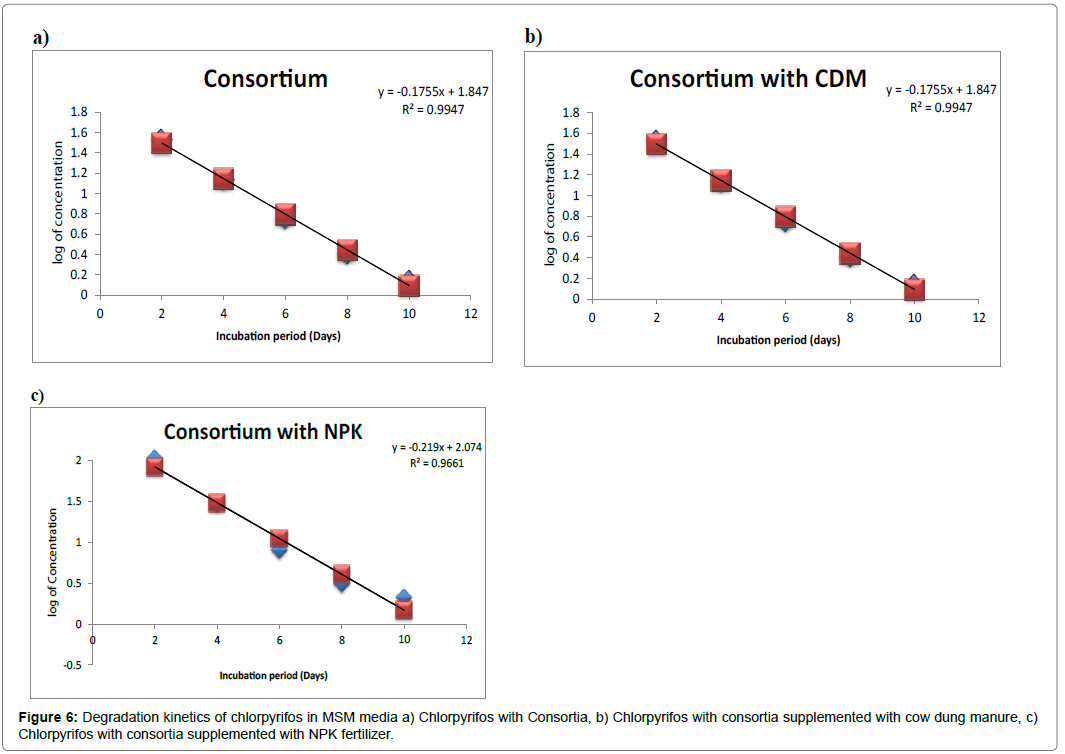
The degradation kinetics (Figure 6) studied shows that the Chlorpyrifos follows a straight line equation. The rate constant K and half-life were determined using first order kinetics. The K value was found to be 0.087 for degradation using consortium and consortium supplemented with cow dung manure. Rate constant for degradation using consortium supplemented with NPK is 0.1095 days-1. The half life for Chlorpyrifos was 4.71, 4.53 and 3,64 hrs respectively for degradation using consortium, consortium supplemented with cow dung manure and NPK respectively.
LC-MS analysis of soil sample
The LC-MS spectra of Chlorpyrifos spiked soil sample (Figure 7) showed a major peak at m/z 350.0 (Figure 7a), with a retention time of 14 min (Figure 8a) at 0 h indicating the presence of Chlorpyrifos. The LC-MS value for the same soil sample after 4 days (Figure 7b) showed a major peak of m/z 334.7 at 12 min (Figure 8b) retention time indicating the presence of Chlorpyrifos oxon [O, O-diethyl O-(3,5,6-trichloro-2 pyridyl) phosphate] [23]. This is in addition to peak at m/z 350 revealing the presence of Chlorpyrifos. The LC-MS spctra of the same soil sample after 8 days (Figure 7c) showed a major peak of m/z 198.2 at a retention time of 8 min (Figure 8c) whose mass value is similar to TCP (3, 5, 6 tricloro 2 pyridinol) [24]. The spectra also indicate the presence of peak at m/z 336, specifies the presence of Chlorpyrifos oxon in the sample at an intensity of 12%. However, no peak is seen at m/z 350 indicating the complete degradation of pesticide, Chlorpyrifos. After 12 days (Figure 7d) a major peak at m/z 172.2 with a retention time of 6 min (Figure 8d) is observed. The mass value of the peak is similar to the mass value diethyltiophosporic acid (DETP) [14]. The spectra also showed peaks at m/z 153.9 with a retention time of 5.8 min (Figure 8d) and 198.2 indicating the presence of diethylposphorothioate and TCP respectively [24]. Peak at m/z 334.2 shows the presence of Chlorpyrifos oxon. The LC-MS of the soil sample after 16 days (Figure 7e) showed peaks at m/z 172.1 and 153.9 indicating the presence of diethyltiophosporic acid (DETP) and diethylposphorothioate respectively in the soil. The appearance of peaks at m/z 198.1 and 334.2 still shows the presence of TCP and Chlorpyrifos oxon respectively. The LC-MS value for the control soil sample after 16 days (Figure 7f) showed a major peak at m/z 350.0 with a retention time of 14 (Figure 8e) min and another peak at 335.2 showing the presence of Chlorpyrifos and Chlorpyrifos oxon [O, O-diethyl O-(3,5,6-trichloro-2 pyridyl) phosphate] respectively in control soil.
Discussion
To isolate potential Chlorpyrifos degrading organism bacterial consortium was developed from agricultural soil by selective enrichment technique by providing Chlorpyrifos as the sole source of carbon. Degradation of Chlorpyrifos by consortium was found to be effective, this could be due to the synergistic effect of various bacterial isolates than degradation by individual organisms.
Many earlier studies have reported the potential bacterial strains that can degrade Chlorpyrifos and TCP in soils. Organisms like Pseudomonas aeruginosa, Pseudomonas fluorescence Bacillus cereus, Brucella melitensis, Bacillus subtilis, Klebsiella sp, Serratia sp [25], Bacillus pumilis [26] Sphingomonas, Stenotrophomonas, Bacillus sp Brevundimonas, Pseudomonas sp [27] Paracoccus sp, Serratia, Tricosporon sp [28], Stenotropomonas sps, Alcaligens fecali [29], Pseudomonas aeruginosa [30], Chlorella vulgaris [31], Pseudomonas putida, Klebsiella sp Pseudomonas stutzeri, Pseudomonas aeruginosa [24] were studied for their efficacy and degradability.
Conclusion
In the present study four organisms; Bacillus aryabhattai, Bacillus drentensis, Bacillus firmus, and Staphylococcus vitulinus were isolated for the first time from the agricultural soils of Visakhapatnam district, Andhra Pradesh. The degradation capabilities of these organisms with reference to Chlorpyrifos in media were studied. In MSM media, the degradation potential of B. drentensis is 63% followed by B. firmus, 59%, B. aryabhattai 48% and S. vitulinus 39%. The degradation potential of B. drentensis and B. firmus and B. aryabhattai increased by 20% while S. vitulinus increased by 10% on the addition of Cow dung manure and the degradation potential increased by 40% for B. drentensis, B. firmus, B. aryabhattai and about 25% for S. vitulinus by the addition of NPK. Moreover the degradation potential of the consortium was found to be higher at about 70% and degradation potential increased for about 10% when Cow dung manure is added and 15% when NPK is added to the media. LC-MS analysis of soil sample suggest that the consortia were able to hydrolyse Chlorpyrifos to Chlorpyrifos oxon ( O, O-diethyl O-(3,5,6-trichloro-2 pyridyl) phosphate within 4 days and 3,5,6-trichloro-2-pyridinol in 8 days and the presence of diethyltiophosporic acid (DETP) and diethylposphorothioate was seen during 16th day in the soil sample. Negligible amounts of Chlorpyrifos oxon and TCP were also observed during 16th day making it clear that the organisms were able to hydrolyse the mother compound but the degradation of Chlorpyrifos oxon and TCP were slow. Degradation rate of the consortia in soil sample was observed to be nearly 80%. The present study reveal that the degradation potential of the isolates follow in the order B. drentensis>B. firmus>B. aryabhattai.>S. vitulinus. The results of the present study reveal that these organisms have the potential to degrade pesticide Chlorpyrifos. Further studies will throw more credible information on the practical application of these isolated organisms in degrading Chlorpyrifos in soil.
References
- Kiely T, Donaldson D, Grube A (2004) Pesticide industry sales and usage: 2000 and 2001 market estimates. Environmental Protection Agency, Washington, USA.
- Shen YJ, Lu P, Mei H, Yu HJ, Hong Q, et al. (2010) Isolation of a methyl parathion-degrading strain Stenotrophomonas sp. SMSP-1 and cloning of the ophc2 gene. Biodegradation 21: 785-792.
- Pakala SB, Gorla P, Pinjari AB, Krovidi RK, Baru R, et al. (2007) Biodegradation of methyl parathion and p-nitrophenol: Evidence for the presence of a p-nitrophenol 2-hydroxylase in a Gram-negative Serratia sp. strain DS001. Applied Microbiol Biotechnol 73: 1452-1462.
- Banerjee I, Tripathi SK, Roy AS, Sengupta P (2014) Pesticide use pattern among farmers in a rural district of West Bengal, India. J Natural Sci Biol Med 5: 313-316.
- Tina E, Metka F (2011) Organophosphorus pesticides -mechanisms of their toxicity: Organophosphorus pesticides, mechanisms of their toxicity. National Institute of Biology pp: 241-260.
- Brown LM, Blair A, Gibson R, Everett GD, Cantor KP, et al. (1990) Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Research 50: 6585-6591.
- Waddell BL, Zahm SH, Baris D, Weisenburger DD, Holmes F, et al. (2001) Agricultural use of organophosphate pesticides and the risk of non-Hodgkin's lymphoma among male farmers (United States). Cancer Causes & Control 12: 509-517.
- Zhang J, Xin Y, Liu H, Wang S, Zhou N (2010) Metabolism-independent chemotaxis of Pseudomonas sp. strain WBC-3 toward aromatic compounds. J Environ Sci 20:1238-1242.
- Ansaruddin PA, Vijayalakshmi K (2003) The womb is not safe anymore. Indegenous Agriculture News.
- Xu G, Zheng W, Li Y, Wang S, Zhang J, et al. (2008) Biodegradation of chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol by a newly isolated Paracoccus sp. strain TRP. Int Biodeter Biodegr 62: 51-56.
- Maya K, Singh RS, Upadhyay SN, Dubey SK (2011) Kinetic analysis reveals bacterial efficacy for biodegradation of chlorpyrifos and its hydrolyzing metabolite TCP. Process Biochemistry 46: 2130-2136.
- Alvarenga N, Birolli WG, Nitschke M, De-O-Rezende MO, Seleghim MH, et al. (2015) Biodegradation of chlorpyrifos by whole cells of marine-derived fungi Aspergillus sydowii and Trichoderma sp. J Microb Biochem Technol 7: 133-139.
- Racke KD (1993) Environmental fate of chlorpyrifos: Review. Environ Contam Toxicol 131: 1-151
- Chen S, Liu C, Peng C, Liu H, Hu M, et al. (2012). Biodegradation of chlorpyrifos and its hydrolysis product 3, 5, 6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PloS One 7: Â e47205.
- Armbrust KL (2001) Chlorothalonil and chlorpyrifos degradation products in golf course leachate. Pest Manage Sci 57: 797-802.
- Mylavarapu RS, Kennelley ED (2002) UF/IFAS Extension soil testing laboratory (ESTL) Analytical Procedures and Training Manual p: 18.
- Official Methods of Analysis (1995) AOAC International. Gaithersburg, MD, sec. 33.2.11, Method 991.20
- Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59: 39-45.
- Black CA (1965) Methods of soil analysis Part I. Am Soc Agron Inc Publi, Madison, Wisconsin, USA.
- Buchanan RE, Gibbons NE (1974) Bergey's manual of determinative bacteriology. Williams & Wilkins Co., Baltimore, Maryland, USA.
- Gong CM (2007) Microbial safety control of compost material with cow dung by heat treatment. J Environ sci 19: 1014-1019.
- Tang J, Ceo Y, Rose RL, Brimfield AA, Dai D, et al. (2001) Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse and rat liver microsomes. Drug Metab Dispos 29: 1201-1204.
- Chitrambalam S, Sonia J (2012) Biodegradation of chlorpyrifos by bacterial consortium isolated from agriculture soil. World J Microbial Biotechnol 28: 1301-1308.
- Vidya Lakshmi C, Kumar M, Khanna S (2008) Biotransformation of chlorpyrifos and bioremediation of contaminated soil. Int Biodeter Biodegrad 62: 204-209.
- Anwar S, Liaquat F, Khan QM, Khalid ZM, Iqbal S (2009) Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. J Hazard Mater 168: 400-405.
- Li J, Liu J, Shen W, Zhao X, Hou Y, et al. (2010) Isolation and characterization of 3,5,6-trichloro-2-pyridinol-degrading Ralstonia sp. strain T6. Bioresour Technol 101: 7479-7483.
- Xu GM, Li YY, Zheng W, Peng X, Li W, et al. (2007) Mineralization of chlorpyrifos by co-culture of Serratia and Trichosporon spp. Biotechnol Lett 29: 1469-1473.
- Yang C, Liu N, Guo X, Qiao C (2006) Cloning of mpd gene from a chlorpyrifos degrading bacterium and use of this strain in bioremediation of contaminated soil. FEMS Microbiol Lett 265: 118-125
- Jayashree R, Vasudevan N (2007) Effect of tween 80 added to the soil on the degradation of endosulfan by Pseudomonas aeruginosa. Int J Environ Sci Tech 4: 203-210.
- Mukherjee I, Gopal M, Dhar DW (2004) Disappearance of chlorpyrifos from cultures of Chlorella vulgaris. Bull Environ Contam Toxicol 73: 358-363
Citation: Balakrishnan SL, Rao PVV (2019) Biodegradation of Chlorpyrifos by Bacterial Strains Isolated from Agricultural Soils of Visakhapatnam District. J Bioremediat Biodegrad 10:459.
Copyright: © 2019 Balakrishnan SL, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3691
- [From(publication date): 0-2019 - Apr 28, 2025]
- Breakdown by view type
- HTML page views: 2818
- PDF downloads: 873