Biodegradability Study of Potassium Hydrogen Phthalate and Benzene Using BOD5 Seed as Inoculum
Received: 12-Mar-2018 / Accepted Date: 10-Mar-2018 / Published Date: 13-Apr-2018 DOI: 10.4172/2155-6199.1000436
Abstract
Benzene and Potassium Hydrogen Phthalate (KHP) with their immeasurable applications lead to tons of generated waste. Biodegradation is considered to be a remedy for this issue and the usage of Activated Sludge is the most dominant method used among these days. However issues of variation in its content arose and thus the usage of standardized inoculum offered opportunities to surpass these disadvantages, this study aims to test the effectivity of BOD5 Seed inoculum in degradation of Benzene and KHP and eventually apply its effectivity to aqueous organic waste. The samples were tested for Dissolved Oxygen (DO) and Chemical Oxygen Demand (COD) Test for initial analysis before it was prepared for the biodegradation process. During the biodegradation process, pH 6-8 was maintained for it was the desired environment of the seed inoculum. Monitoring was conducted through COD test. The degraded benzene sample was then subjected for GC-MS analysis. The 1000 ppm benzene sample was 99.79% degraded from an initial COD concentration of 1112.545 ppm to 2.352 ppm. On the other hand, 1000 ppm KHP sample was 47% degraded from an initial concentration of 1271.6 ppm to 673.99 ppm. There was an effect of filtration on the COD concentration obtained. In conclusion, BOD5 seed inoculum was capable of an effect of filtration on the COD concentration obtained. In conclusion, BOD5 seed inoculum was capable of degrading 1000 ppm benzene and 1000 KHP sample. However, 1000 ppm benzene sample were degraded faster than 1000 ppm KHP sample. On the other hand, higher concentrations of KHP sample were not degraded. Furthermore, treatment/degradation of aqueous organic waste using BOD5 seed inoculum is possible. Unseeded samples were also eventually degraded however slower than seeded samples.
Keywords: Benzene; Potassium hydrogen pthalate; Aqueous organic waste; Biodegradation process; BOD5 seed inoculum; Chemical oxygen demand
Introduction
One of the mainstream problems of today is the generation of too much waste coming from the rapid growth of population together with the continuous industrialization. Wastes in water bodies are one of the worldwide issues that create a direct impact to the environment. Existence of organic pollutants in water bodies and to the environment itself has been recently studied. Among these organic pollutants were: Benzene and Potassium Hydrogen Phthalate.
Potassium Hydrogen Phthalate (KHP) is the monopotassium salt of phthalic acid. Due to its astonishing properties such as high purity, readily soluble in water, easy to dry, air-stable, not hygroscopic, and not affected by carbon dioxide, industries use large quantities of KHP in the manufacture and quality control of its products thus large quantities of organic waste containing KHP is also generated.
On the other hand, Benzene is a cyclic hydrocarbon with a molecular formula of C6H6. It is a clear, colorless, highly flammable, volatile and toxic, liquid aromatic hydrocarbon. Benzene is a valuable industrial raw material. It is extensively used in the synthesis of various organic compounds such as ethylbenzene, phenol and cyclohexane [1]. It is also used as an organic solvent for various experiments and as a raw material for the manufacture of plastic, paper, detergents, and pesticides [2]. Vast applications of benzene in the chemical and petrochemical industry result to generation of large volumes of organic waste containing benzene. Both Benzene and Potassium Hydrogen Phthalate have been constantly reported as an environmental contaminant. Because of this there is a great need for scientists to develop and devise new methods of waste degradation.
Degradation using microbes is one of the most efficient ways in degradation of chemical waste as it is cost-effective and eco-friendly. Biodegradation uses pure microbial structure as well as mixed microbial cultures. However the usage of mixed microbial cultures is more advantageous, it’s because mixed microbial communities have greater ability in biodegradation of organic pollutants compared to a single bacterium. In biodegradability testing, important factors that cannot be easily standardized should be given enough attention for it has a great influence in the biodegradation results; which includes the inoculum, the source of the microorganisms for the test, and its state of acclimatization and adaptation.
Assessment of the biodegradability of chemicals through developed laboratory standard methods plays an essential role in nature’s waste management and recycling system. Usage of activated sludge has been the most dominant method used in measuring the inherent biodegradability of chemicals. Activated sludge is a complex and dynamic mixture of suspended solids, various microorganisms, and extracellular material made up of polysaccharides, organic acids, proteins, and lipids. However, the characteristics of the activated sludge microbial population depend on the waste composition and operation mode of the plant [3]. The variation in microbial composition and physical-chemical characteristics of the activated sludge used in carrying experiments make it almost impossible to have comparable results. In addition to this, the use of a mixed microbial inoculum from an activated sludge treatment plant, leads to concerns with regards to inoculum sampling, difficulty in maintaining viability and controlling quantitative and qualitative characteristics, and the possible presence of pathogenic microorganisms [4,5].
The definition of a ‘standard’ inoculum could be the best solution to this potential problem. The ‘standard’ inoculum could be formed by mixing specific microbial strains conserved in microbial collections with fine knowledge of its relevant biochemical properties. Standardized microbial communities as inoculum give way to more homogenous and comparable results that eliminates bias with the results. An example of a standardized inoculum is the BOD5 Seed Inoculum which is known as the standard inoculum use in BOD5 test.
In this present work, BOD5 Seed Inoculum had been used as a standardized seed inoculum for biodegradation of Benzene and Potassium Hydrogen Phthalate. The seed was composed of specialized microbial cultures which contain species from the genera Pseudomonas, Nocardia, Streptomyces, Bacillus and Micromonospora. BOD5 seeds inoculums are non-pathogenic, convenient, fast and costeffective thus such seeds have the potential for widespread use in water pollution laboratories [6]. The BOD5 Seed Inoculum had also been used to treat aqueous organic waste sample to test the effectivity of its biodegradation for aqueous organic waste.
Methodology
In order to monitor the degradation of the samples, various wastewater analyses are used to identify the initial and final content through the biodegradation process. This includes the Chemical Oxygen Demand (COD), Dissolved Oxygen (DO) and pH.
Materials
The chemical and reagents used throughout the research were analytical grade and was available in the Philippine market and PUP Chemical storage. Potassium Hydrogen Pthalate (Himedia, India) from the PUP laboratory and 99.5% Benzene (LobaChemie Pvt. Ltd, India) bought from Belman Laboratories (Quezon City, Metro Manila) was used as the primary sample for the biodegradation. For the Dissolved Oxygen Analysis, Manganese Sulfate, Potassium Iodide, Sodium Azide, Sulfuric Acid, Starch indicator, Sodium Thiosulfate (all were LobaChemie Pvt. Ltd, India), provided by Scientia Tech Laboratory, was prepared. While on the other hand, Potassium Dichromate (LobaChemie Pvt. Ltd, India), bought from Belman Laboratories, was used as the primary reagent for oxidation of the organic wastes. Sulfuric acid (RCI Labscan, Thailand) bought from Belman Laboratories and Silver Sulfate (Univar, USA) provided by the PUP Chemical Storage was used for the Chemical Oxygen Analysis. While for the BOD test, the BOD seed inoculums, Polyseed® (InterLab Supply, USA), bought from Scientia Tech laboratories, was used as the seed for the biodegradation of samples. The additional nutrients such as Phosphate buffer, Magnesium Sulfate (LobaChemie Pvt. Ltd, India) solution, Calcium Carbonate (Scharlau S.L., Scharab, Spain) Solution and Ferric Sulfate (J.T. Baker, J.T. baker Inc., USA) Solution, that are bought from Scientia Tech Laboratories and provided by the PUP Chemical Storage, were needed for the dilution water. The neutralization of the sample was obtained by adjustment of pH with the use of Sulfuric Acid and Sodium Hydroxide (LobaChemie Pvt. Ltd, India). The instruments used were HITACHI 5900 UV-Vis Spectrophotometer model that was used to determine the COD value after the digestion of the samples and the pH meter was used for the monitoring of the pH daily. A Gas Chromatography Mass Spectroscopy was used for the analysis of benzene to the treated Benzene sample which was available at Philippine Institute of Pure and Applied Chemistry.
Sample preparation
Concentrated benzene solution (99.98%) and Potassium Hydrogen Phthalates samples were obtained from Belman Corp. and PUP Chemical storage, respectively. These samples are prepared in an initial concentration of 1000 mg/L. Anhydrous Potassium Hydrogen Phthalate (KHP) was prepared by diluting 1 g of oven-dried KHP with 1 L distilled water. While for benzene sample, a gram of the concentrated benzene solution was diluted with distilled water. Samples were stored at temperature of 10°C up to 20°C in amber bottles. On the other hand, the chemical waste samples were obtained from the PUP chemical storage. This was prepared using random sampling and storing samples up to 20°C.
Initial analyses
The initial analyses consists three (3) water analyses to determine the initial content of the samples before the biodegradation process. This consists of Chemical Oxygen Demand (COD), Dissolved Oxygen (DO) and pH. The pH was monitored to treat the sample into neutral in case of the sample is in the acidic or basic condition due it will interfere the activity of the microorganism.
Dissolved Oxygen (DO): The Winkler method with Azide modification was used for the determination of DO. The samples were transferred to a standard BOD bottle and were added with 1 mL of Manganese Sulfate and Iodide-Azide reagent, wherein the tip of the pipette was below the liquid level. The sample was stoppered immediately and was inverted two to three times gently. A precipitation was formed and was treated with 1 mL Sulfuric Acid, stoppered and inverted. A 201 mL of treated sample was then transferred in an Erlenmeyer flask and titrated with 0.0025 N Sodium Thiosulfate with the Starch indicator. The endpoint was reached when the initial color of the untitrated sample turned colorless after the titration (SMEWW 4500-O C).
Chemical Oxygen Demand (COD)
Chemical oxygen demand of samples was determined according to the closed reflux colorimetric method of Standard Methods for the Examination of Water and Wastewater (SMEWW No. 5200 D).
A suitable volume of sample and reagents was measured, then digested and cooled including the standards. The standards, samples and controls were measured at 600 nm. A digested blank was used as the reference solution and as a blank. The absorption measurement of the digested blank containing gave the initial dichromate absorption. Afterwards, the calibration curve was determined then the samples and controls were analyzed. The COD values of the samples were then calculated using the formula:
COD as mg O2/L=(mg O2 final vol × 1000)(mL of sample)
Biodegradation
The biodegradation process was consisted of three steps, the pretreatment of the samples, preparation of dilution water and seed solution and the seeding of the samples. The pre-treatment of the samples was done by adjusting the pH to neutral and lowering the temperature up to 20°C to preserve the content of the sample. The dilution water was prepared by adding the nutrients needed by the microbes for biodegradation. Magnesium sulfate solution, Calcium carbonate solution and Ferric sulfate solution were added for the nutrients of the microbe. All the components were mixed and preserved up to 20 ± 3°C before analysis. The seed solution was then prepared by pouring entire content of the Polyseed® capsule in a half liter of dilution water, aerated and stirred for an hour then settled for 5 to 15 minutes. A Bran, which acts as the carrier for the microorganisms, will neither dissolve or nor inhibit microbial activity, this must be settled out of the solution necessarily. When the solution is settled, decant the supernatant carefully then transfer decanted solution to a clean beaker and was gently stirred in the remainder of the test. The prepared samples were transferred in an amber bottle and added with 300 mL dilution water along with 4 mL of the seed solution added with 300 mL dilution water along with 4 mL of the seed solution which is prescribed to the method of the BOD. Aside from the biodegradability of the samples, a negative, blank, and sample control were used. The Negative control was used to determine the possible abiotic degradation wherein the test water and seed was used as the negative control. The blank was used to check the possible effect between the seed and dilution water only. The sample control is the unseeded samples which is composed of two: the aerated and sealed. The first COD monitoring started after three (3) to fifteen (15) hours of the completion of the set-up. The Chemical Oxygen demand was used to monitor the biodegradation of the samples and controls.
Set-up: Four (4) Amber bottles with a capacity of four (4) liters were filled with the one liter of sample. A silicone tube with a small diameter is dipped in the samples and connected with a splitter connector for aeration of samples. The silicone tubes are connected along the other sample’s splitter and to the air pump, which is elevated from the samples, for the distribution of air. The set up consists for degradations of the negative, blank, sample controls and the samples.
Final analysis
After the biodegradation of the samples, samples then undergone final analysis. Similar in the initial analyses, the pH, dissolved oxygen content, and chemical oxygen demand was determined.
Results and Discussion
The biodegradation process was caused mainly by the microorganisms in the BOD5 Seed Inoculum, wherein they consume the organic pollutants as a part of their metabolism thus leading to its growth and to the biodegradation of the samples.
Initial and final dissolved oxygen
This section reveals the result obtain from the biodegradation of the samples using BOD5 seed inoculum. The biodegradation of the samples was assessed using COD concentration which was measured at time intervals throughout the biodegradation.
The Dissolved Oxygen (DO) was determined using the Winkler- Azide Modification Method. The results were obtained before and after the biodegradation process to compare and prove the degradation method proposed. Table 1 shows the initial and final Dissolved Oxygen of benzene and KHP and waste. As shown in Table 1, DO concentration of benzene increased from 3.8 mg/L to 6.1 mg/L while DO of KHP increased from 2.4 mg/L to 8.6 mg/L. Same instance was observed for the waste sample in which its DO concertation increased from 1.1 mg/L to 1.7 mg/L.
| Sample | Dissolved Oxygen(DO) (mg/L) | |
|---|---|---|
| Initial | Final | |
| Benzene | 3.8 | 6.1 |
| KHP | 2.4 | 8.6 |
| Waste | 1.1 | 1.7 |
Table 1: Dissolved Oxygen.
The results obtained showed that dissolved oxygen content of the samples. The increased DO indicate that the samples were degraded since increased DO means that organic substances present in the sample have decreased which allows more oxygen to be dissolved in the solution. In DO analysis, organic substances present in the sample tend to snatched dissolved oxygen in the sample.
Biodegradation of 1000 ppm benzene and 1000 ppm KHP
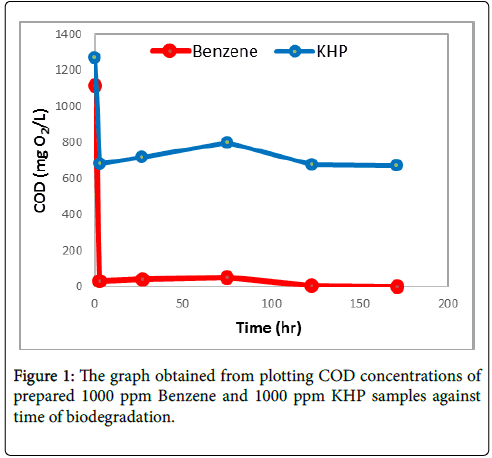
Figure 1 shows that the COD concentration of 1000 ppm benzene (Benzene 1) and 1000 ppm KHP (KHP 1) samples slightly decreases throughout the biodegradation process. After 171 hours of biodegradation, COD concentration of Benzene 1 was reduced by 99.79% from the initial concentration of 1271.6 mg O2/L to 2.3520 mg O2/L while COD concentration of KHP 1 sample was reduced by 47.00% from the initial concentration of 1112.5 mg O2/L to 673.99 mg O2/L. Based on the obtained results, KHP 1 has a slower biodegradation process compared to that of 1000 ppm Benzene 1.
In Figure 1, an exponential decreased in the concentration of Benzene 1 and KHP from initial COD of 1112.5 mg O2/L and 1271.6 mg O2/L to 31.850 mg O2/L and 683.31 mg O2/L, respectively, was observed after 3 hours of biodegradation. COD concentration of benzene sample was reduced by 97.14% after 3 hours while COD of KHP was reduced by 47.00%.
However, after that period, COD concentration of both benzene and KHP then decreased slightly as biodegradation proceeds. Furthermore, Figure 2 also shows that the COD concentration of both samples slightly increased at time interval 3 to 75 hours. In addition to the degradation caused by the seed, the exponential decreased of COD concentration observed after 3 hours of biodegradation may also be due to the addition of 300 mL dilution water which contains the nutrients needed by the microorganisms to survive. On the other hand, the sudden increase of COD at time interval 3 to 75 hours could be explained by the factor caused by filtration in obtaining samples. Filtration eliminates interferences from the seed itself. The significance of the filtration of the samples was then justified by the result obtained after 123 hours which shows a 92.99% decrease in the COD concentration of Benzene 1 and 14.82% in KHP 1.
Biodegradation of aqueous organic waste
The biodegradability of aqueous organic waste sample using BOD seed inoculum in 250 hours (10 days) was studied. As shown in Figure 2, COD concentration of aqueous organic waste sample continuously decreases as biodegradation proceeds. It is shown that COD of the waste sample was reduced by 68.71% from the initial COD of 48360 mg O2/L to final COD of 15131 mg O2/L after 243 hours or 10 days of biodegradation. At first COD monitoring (3 hours after seeding), 22.95% COD reduction was observed. While at second COD monitoring, 17.54% of COD was reduced after 24 hours from first COD monitoring. On the other hand, at third COD monitoring (after 96 hours of biodegradation), 41.37% of COD was reduced from COD concentration at second monitoring which may suggest that about 10% COD was reduced every 24 hours of biodegradation from third COD monitoring to second COD monitoring. Moreover, 15.99% of COD was reduced from COD concentration at third monitoring after another 120 hours of biodegradation which may also suggest that about 3% COD was reduced every 24 hours of biodegradation from fourth COD monitoring to third COD monitoring. This implies that the rate of COD concentration reduction decreases the longer the biodegradation of the aqueous organic waste sample.
The initial concentration of 48360 ppm of the organic waste depletes rapidly at the irst COD (3 hours) due to the two factors: the addition of the dilution water and the effectivity of the seed inoculum. A ter 24 hours, a gradual decrease in COD was showed indicating that the organic compounds are being slowly consumed yet arriving at the third analysis (123 hours), the COD value decreased vigorously up to 50%. This is due to the increase of the microbial population and hence increasing the consumed organic substance. The aqueous organic waste was reduced by a total of 68.71% after 243 hours which was faster and more efficient compared to the study of Sphiner et al., using Rhodococcus rhodochrous , Rhodococcus ruber , Ralstonia sp., Acinetobacter venetianus , Paenibaccilus naphtalenovo rans, Pseudomonas putida , and Marinobacter hydrocarbonclausticus as mixed cultures resulting to a biodegradation time of 160 days and was able to degrade >82% of their waste sample. This only implies the effectivity of the BOD5 Seed Inoculum in the biodegradation of aqueous organic waste.
Biodegradation of higher concentration of KHP
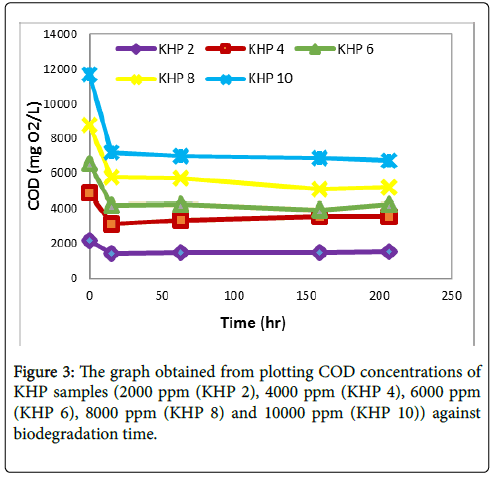
The biodegradability of KHP samples (2000 ppm, 4000 ppm, 6000 ppm, 8000 ppm and 10000 ppm) using BOD seed inoculum was studied for 207 hours (9 days). Figure 3 shows that after 15 hours of biodegradation (1st COD monitoring), COD concentrations of 2000 ppm, 4000 ppm, 6000 ppm, 8000 ppm, and 10000 ppm KHP samples were reduced by 34.76%, 36.28%, 35.74%, 34.10% and 38.40% respectively. Beyond the first COD monitoring, proceeding results showed that COD concentrations of these samples reached a plateau.
Figure 3 shows that after the first COD monitoring, it eventually approached to an abrupt halt. The variations with their values are almost insignificant and may be caused by the aerator and even the environment of the samples. Possibilities may also arise from the nutrients for the seed which may not be enough to provide for samples with higher concentrations. Unfortunately, in this study no experimental procedure was conducted to validate this concept. Benzene in higher concentration was not studied because it was not completely soluble in water. Within the biodegradation process the pH of the seeded sample ranges from 6-8 which is the required pH for the continuous survival of the seed.
Controls
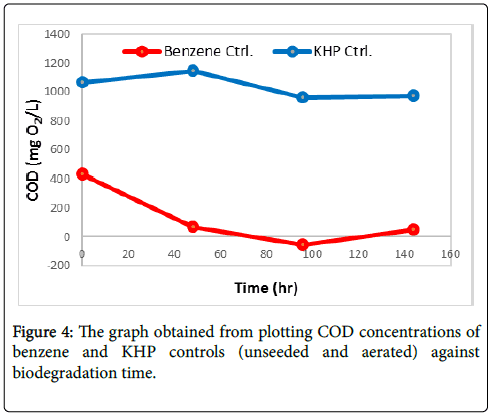
Degradation of benzene and KHP controls (unseeded and aerated): The effect of aeration was determined by investigating the degradation of 1000 ppm benzene and 1000 ppm KHP control for 6 days. For the investigation, controls were not seeded instead they were aerated together with the seeded 1000 ppm benzene and 1000 ppm KHP samples. The degradation of the controls was assessed through measurement of their COD concentration at time intervals throughout the degradation process. Figure 4 reveals that COD concentrations of benzene and KHP control progressively decreases all through degradation process. COD concentration of these controls was reduced by 89.10% and 8.74% at the end of degradation time. This implies that aeration affects degradation of samples.
The initial concentration of 1000 ppm of Benzene and KHP, a first COD analysis started at 48 hours after the initial COD analysis and showed vigorous changes. The KHP value started to increase, on the other hand, Benzene depleted rapidly only through aeration process. The possible explanation of this is the volatile property of Benzene, the aeration process also affected its concentration. The bacteria’s presence in the air that is pumped by the aerator or the transfer of the bacteria’s from the seeded sample due to its interconnection lead as well to the decrease on the sample’s concentration.
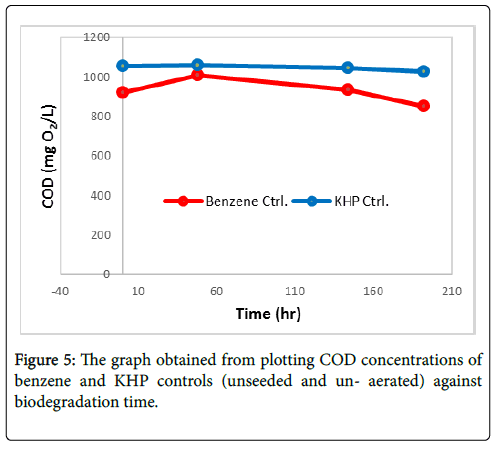
Degradation of benzene and KHP controls (unseeded and unaerated): The abiotic degradation of benzene (1000 ppm) and KHP control (1000 ppm) was studied for 192 hours (8 days). To study their abiotic degradation, the controls were not seeded and not aerated throughout the degradation process. Figure 5 shows that COD concentrations of the controls slightly decreased as time increased. At final COD monitoring, initial COD concentration of benzene and KHP control was reduced by 8.09% and 3.29%, respectively. Obtained results suggest that samples are degraded as time proceeds even without the use of the BOD seed inoculum and aeration. However, degradation of samples without seed is slower than the degradation of samples with seed and aeration.
Benzene and KHP control without aerator exhibited minimal change in its concentration which could emphasize the significance of the seed and aerator in the biodegradation of the samples.
Conclusion
In conclusion, BOD5 seed inoculum was capable of degrading 1000 ppm benzene and 1000 KHP sample. However, 1000 ppm benzene sample are degraded faster than 1000 ppm KHP sample. On the other hand, higher concentrations of KHP sample were not degraded. Furthermore, treatment/degradation of aqueous organic waste using BOD5 seed inoculum is possible. Unseeded samples are also eventually degraded however slower than seeded samples.
References
- Kureel MK, Geed SR, Giri BS, Rai BN, Singh RS (2017) Biodegradation and kinetic study of benzene in bioreactor with PUF and alginate beads and immobilized with Bacillus sp. M3. Bioresource Technology 242: 92-100.
- Kureel MK, Geed SR, Giri BS, Shukla AK, Rai BN, et al. (2016) Removal of aqueous benzene in the immobilized batch and continuous packed bed bioreactor by isolated Bacillus sp. M1. Resource Efficient Technologies 2: 87-95.
- Paixao SM, Santos P, Baeta-Hall L, Tenreiro R, Anselmo AM (2003) Alternative inocula as activated sludge surrogate culture for a toxicity test. Environmental Toxicology 18: 37-44.
- Ormeci B, Vesilind PA (2000) Development of an improved synthetic sludge: a possible surrogate for studying activated sludge dewatering characteristics. Water Research 34: 1069-1078.
- CORDIS (2000) Biological material exhibiting the same metabolic behaviour as activated sludge.
- Fitzmaurice GD, Gray NF (1989) Evaluation of Manufactured Inocula For Use in The BOD test. Water Research 23: 655-657.
Citation: Berina LIM, Ricohermoso SDA, Tejada VAC, Bautista CCJ, Abigail CAP (2018) Biodegradability Study of Potassium Hydrogen Phthalate and Benzene Using BOD5 Seed as Inoculum. J Bioremediat Biodegrad 9: 436. DOI: 10.4172/2155-6199.1000436
Copyright: © 2018 Berina LIM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8425
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 7526
- PDF downloads: 899